Abstract
BACKGROUND
The nucleus accumbens is a critical mediator of depression-related outcomes to social defeat stress. Previous studies demonstrate distinct neuroplasticity adaptations in the two medium spiny neuron (MSN) subtypes, those enriched in dopamine receptor D1 versus dopamine receptor D2, in reward and reinforcement leading to opposing roles for these MSNs in these behaviors. However, the distinct roles of nucleus accumbens MSN subtypes, in depression, remain poorly understood.
METHODS
Using whole-cell patch clamp electrophysiology, we examined excitatory input to MSN subtypes and intrinsic excitability measures in D1-green fluorescent protein and D2-green fluorescent protein bacterial artificial chromosome transgenic mice that underwent chronic social defeat stress (CSDS). Optogenetic and pharmacogenetic approaches were used to bidirectionally alter firing of D1-MSNs or D2-MSNs after CSDS or before a subthreshold social defeat stress in D1-Cre or D2-Cre bacterial artificial chromosome transgenic mice.
RESULTS
We demonstrate that the frequency of excitatory synaptic input is decreased in D1-MSNs and increased in D2-MSNs in mice displaying depression-like behaviors after CSDS. Enhancing activity in D1-MSNs results in resilient behavioral outcomes, while inhibition of these MSNs induces depression-like outcomes after CSDS. Bidirectional modulation of D2-MSNs does not alter behavioral responses to CSDS; however, repeated activation of D2-MSNs in stress naïve mice induces social avoidance following subthreshold social defeat stress.
CONCLUSIONS
Our studies uncover novel functions of MSN subtypes in depression-like outcomes. Notably, bidirectional alteration of D1-MSN activity promotes opposite behavioral outcomes to chronic social stress. Therefore, targeting D1-MSN activity may provide novel treatment strategies for depression or other affective disorders.
Keywords: Depression, Medium spiny neurons, Nucleus accumbens, Optogenetics, Pharmacogenetics, Social defeat stress
Converging human and rodent studies demonstrate a critical role for the nucleus accumbens (NAc) in depression symptomatology including reduced motivation and anhedonia (1–6). The NAc integrates information from afferent inputs leading to motivation and reward functions (7,8). Recent studies demonstrate these NAc afferent inputs become dysfunctional after stressful stimuli resulting in altered cellular and molecular mechanisms in NAc that mediate depression-like outcomes (1–4,9–14). Additionally, clinical studies demonstrate a role for altered NAc activity in depression since high-frequency deep brain stimulation (DBS) has antidepressant effects in individuals with treatment-resistant depression (15–19). Despite the important role for NAc in mediating emotional behaviors in depression, the function of the two main projection neuron subtypes, the medium spiny neurons (MSNs), in affective behaviors are poorly understood.
The MSNs in NAc and dorsal striatum are differentially enriched in dopamine receptor D1 versus dopamine receptor D2, as well as other genes (20–22), and they send distinct projections to downstream basal ganglia and reward structures (23–25). NAc D1-MSNs project to the ventral pallidum, globus pallidum internal, ventral tegmental area (VTA), and substantia nigra, while NAc D2-MSNs project to the ventral pallidum (24,25). These two neuronal populations work together to promote normal behavioral output, while imbalance of one MSN subtype can drive dysfunctional motivational states (26–29). This network balance model is supported by positive and negative outcome tasks in humans and studies in rodents, which demonstrate a role for activation of the D1-MSN pathway in positive reward and activation of the D2-MSN pathway in aversion, while inhibition of these pathways produces opposing outcomes (28,30–32), implicating imbalance of these MSN subtypes in psychiatric and neurological disease. Indeed, rodent studies shed light on D1-MSN versus D2-MSN function, demonstrating that both NAc and dorsal striatum MSN subtypes have opposing roles in reward, action-value, reinforcement, motor function, and sensitized responses to drugs of abuse (26,29,33–40). Additionally, a recent study demonstrated that decreased excitatory synaptic strength of NAc D1-MSN synapses, but not D2-MSN synapses, mediates anhedonia after restraint stress (5). Furthermore, using a highly validated model of depression, chronic social defeat stress (CSDS) (1,2,41), we demonstrated opposite molecular properties in MSN subtypes in mice that are susceptible (those displaying depression-like behaviors) versus resilient (those that do not display depression-like behaviors) to CSDS (14).
To provide new insight into the function of the NAc MSN subtypes in depression, we examined excitatory synaptic input and intrinsic excitability in MSNs after CSDS. We then used optogenetic or pharmacogenetic approaches to test, for the first time in vivo, how repeated manipulation of activity in the MSN subtypes alters depression-like outcomes to social defeat stress. Our data demonstrate that excitatory input onto MSN subtypes, in mice displaying susceptible phenotypes after CSDS, is bidirectionally altered in NAc MSNs. Further, repeated bidirectional control of activity in D1-MSNs can oppositely alter behavioral responses to CSDS, whereas repeated activation of D2-MSNs in stress naïve mice can induce a depression-like behavior to a subthreshold social defeat stress (SSDS). Our findings provide new insight into the role of the two NAc MSN subtypes in depression-like outcomes to social stress.
METHODS AND MATERIALS
Experimental Subjects
Male D1-GFP and D2-GFP hemizygote mice (42) (gensat.org) on a C57BL/6J background were used for electrophysiological recordings. Male Drd1a-Cre (D1-Cre, Line FK150) or Drd2a-Cre (D2-Cre, Line ER44) hemizygote bacterial artificial chromosome transgenic mice (42,43) on a C57BL/6J background were used for optogenetic and pharmacogenetic experiments. CD1 retired breeders (Charles River, Raleigh, North Carolina) were obtained at ~3 months old. Experimental mice were 8 weeks of age and CD1 retired breeders were ≥3 months of age at the start of the experiment. Mice were maintained on a 12-hour light/dark cycle with ad libitum food and water. All studies were conducted in accordance with the guidelines set up by the Institutional Animal Care and Use Committee at the University of Maryland School of Medicine.
Adeno-Associated Virus and Optic Fiber Stereotaxic Surgeries
Mice were anesthetized with 3% isoflurane and underwent stereotaxic surgery to inject serotype 5 adeno-associated viruses (AAV) (UNC Viral Vector Core, Chapel Hill, North Carolina) and implant optic fibers. For stimulation experiments, D1-Cre and D2-Cre mice were stereotaxically injected bilaterally into the NAc (anterior/posterior: +1.6, lateral: +1.5, dorsal/ventral: −4.4 from top of skull) with a double inverted open reading frame (DIO) AAV (36,40,44). For cell-type specific expression, D1-Cre and D2-Cre mice were injected with DIO-AAVs containing ChR2(E123A)-enhanced yellow fluorescent protein (EYFP), also known as ChETAA-EYFP, EYFP, or the inhibitory designer receptor exclusively activated by a designer drug (DREADD) construct hM4(Gi)-mCherry (45). Virus was infused at a rate of .1 μL per minute. The injection needle was left in place for 5 to 10 minutes following the infusion. For optogenetics, mice were implanted with 4 mm chronically implantable fibers (.22 numerical aperture, 105 micrometer core) following CSDS or before SSDS (40,44,46).
Slice Electrophysiological Recordings
Whole-cell patch clamp recordings occurred 2 to 4 weeks following social interaction (SI). D1-GFP or D2-GFP mice were perfused with oxygenated (95% oxygen, 5% carbon dioxide) ice-cold artificial cerebrospinal fluid (ACSF) containing (in mmol/L): 125 sodium chloride, 25 sodium bicarbonate, 10 glucose, 3.5 potassium chloride, 1.25 monosodium phosphate, .1 calcium chloride, 3 magnesium chloride, pH 7.45; osmolarity 285 to 295 mOsm. Brains were rapidly extracted and 300 μm coronal sections containing the NAc were cut with a vibratome in ice-cold ACSF. Slices were incubated for 1 hour at 33°C before recording. For recording, ACSF calcium ion concentrations were altered (in mmol/L): 2 magnesium chloride and 1 calcium chloride. All recordings were performed at 33°C in oxygenated ACSF.
MSNs were visualized under differential interference contrast with a 40× water-immersion objective (Olympus, Center Valley, Pennsylvania). Patched cells were verified to be D1-MSNs or D2-MSNs by green fluorescent protein (GFP), EYFP, or mCherry fluorescence using a QiClick camera (Q-Imaging, Surrey, Canada). Recordings were obtained using a computer-controlled amplifier (MultiClamp 700B; Molecular Devices, Sunnyvale, California). Signals were digitized at 20 kHz (Digidata 1322; Molecular Devices) and acquired with Axo-Scope 9 (Molecular Devices). Patch pipettes were pulled with resistances of 3 to 7 MΩ. For intrinsic excitability recordings and validation recordings, patch pipettes were filled with (in mmol/L): 115 potassium-gluconate, 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 2 magnesium chloride, 20 potassium chloride, 2 magnesium adenosine triphosphate, 2 adenosine-5′-triphosphate disodium salt, .3 guanosine-5′-triphosphate, pH 7.4; 285 to 295 mOsm.
In current clamp, current was injected to hold cells at −75 mV. Cells with resting membrane potentials > −70 mV were excluded. Rheobase was determined by injecting a ramp of current and examining the current needed to elicit the first spike. The number of spikes elicited from current injections was determined by injecting 500 millisecond square currents from 50 to 400 pA. For voltage clamp recordings, a cesium-gluconate internal solution was used (in mmol/L): 115 cesium-gluconate, 20 cesium chloride, 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 2 magnesium chloride, 2 magnesium adenosine triphosphate, 2 adenosine-triphosphate sodium salt, .3 guanosine-5′-triphosphate, pH brought to 7.3 with cesium hydroxide (290–295 mOsm). In voltage clamp recordings, miniature excitatory postsynaptic current (mEPSC) was observed at a −70 mV holding potential and performed in the presence of 50 mmol/L picrotoxin and 1.5 μmol/L tetrodotoxin. Responses were verified to be excitatory by wash in 20 μmol/L of 6-cyano-7-nitroquinoxaline-2,3-dione and 100 μmol/L of 2-amino-5-phosphonopentanoic acid (data not shown). If series resistance was altered by more than 20%, recordings were discarded.
D1-Cre and D2-Cre mice were injected with AAV-DIO-ChETAA-EYFP for opsin validation or AAV-DIO-hM4(Gi)-mCherry for DREADDs validation. Cells were tested for a response in current clamp with a short 5-millisecond 473 nm blue light pulse and stimulated at 50 Hz for 5 minutes. For DREADDs validation, patched cells were exposed to a pulse ramp protocol, similar to excitability recordings, before and 1 hour after 1 μmol/L clozapine-N-oxide (CNO) (LKT Laboratories, Inc., St. Paul, Minnesota) wash on.
CSDS and Social Interaction Test
D1-Cre, D2-Cre, D1-GFP, and D2-GFP mice underwent 10-day CSDS. This is a well-established protocol that yields stress susceptible (mice displaying depression-like behaviors) or resilient cohorts (1,2,41). Experimental mice were exposed to an aggressive retired CD1 breeder for 10 minutes per day and then housed within the same cage as the CD1 on the opposite side of a perforated divider to maintain sensory contact. This was repeated for 10 days with a novel CD1 mouse on each day. On day 11, mice were screened in a SI behavior. Animals were placed in an open field with a perforated box located in a designated interaction zone for 2.5 minutes and assessed for time spent in the interaction zone using TopScan video tracking software (CleverSys, Reston, Virginia). Subsequently, a novel CD1 was placed in the perforated box and experimental mice were assessed for time spent interacting with the novel social target in the interaction zone. Mean interaction time was assessed in each experiment. For each experiment, animals were deemed susceptible if interaction times were two standard deviations below the mean of nondefeated control animals or resilient if they did not fall into this category. Mice that received CSDS and optogenetic or pharmacogenetic manipulations underwent a second SI.
Sucrose Preference Test
Sucrose preference was examined the 2 days following the second SI test for CSDS mice or the first SI test for SSDS mice (see below) using a two-bottle choice preference paradigm. Two days before sucrose, animals were habituated to two bottles filled with water. For testing, bottles were filled with either 1% sucrose or water and placed evenly on two sides of a cage top. Bottles were alternated to the opposite side following each daily measurement. Preference was measured by examining the change in liquid weight of sucrose-containing bottles compared with water bottles (sucrose day 1 − sucrose day 2)/[(sucrose day 1 − sucrose day 2) + (water day 1 − water day 2)].
In Vivo Optogenetic Stimulation After CSDS
Following CSDS, D1-Cre and D2-Cre mice were implanted with chronic fibers (see above) and stimulated with 473 nm blue light two times a day (morning and evening), 15 minutes per session, for 5 days at 50 Hz, 5-second on/off cycles, 50% duty cycle, 3 to 5 mW from tip of fiber using 473 nm diode-pumped solid-state lasers obtained from OEM Laser Systems (Midvale, Utah). On day 26, mice were stimulated once in the morning before SI in their home cage, which was placed adjacent to the CD1 aggressor cage.
SSDS and Optogenetic Stimulation
On day 1, D2-Cre mice were injected with AAV-DIO-ChETAA-EYFP and implanted with 4 mm chronically implantable fibers. On days 4 to 17, mice were stimulated morning and evening with 50 Hz, 473 nm blue light stimulation, 5-second on/off cycles, 50% duty cycle (see above). On day 18, mice were exposed to three social defeat sessions: 5 minutes of physical defeat followed by 15 minutes with no defeat. During the 15 minutes of no social defeat, mice received blue light stimulation.
Pharmacogenetic Inhibition After CSDS
For repeated inhibition experiments, D1-Cre and D2-Cre mice were stereotaxically injected bilaterally with AAV-DIO-hM4D (Gi)-mCherry and subsequently run through CSDS on days 7 to 17. On days 19 to 22, animals were injected at 8:00 AM and 6:00 PM intraperitoneally with 5 mg/kg of CNO (LKT Laboratories), the ligand designed to activate the receptors (37,45,47,48), or .9% saline. On day 23, animals were injected at 8:00 AM before SI.
Immunohistochemistry
D1-Cre and D2-Cre mice were perfused with 4% paraformaldehyde in 1× phosphate buffered saline, and brains were extracted and left in paraformaldehyde solution overnight. Brains were cryoprotected in 30% sucrose and cryosectioned at 35 μm (Leica, Buffalo Grove, Illinois). Immunofluorescence was performed according to previous studies (36,40). Sections were blocked for 1 hour in 3% normal donkey serum with .3% Triton-X then incubated in 1:5000 chicken anti-GFP primary antibody (Aves Labs, Tigard, Oregon) for EYFP or 1:1000 rabbit anti-dsRed primary antibody (Clontech, Mountain View, California) for mCherry overnight. Brain sections were rinsed with 1× phosphate buffered saline and underwent 1 hour incubation in secondary antibody: 1:1000 donkey anti-chicken Alexa488 (Jackson ImmunoResearch, West Grove, Pennsylvania) or 1:500 Cy3 anti-rabbit (Jackson ImmunoResearch). Immunofluorescence imaging was performed on an Olympus Bx61 confocal microscope.
Statistical Analyses
Analyses were performed using Graphpad Prism 5 software (La Jolla, California). Behavioral data from one SI session and electrophysiological data were analyzed by one-way analysis of variance (ANOVA) analyses. Two-way repeated measure ANOVA analysis was used to analyze behavioral changes for pretreatment and posttreatment (light or CNO; i.e., two SI sessions). Two-way ANOVA tests were used to analyze differences in sucrose preference. Differences between the nondefeated group and other groups were determined by Bonferroni post hoc tests.
RESULTS
Bidirectional Changes in Excitatory Input in MSN Subtypes from Susceptible Mice
Using whole-cell patch clamp recordings, we examined physiological properties in MSN subtypes in D1-GFP or D2-GFP bacterial artificial chromosome transgenic mice (42) that are susceptible (those displaying depression-like behaviors) or resilient (those that do not display depression-like behaviors) to a 10-day CSDS (1,2). CSDS is a well-validated stress-induced model of depression (1–4,12) in which experimental mice undergo 10-minute social defeat episodes with a novel retired CD1 breeder each day for 10 days. On day 11, mice were assayed in a SI test and separated into susceptible and resilient cohorts based on their SI scores (2) (Figure S1 in Supplement 1). We examined excitatory synaptic input onto MSN subtypes after CSDS. Susceptible mice display decreased time interacting with a novel social target, while resilient mice interact with the social target similar to non-defeated animals (Figure 1A). There was no difference in interaction time without the target present between all groups (Figure 1A). D1-MSN mEPSC frequency was decreased in susceptible conditions, while amplitude remained unchanged (Figure 1B). In contrast, the frequency of mEPSCs increased in D2-MSNs from susceptible mice, with no alterations in mEPSC amplitude (Figure 1C).
Figure 1.
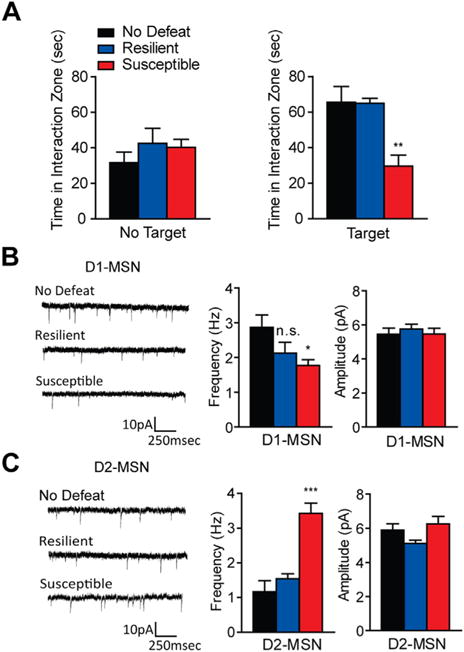
Excitatory input is altered in medium spiny neuron (MSN) subtypes after chronic social defeat stress. (A) Susceptible mice, used to examine alterations in excitatory input, spend significantly less time interacting with a novel mouse, while resilient mice used in these measures interact the same as control (no defeat) animals (one-way analysis of variance [ANOVA] F2,19 = 12.00, p < .01, n = 6–8 per group). No differences were observed when a social target was not present (one-way ANOVA F2,19 = .80, p > .05). (B–C) Excitatory synaptic input measures in dopamine receptor D1-MSNs and dopamine receptor D2-MSNs in susceptible and resilient mice after chronic social defeat stress. (B) Susceptible mice displayed decreased miniature excitatory postsynaptic current (mEPSC) frequency in D1-MSNs (one-way ANOVA F2,33 = 3.49, p < .05, n = 9–13 cells per group) with no change in mEPSC amplitude (one-way ANOVA F2,33 = .29, p > .05). (C) D2-MSNs from susceptible animals displayed increased mEPSC frequency in D2-MSNs (one-way ANOVA F2,23 = 23.87, p < .0001, n = 7–9 cells per group) but not in mEPSC amplitude (one-way ANOVA F2,23 = .23, p > .05). Groups were compared to No Defeat group: *p < .05, **p < .01, ***p < .001. Error bars represent standard error measure (SEM). n.s., nonsignificant.
Intrinsic Excitability Is Altered in D1-MSNs from Susceptible Animals
To determine if the change in excitatory synaptic input corresponds to excitability changes in MSN subtypes after CSDS, we measured the intrinsic excitability of these MSN subtypes. Susceptible animals displayed significantly decreased time interacting with a novel target as compared with no defeat and resilient animals (Figure 2A). In D1-MSNs from susceptible mice, we observed an increase in current-induced neuronal firing at 200 to 300 pA, with a decrease in rheobase (the current needed to elicit firing) (Figure 2B), while resilient mice displayed no change in D1-MSN intrinsic excitability (Figure 2B). Intrinsic excitability and current-induced neuronal firing were unaltered in D2-MSNs in susceptible and resilient mice (Figure 2C).
Figure 2.
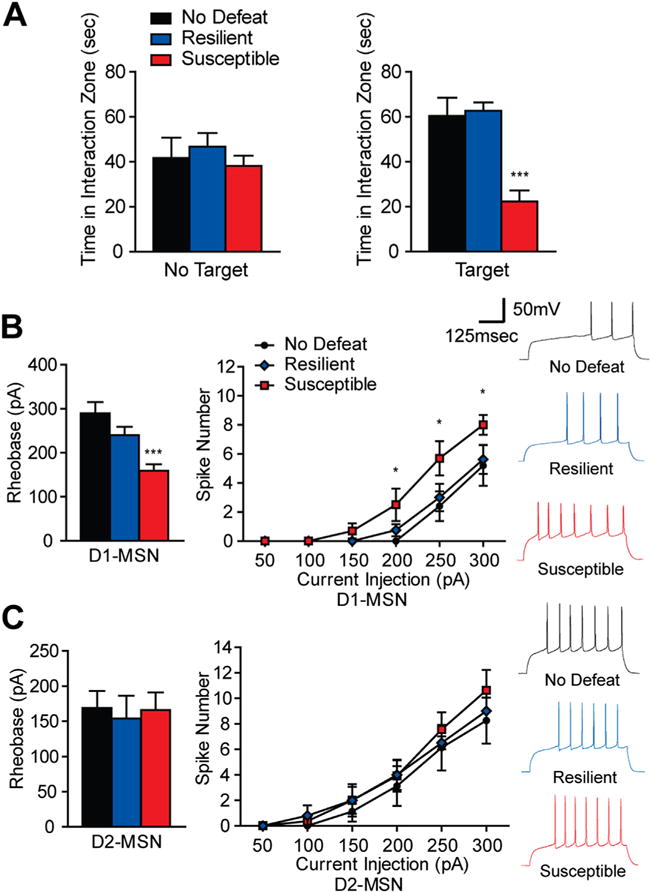
Intrinsic excitability is altered in dopamine receptor D1-medium spiny neurons (MSNs) after chronic social defeat stress (CSDS). (A) Susceptible mice, used to examine intrinsic excitability, spend significantly less time in the interaction zone, while resilient mice interact the same as control animals (one-way analysis of variance [ANOVA] F2,21 = 16.50, p < .0001, n = 6–10 animals per group). No target conditions were similar across groups (one-way ANOVA F2,21 = .54, p > .05). (B–C) Intrinsic excitability measures of D1-MSNs and D2-MSNs from susceptible and resilient mice after CSDS. (B) Susceptible mice display increased rheobase, the amount of current needed to elicit an action potential (one-way ANOVA F2,30 = 10.71, p < .001, n = 8–13 cells per group) and current-evoked spikes in D1-MSNs (two-way ANOVA with a main effect of CSDS phenotype F2,119 = 8.15, p < .001, n = 5–8 cells per group). (C) In D2-MSNs, no changes were observed in rheobase (one-way ANOVA F2, 27 = .08, p > .05, n = 8–12 cells per group) or current-evoked spiking (two-way ANOVA no effect of CSDS phenotype F2,115 = 1.23, p > .05, n = 6–11 cells per group). Groups were compared to No Defeat group: *p < .05, **p < .01, ***p < .001. Error bars represent SEM.
Optogenetic Stimulation of D1-MSNs After CSDS Promotes Resilience
Since we observed altered excitatory synaptic input and/or intrinsic excitability in MSN subtypes after CSDS, we next used an optogenetic strategy to enhance activity of D1-MSNs or D2-MSNs in susceptible or resilient mice (34–36,38,39,47). We performed blue (473 nm) light stimulation of the channelrhodopsin ChR2(H134) and ChETAA-expressing D1-MSNs, D2-MSNs, or total NAc neurons (Figure 3; Figure S2 in Supplement 1). We previously demonstrated selective expression of ChR2(H134)-EYFP in MSN subtypes of D1-Cre and D2-Cre mice (36) and observed ChETAA-EYFP expression in NAc cell bodies and appropriate terminals of D1-Cre and D2-Cre mice (Figure 3A). Blue light (473 nm) stimulation reliably activated MSNs expressing ChETAA (Figure 3B). Acute stimulation of all NAc neurons or NAc MSN subtypes in susceptible mice during SI produced no behavioral changes (Figure S2A in Supplement 1).
Figure 3.
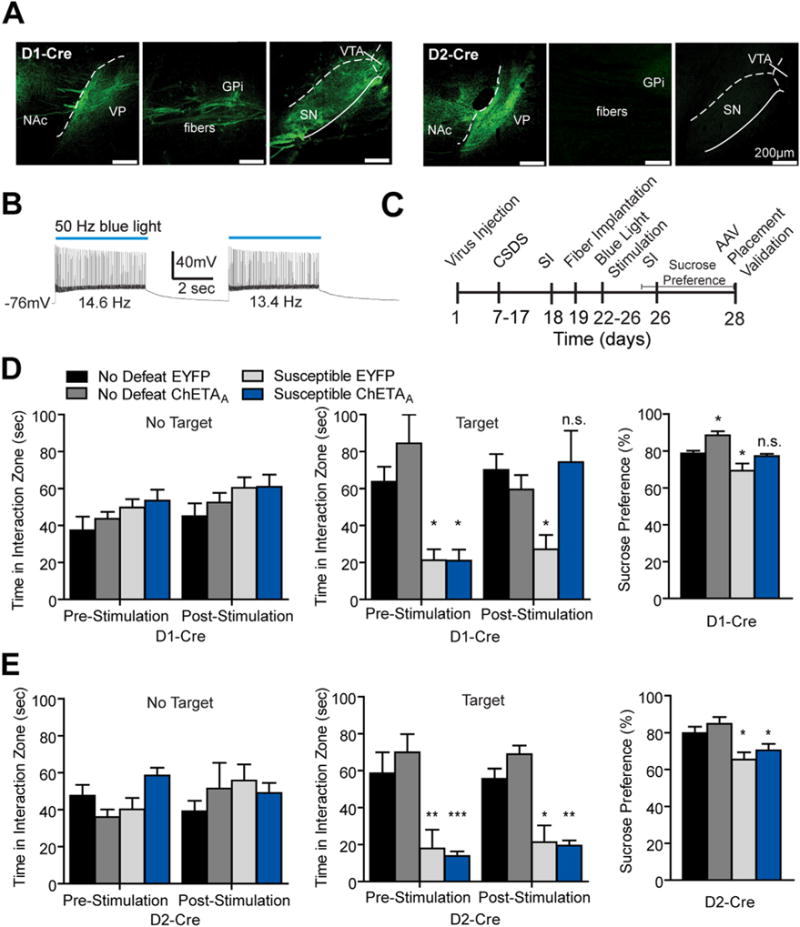
Repeated activation of dopamine receptor D1-medium spiny neurons (MSNs) but not dopamine receptor D2-MSNs following chronic social defeat stress (CSDS) promotes resilience. (A) Sagittal brain images in D1-Cre and D2-Cre animals demonstrating ChETAA expression in appropriate terminals of each MSN subtype. ChETAA expressing D1-MSNs project to ventral pallidum (VP), globus pallidus internal (GPi), ventral tegmental area (VTA), and substantia nigra (SN) and D2-MSNs project to VP (scale bar 200 μm). (B) 50-Hz blue light stimulation of ChETAA elicits 13 Hz to 14 Hz firing frequencies in a representative MSN. (C) Experimental timeline of CSDS, repeated optogenetic activation, and behavioral assays in D1-Cre and D2-Cre mice. (D) Repeated 50-Hz blue light pulses to the nucleus accumbens (NAc) of susceptible D1-Cre mice expressing ChETAA produces an increase in time spent interacting with a novel CD1 target (two-way repeated measures analysis of variance [ANOVA] significant interaction F3,20 = 6.26, p < .01, with a main effect of stimulation F3,20 = 7.88, p < .01, n = 4–8 animals per group) but does not alter interaction time without the social target (two-way repeated measures ANOVA nonsignificant (n.s.) interaction F3,20 = 1.52, p > .05). Repeated 50-Hz blue light pulses to D1-MSNs also enhanced sucrose preference in susceptible and no defeat control animals (two-way ANOVA main effect of stimulation F1,24 = 13.96, p = .001 and CSDS F1,24 = 18.81, p < .001). (E) Repeated 50-Hz blue light pulses to the NAc of susceptible D2-Cre animals does not alter time spent interacting with the target or no target (two-way repeated measures ANOVA nonsignificant interaction: target F3,19 = .21, p > .05; no target: F3,19 = 2.28, p > .05) and mice remained anhedonic as measured by sucrose preference (two-way ANOVA nonsignificant interaction F1,19 = .00003, p > .05; n = 4–8 per group). Groups were compared with no defeat enhanced yellow fluorescent protein (EYFP) group using a Bonferroni post hoc test: *p < .05, **p < .01, ***p < .001. Error bars represent SEM. AAV, adeno-associated virus; SI, social interaction.
Recent studies demonstrate that repeated optogenetic stimulation paradigms alter emotional behaviors and molecules implicated in such behaviors (14,36,49,50). Furthermore, repeated high-frequency stimulation (HFS) of the NAc in DBS patients promotes antidepressant effects in individuals with treatment-resistant depression (15,17,18). Therefore, we assessed a range of stimulation frequencies of NAc neurons after CSDS in mice injected with ChETAA or ChR2(H134) using a repeated stimulation paradigm (Figure 3C; Figure S2B in Supplement 1). Five days of repeated high-frequency (≥50 Hz) blue light pulses to D1-MSNs restored SI time to no defeat control levels in mice susceptible to CSDS (Figures 3D; Figure S2B in Supplement 1) and this stimulation did not alter interaction time when the novel social target was not present. Repeated 50-Hz activation of D1-MSNs promoted similar sucrose consumption in susceptible mice compared with EYFP no defeat control animals. However, we also observed an increase in sucrose preference when activating D1-MSNs in no defeat control animals (Figure 3D), suggesting increases in sucrose preference was a result of stimulation alone in defeated and nondefeated groups. Additionally, repeated HFS (≥50 Hz) of total NAc expressing AAV-hSyn-ChETAA-EYFP neurons enhanced the SI time in susceptible mice, similar to the D1-MSN results (Figure S2B in Supplement 1). We observed no change in SI or sucrose preference following 50 Hz repeated stimulation in MSN subtypes of susceptible D2-Cre and resilient D1-Cre and D2-Cre mice (Figure 3E; Figure S3A in Supplement 1). There was no observed change in locomotor activity in D1-Cre and D2-Cre mice with the 50 Hz stimulation paradigm (Figure S3B in Supplement 1). Furthermore, 50 Hz stimulation had no effects on expression of cell death markers and NeuN in NAc (Figure S3C in Supplement 1).
Pulses (50 Hz) to ChETAA-expressing MSNs does not elicit these higher frequency firing rates in whole-cell patch clamp slice recordings but instead firing rates of ~13 to 14 Hz (Figure 3B). For this reason, we assayed MSN neuronal firing in vivo using multi-unit recordings. We observed ~30 Hz maximal firing rates in the overall responding neuronal population with 50 Hz blue light pulses in representative multi-unit recordings (Figure S4A in Supplement 1). Furthermore, 5 days of repeated 50 Hz blue light pulses to D1-MSNs produced no difference between overall firing rates and the averaged multi-unit firing rates of MSNs on day 1 versus day 5 (Figure S4B in Supplement 1), suggesting ChETAA-mediated firing rates are not hindered by repeated optogenetic stimulation.
Stimulation of D2-MSNs in Stress Naïve Mice Promotes Susceptibility to SSDS
Since we observed a change in excitatory input onto D2-MSNs in susceptible mice (Figure 1C) but were unable to alter behavioral outcomes to CSDS when activating these MSNs (Figure 3E), we then tested whether repeated activation of D2-MSNs in stress naïve mice would confer vulnerability to a 1-day SSDS (2–4,12,13,51). D2-Cre mice received blue light, 50-Hz priming pulses to ChETAA-expressing NAc D2-MSNs for 4 days before SSDS (Figure 4). Mice then underwent three 5-minute social defeat episodes with 15-minute time periods during which D2-MSNs received blue light pulses in their home cages. We found that this priming stimulation paired with stimulation of D2-MSNs between SSDS sessions could induce susceptibility in SI with no change in sucrose preference (Figure 4B). This stimulation paradigm had no effect on time spent in the interaction zone without the novel social target present or on locomotor behavior (Figure 4B; Figure S4B in Supplement 1).
Figure 4.
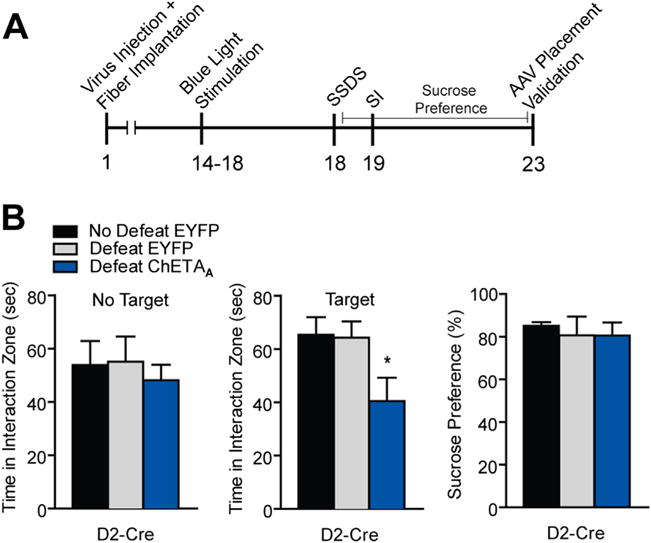
Repeated activation of dopamine receptor D2-medium spiny neurons (MSNs) in stress naïve mice induces susceptibility to subthreshold social defeat stress (SSDS). (A) Experimental timeline of D2-Cre optogenetic stimulation and SSDS. (B) D2-Cre mice receiving repeated priming 50-Hz blue light pulses to nucleus accumbens (NAc) before SSDS and during SSDS displayed reduced social interaction (SI) (one-way analysis of variance [ANOVA] F2,28 = 3.75, p < .05, n = 6–8 animals per group) with no alteration in no target interaction (one-way ANOVA F2,28 = .26, p > .05). Sucrose preference was unaltered in these conditions (one-way ANOVA F2,13 = .10, p > .05). Groups were compared with no defeat enhanced yellow fluorescent protein (EYFP) group using a Bonferroni post hoc test: *p < .05. Error bars represent SEM. AAV, adeno-associated virus.
Pharmacogenetic Inhibition of D1-MSNs Shifts Resilient Mice to a Susceptible Phenotype
To determine if inhibiting NAc neurons could alter depression-like outcomes to CSDS, we first examined acute optogenetic inhibition of NAc during SI. C57BL/6J CSDS mice were injected in the NAc with AAV-hsyn-eNpHR3.0. No effects were observed from acute inhibition of the NAc in both susceptible and resilient animals (Figure S5 in Supplement 1). Next, we took a pharmacogenetic approach to repeatedly silence MSN subtypes using the hM4(Gi) inhibitory DREADD (37,39,47,48). D1-Cre or D2-Cre mice received stereotaxic infusion of the DIO-AAV-hM4(Gi)-mCherry to NAc. We observed hM4(Gi)-mCherry expression in NAc cell bodies and respective D1-MSN and D2-MSN terminals (Figure 5A) and we reliably inhibited neuronal firing with the hM4(Gi) ligand, CNO (1 μmol/L; Figure 5B). Following CSDS, CNO (5 mg/kg) or saline was administered (intraperitoneal injections) (48) for 5 days to CSDS D1-Cre and D2-Cre mice (Figure 5C). Repeated inhibition of D1-MSNs in resilient mice decreased SI time and sucrose preference (Figure 5D). No effect was observed with CNO administration to resilient D2-Cre mice (Figure 5E) or susceptible mice of both genotypes (Figure S5B,C in Supplement 1). CNO treatment did not alter interaction zone times when the novel social target was not present (Figure 5D,E and Figure S6A in Supplement 1), nor did it have effects on locomotion (Figure S5B in Supplement 1). NeuN and cell death markers were unaltered after this 5-day inhibition paradigm (Figure S6C in Supplement 1).
Figure 5.
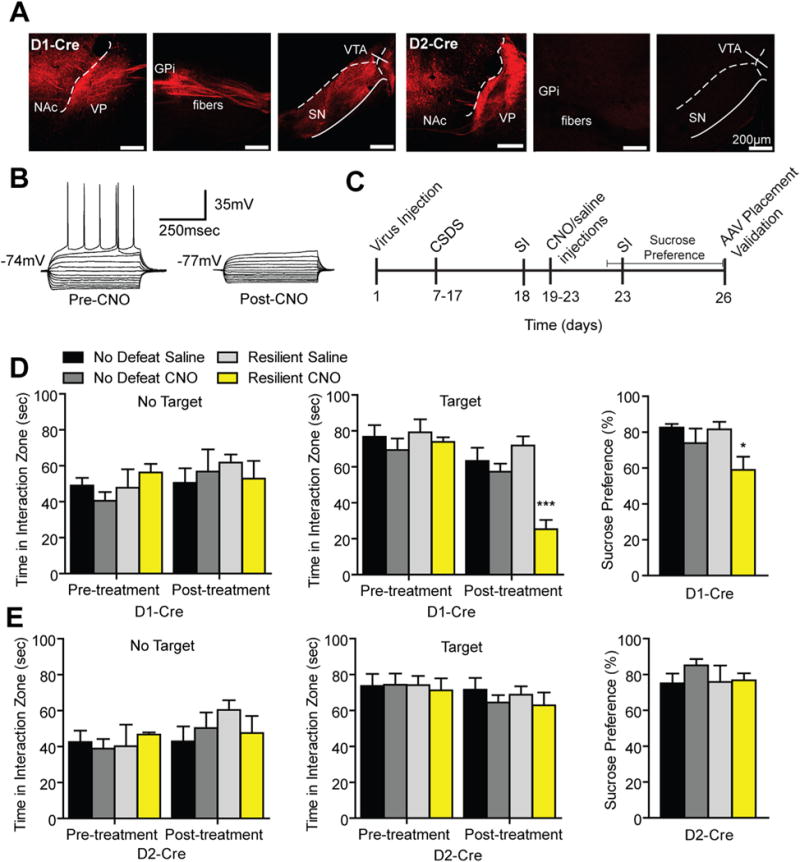
Repeated pharmacogenetic inhibition of dopamine receptor D1-medium spiny neurons (MSNs) but not dopamine receptor D2-MSNs induces a prodepressive phenotype. (A) Experimental timeline of chronic social defeat stress (CSDS) and repeated pharmacogenetic inhibition of D1-Cre and D2-Cre mice. (B) Sagittal confocal images of hM4(Gi)-mCherry expression D1-MSNs and D2-MSNs in the nucleus accumbens (NAc). hM4(Gi) expressing D1-MSNs project to ventral pallidum (VP), globus pallidus internal (GPi), ventral tegmental area (VTA), and substantia nigra (SN) and D2-MSNs project to VP (scale bar 200 μm). (C) Representative traces from a current clamp slice recording of an MSN expressing the hM4(Gi) receptor. A decrease in spiking and input resistance was observed 1 hour following clozapine-N-oxide (CNO) wash on. (D) In resilient D1-Cre mice, repeated CNO injections reduced social interaction (SI) time (two-way repeated measures analysis of variance [ANOVA] significant interaction F3,21 = 5.40, p < .01 with a main effect of treatment F3,21 = 6.354, p < .01; n = 4–8 animals per group) and decreased sucrose preference (two-way ANOVA significant interaction F1,21 = 11.19, p < .01 with a main effect of treatment F1,21 = 11.65, p < .01) without altering interaction zone times without the target present (two-way repeated measures ANOVA nonsignificant interaction F3,21 = 1.13, p > .05). (E) No effects were observed in time spent in the interaction zone with the target or no target (two-way repeated measures ANOVA nonsignificant interaction: target F3,15 = .26, p > .05; no target F3,15 = 1.28, p > .05) or in sucrose preference (two-way ANOVA nonsignificant interaction F1,15 = .52, p > .05) in resilient D2-Cre mice. Groups were compared with no defeat saline group using a Bonferroni post hoc test: *p < .05, ***p < .001. Error bars represent SEM. AAV, adeno-associated virus.
DISCUSSION
Our study validates distinct roles for NAc MSN subtypes in mediating behavioral outcomes to social defeat stress. We demonstrate that repeated optogenetic enhancement of D1-MSN firing restores normal social interaction and sucrose preference, whereas pharmacogenetic-mediated inhibition of these neurons produces social avoidance and anhedonia. Furthermore, repeated stimulation of D2-MSNs produces social avoidance following a SSDS paradigm without changing sucrose preference. These results agree with findings demonstrating repeated restraint stress selectively produces anhedonia through attenuation of the strength of NAc D1-MSNs excitatory synapses (5). We observed a reduction in excitatory signaling to D1-MSNs following CSDS, which further supports this point. In contrast, recent studies demonstrate enhancement of excitatory signaling and spine growth in MSNs of susceptible mice (4,52). Our findings suggest these observed plasticity changes are weighted toward D2-MSNs, since we identified enhanced excitatory input to these MSNs in susceptible mice.
It is unclear which excitatory afferents regulate the bidirectional change in excitatory synaptic input onto MSN subtypes in susceptible conditions. The medial prefrontal cortex (mPFC) is a likely candidate because previous studies demonstrate optogenetic manipulation of mPFC or mPFC-NAc terminals promotes resilient responses after CSDS (10,11,53,54). Other brain regions, including the ventral hippocampus (vHipp), are potentially responsible for these cell-type specific excitatory input adaptations through differences in dendritic subcellular connectivity (55). Similar enhancement in reward and reinforcement outcomes of driving vHipp input (8) and D1-MSNs (36) may suggest a role for vHipp afferent signaling to D1-MSNs in stress behaviors. Furthermore, chronic activation of vHipp, as well as mPFC, results in distinct patterns of induction of the transcription factor ΔFosB in NAc MSN subtypes, and ΔFosB mediates resiliency through D1-MSNs, while ΔFosB is induced in D2-MSNs in susceptible mice (3,14). These findings are consistent with our results showing repeatedly driving D1-MSN firing promotes positive, resilient outcomes following stress, while driving D2-MSNs promotes susceptibility.
Surprisingly, acute activation of MSN subtypes or total NAc had no effect on behavioral outcomes to CSDS. It is plausible that repeated stimulation or repeated inhibition, which alters behavioral responses to CSDS or SSDS, results in long-term plasticity adaptations in the NAc (3,4,14,52,56,57) that regulate stress outcomes. Analogously, patients with treatment-resistant depression receiving HFS DBS display larger improvements in depression symptoms after repeated stimulation as compared with acute stimulation (15,17,18). These effects are similar to the resilient outcomes observed from repeated optogenetic HFS to the total NAc or D1-MSNs. It is also unclear as to why HFS is effective, whereas <50 Hz stimulation is not. The effectiveness of HFS DBS could be potentially mediated by frequency band shifts in local field potential oscillations and mesolimbic synchrony as a result of HFS (58).
The observed enhanced intrinsic excitability in susceptible D1-MSNs was surprising. However, this alteration could reflect a homeostatic plasticity mechanism occurring in these cell subtypes to compensate for attenuation in excitatory synaptic input. It is plausible that neuromodulatory signaling, via increased phasic VTA dopamine neuron firing, release of dopamine, or brain-derived neurotrophic factors from VTA inputs, which has been demonstrated in animals susceptible to CSDS (1,2,12,13,59), mediates this potential homeostatic mechanism.
Our study demonstrates a dynamic role for MSN subtypes in depression-like outcomes to social defeat stress. Intriguingly, enhancement of D1-MSN activity reverses the social avoidance and anhedonia behaviors characteristic of susceptible mice after CSDS, while enhancement of D2-MSN activity promotes susceptibility to SSDS. Collectively, our study reveals opposing actions of these MSN subtypes in behavioral outcomes to social stress.
Supplementary Material
Acknowledgments
This study was supported by the Brain and Behavioral Research Foundation National Alliance for Research on Schizophrenia and Depression Young Investigator grant.
We thank S. Thompson and B. Mathur at University of Maryland School of Medicine and D. Chaudhury at Mount Sinai School of Medicine for discussion on slice physiology experiments. We thank M. Sidor at University of Pittsburgh for advice on pharmacogenetic studies. We thank J. Cheer at University of Maryland School of Medicine for helpful discussion about this manuscript.
Footnotes
DISCLOSURES
All authors report no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2014.07.021
References
- 1.Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, et al. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- 2.Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, et al. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, et al. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, et al. IκB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci. 2011;31:314–321. doi: 10.1523/JNEUROSCI.4763-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lim BK, Huang KW, Grueter BA, Rothwell PE, Malenka RC. Anhedonia requires MC4R-mediated synaptic adaptations in nucleus accumbens. Nature. 2012;487:183–189. doi: 10.1038/nature11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Covington HE, 3rd, Maze I, LaPlant QC, Vialou VF, Ohnishi YN, Berton O, et al. Antidepressant actions of histone deacetylase inhibitors. J Neurosci. 2009;29:11451–11460. doi: 10.1523/JNEUROSCI.1758-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Covington HE, 3rd, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci. 2010;30:16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar S, Black SJ, Hultman R, Szabo ST, DeMaio KD, Du J, et al. Cortical control of affective networks. J Neurosci. 2013;33:1116–1129. doi: 10.1523/JNEUROSCI.0092-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh JJ, Friedman AK, Sun H, Heller EA, Ku SM, Juarez B, et al. Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat Neurosci. 2014;17:27–29. doi: 10.1038/nn.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lobo MK, Zaman S, Damez-Werno DM, Koo JW, Bagot RC, DiNieri JA, et al. DeltaFosB induction in striatal medium spiny neuron subtypes in response to chronic pharmacological, emotional, and optogenetic stimuli. J Neurosci. 2013;33:18381–18395. doi: 10.1523/JNEUROSCI.1875-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- 16.Mayberg HS. Targeted electrode-based modulation of neural circuits for depression. J Clin Invest. 2009;119:717–725. doi: 10.1172/JCI38454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bewernick BH, Kayser S, Sturm V, Schlaepfer TE. Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: Evidence for sustained efficacy. Neuropsychopharmacology. 2012;37:1975–1985. doi: 10.1038/npp.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schlaepfer TE, Bewernick BH. Neuromodulation for treatment resistant depression: State of the art and recommendations for clinical and scientific conduct. Brain Topogr. 2014;27:12–19. doi: 10.1007/s10548-013-0315-9. [DOI] [PubMed] [Google Scholar]
- 19.Nauczyciel C, Robic S, Dondaine T, Verin M, Robert G, Drapier D, et al. The nucleus accumbens: A target for deep brain stimulation in resistant major depressive disorder. J Mol Psychiatry. 2013;1:17. doi: 10.1186/2049-9256-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- 21.Lobo MK, Karsten SL, Gray M, Geschwind DH, Yang XW. FACS-array profiling of striatal projection neuron subtypes in juvenile and adult mouse brains. Nat Neurosci. 2006;9:443–452. doi: 10.1038/nn1654. [DOI] [PubMed] [Google Scholar]
- 22.Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–748. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerfen CR. The neostriatal mosaic: Multiple levels of compartmental organization. Trends Neurosci. 1992;15:133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- 24.Nicola SM. The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology (Berl) 2007;191:521–550. doi: 10.1007/s00213-006-0510-4. [DOI] [PubMed] [Google Scholar]
- 25.Smith RJ, Lobo MK, Spencer S, Kalivas PW. Cocaine-induced adaptations in D1 and D2 accumbens projection neurons (a dichotomy not necessarily synonymous with direct and indirect pathways) Curr Opin Neurobiol. 2013;23:546–552. doi: 10.1016/j.conb.2013.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lenz JD, Lobo MK. Optogenetic insights into striatal function and behavior. Behav Brain Res. 2013;255:44–54. doi: 10.1016/j.bbr.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 27.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 28.Maia TV, Frank MJ. From reinforcement learning models to psychiatric and neurological disorders. Nat Neurosci. 2011;14:154–162. doi: 10.1038/nn.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kravitz AV, Kreitzer AC. Striatal mechanisms underlying movement, reinforcement, and punishment. Physiology (Bethesda) 2012;27:167–177. doi: 10.1152/physiol.00004.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlezon WA, Jr, Thomas MJ. Biological substrates of reward and aversion: A nucleus accumbens activity hypothesis. Neuropharmacology. 2009;56(suppl 1):122–132. doi: 10.1016/j.neuropharm.2008.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lobo MK, Nestler EJ. The striatal balancing act in drug addiction: Distinct roles of direct and indirect pathway medium spiny neurons. Front Neuroanat. 2011;5:41. doi: 10.3389/fnana.2011.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Freeze BS, Kravitz AV, Hammack N, Berke JD, Kreitzer AC. Control of basal ganglia output by direct and indirect pathway projection neurons. J Neurosci. 2013;33:18531–18539. doi: 10.1523/JNEUROSCI.1278-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron. 2010;66:896–907. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466:622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, et al. Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci. 2011;14:22–24. doi: 10.1038/nn.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tai LH, Lee AM, Benavidez N, Bonci A, Wilbrecht L. Transient stimulation of distinct subpopulations of striatal neurons mimics changes in action value. Nat Neurosci. 2012;15:1281–1289. doi: 10.1038/nn.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, et al. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci. 2013;16:632–638. doi: 10.1038/nn.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chandra R, Lenz JD, Gancarz AM, Chaudhury D, Schroeder GL, Han MH, et al. Optogenetic inhibition of D1R containing nucleus accumbens neurons alters cocaine-mediated regulation of Tiam1. Front Mol Neurosci. 2013;6:13. doi: 10.3389/fnmol.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 43.Gerfen CR, Paletzki R, Heintz N. GENSAT BAC crerecombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron. 2013;80:1368–1383. doi: 10.1016/j.neuron.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Francis TC, Lobo MK. Optogenetic regulation of dopamine receptor-expressing neurons. In: Wolfgang W, Mario T, editors. Dopamine Receptor Technologies, Neuromethods ed Springer Protocols. in press. [Google Scholar]
- 45.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci U S A. 2007;104:5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sparta DR, Stamatakis AM, Phillips JL, Hovelso N, van Zessen R, Stuber GD. Construction of implantable optical fibers for long-term optogenetic manipulation of neural circuits. Nat Protoc. 2011;7:12–23. doi: 10.1038/nprot.2011.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferguson SM, Phillips PE, Roth BL, Wess J, Neumaier JF. Direct-pathway striatal neurons regulate the retention of decision-making strategies. J Neurosci. 2013;33:11668–11676. doi: 10.1523/JNEUROSCI.4783-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogan SC, Roth BL. Remote control of neuronal signaling. Pharmacol Rev. 2011;63:291–315. doi: 10.1124/pr.110.003020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmari SE, Spellman T, Douglass NL, Kheirbek MA, Simpson HB, Deisseroth K, et al. Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science. 2013;340:1234–1239. doi: 10.1126/science.1234733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sidor MM, McClung CA. Timing matters: Using optogenetics to chronically manipulate neural circuitry and rhythms. Front Behav Neurosci. 2014;8:41. doi: 10.3389/fnbeh.2014.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.LaPlant Q, Vialou V, Covington HE, 3rd, Dumitriu D, Feng J, Warren BL, et al. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci. 2010;13:1137–1143. doi: 10.1038/nn.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Golden SA, Christoffel DJ, Heshmati M, Hodes GE, Magida J, Davis K, et al. Epigenetic regulation of RAC1 induces synaptic remodeling in stress disorders and depression. Nat Med. 2013;19:337–344. doi: 10.1038/nm.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Challis C, Beck SG, Berton O. Optogenetic modulation of descending prefrontocortical inputs to the dorsal raphe bidirectionally bias socioaffective choices after social defeat. Front Behav Neurosci. 2014;8:43. doi: 10.3389/fnbeh.2014.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vialou V, Bagot RC, Cahill ME, Ferguson D, Robison AJ, Dietz DM, et al. Prefrontal cortical circuit for depression- and anxiety-related behaviors mediated by cholecystokinin: Role of DeltaFosB. J Neurosci. 2014;34:3878–3887. doi: 10.1523/JNEUROSCI.1787-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.MacAskill AF, Little JP, Cassel JM, Carter AG. Subcellular connectivity underlies pathway-specific signaling in the nucleus accumbens. Nat Neurosci. 2012;15:1624–1626. doi: 10.1038/nn.3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilkinson MB, Xiao G, Kumar A, LaPlant Q, Renthal W, Sikder D, et al. Imipramine treatment and resiliency exhibit similar chromatin regulation in the mouse nucleus accumbens in depression models. J Neurosci. 2009;29:7820–7832. doi: 10.1523/JNEUROSCI.0932-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wilkinson MB, Dias C, Magida J, Mazei-Robison M, Lobo M, Kennedy P, et al. A novel role of the WNT-dishevelled-GSK3beta signaling cascade in the mouse nucleus accumbens in a social defeat model of depression. J Neurosci. 2011;31:9084–9092. doi: 10.1523/JNEUROSCI.0039-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McCracken CB, Grace AA. Nucleus accumbens deep brain stimulation produces region-specific alterations in local field potential oscillations and evoked responses in vivo. J Neurosci. 2009;29:5354–5363. doi: 10.1523/JNEUROSCI.0131-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barik J, Marti F, Morel C, Fernandez SP, Lanteri C, Godeheu G, et al. Chronic stress triggers social aversion via glucocorticoid receptor in dopaminoceptive neurons. Science. 2013;339:332–335. doi: 10.1126/science.1226767. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


