Abstract
Rationale
A common treatment strategy for pediatric attention deficit/hyperactivity disorder (ADHD) and major depressive disorder (MDD) is combined methylphenidate (MPH) and fluoxetine (FLX). This has raised concerns because MPH+FLX treatment may have pharmacodynamic properties similar to cocaine, potentially increasing drug abuse liability.
Objectives
To examine the short- and long-term consequences of repeated vehicle, MPH, FLX, MPH+FLX, and cocaine treatment on gene expression in juvenile (postnatal days [PD] 20–34) and adult (PD 70–84) male mice. We further assessed whether juvenile drug treatment influenced subsequent sensitivity for nicotine in adulthood.
Methods
Juvenile and adult C57BL/6J mice received vehicle, MPH, FLX, MPH+FLX, or cocaine twice-daily for 15 consecutive days. Mice were sacrificed 24 h or 2 months after the last drug injection to assess drug-induced effects on the extracellular signal-regulated protein kinase-1/2 (ERK) pathway within the ventral tegmental area. Subsequent sensitivity for nicotine (0.05, 0.07, and 0.09 mg/kg) was measured using the place-conditioning paradigm (CPP) 24 h and 2 months after juvenile drug exposure.
Results
MPH+FLX, or cocaine exposure in juvenile mice increased mRNA expression of ERK2 and its downstream targets (CREB, cFos, and Zif268), and increased protein phosphorylation of ERK2 and CREB 2 months after drug exposure. Similar mRNA findings were observed in the adult-treated mice. Findings on gene expression 24 h following drug treatment were variable. Juvenile drug exposure increased preference for nicotine when tested in adulthood.
Conclusions
Early-life MPH+FLX, or cocaine exposure similarly disrupts the ERK pathway, a signaling cascade implicated in motivation and mood regulation, and increases sensitivity for nicotine in adulthood.
Keywords: ADHD, Depression, Comorbidity, ERK, mTOR, Methylphenidate, Fluoxetine, Cocaine, Nicotine, Conditioned place preference
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is among the most prevalent neuropsychiatric disorders in children and adolescents (Polanczyk et al. 2007; Wigal et al. 2010). Youth with ADHD are at a significantly increased risk for the development of major depressive disorder (MDD) and dysthymia relative to youth without ADHD (Chronis-Tuscano et al. 2010; Daviss 2008a; Spencer 2006). The co-occurrence of ADHD and MDD has a prevalence rate of up to 40 % (Spencer 2006; Waxmonsky 2003), and individuals with comorbid ADHD and MDD have greater psychosocial impairment, experience enhanced sensitivity to stress, higher rates of depression recurrence, and greater health care costs (Chronis-Tuscano et al. 2010; Daviss 2008a; Fishman et al. 2007; Seymour et al. 2011). Thus, comorbid ADHD and MDD is a serious disorder necessitating timely and appropriate therapeutic intervention. The stimulant methylphenidate (MPH; Ritalin), an effective agent for the management of ADHD (Kaplan and Newcorn 2011; Rowles and Findling 2010), and the antidepressant fluoxetine (FLX; Prozac), the only drug approved for treatment of pediatric depression (Safer 2006), are therapeutic agents often combined as a treatment strategy for comorbid ADHD and depression (Efron et al. 2003; Olfson et al. 2002; Rushton and Whitmire 2001). Nevertheless, concerns regarding the safety, adverse effects, and possible sensitivity to other drugs associated with concomitant drug use in youths have emerged (Greenhill et al. 2003; Wolraich 2003; Zito et al. 2008).
Combined MPH and FLX (MPH+FLX) treatment is surprising given the scarcity of clinical and pre-clinical studies assessing drug efficacy, and because together, these drugs may also have a pharmacodymanic profile similar to cocaine (Bhatara et al. 2004; Safer et al. 2003; Steiner et al. 2010). MPH and cocaine inhibit dopamine transporters to increase dopamine tone in mesolimbic brain regions (Bolaños and Nestler 2004; Volkow et al. 2002), and the mechanistic differences between these drugs may be due to inhibition of serotonin transporters by cocaine, because MPH has significantly lower affinity for the serotonin transporter (Han and Gu 2006; Yano and Steiner 2007). Thus, the concurrent use of MPH with the serotonin transporter blocker, FLX, may induce effects similar to those of cocaine by simultaneously inhibiting the reuptake of both serotonin and dopamine as cocaine does. This is especially worrisome because repeated exposure to psychomotor stimulants results in long-lasting neurobiological adaptations implicated in the transition from drug use to abuse (Bolaños and Nestler 2004; Koob and Nestler 1997; Volkow and Li 2004). Additionally, acute and repeated exposure to MPH+FLX results in a robust pattern of immediate early gene (IEG) expression and behavioral reactivity similar to cocaine (Steiner et al. 2010; Van Waes et al. 2010; Warren et al. 2011).
Given that MPH and FLX interact with mesolimbic reward pathways known to control mood and motivation under normal conditions (Bolaños et al. 2003b; Kelley and Berridge 2002; Koob and Le Moal 2008; Naranjo et al. 2001; Nestler and Carlezon 2006), we assessed the neurobiological consequences of repeated MPH, FLX, MPH+FLX, and cocaine exposure in either juvenile (postnatal days [PD] 20–34) or adult male mice (PD70-84). Drug-induced biochemical changes within the ventral tegmental area (VTA), a neural substrate heavily implicated in both drug reward (Iñiguez et al. 2008; Iñiguez et al. 2010c; Lu et al. 2009; Ortiz et al. 1995; Russo et al. 2007) and mood disorders (Duman and Monteggia 2006; Iñiguez et al. 2010a; Krishnan et al. 2008; Nestler and Carlezon 2006) were of particular interest. Specifically, we measured the expression of brain-derived neurotrophic factor’s (BDNF) downstream target, extracellular signal-regulated protein kinase-1/2 (ERK). To further evaluate drug-induced changes in this signaling pathway, we also looked at other targets of ERK, such as cAMP response element-binding protein (CREB), mammalian target of rapamycin (mTOR), and the IEGs cFos and Zif268. Although drug-induced changes in behavior are likely modulated by a multitude of genes through various interacting signaling pathways, the ERK-CREB-mTOR pathway has been implicated in mood regulation and drug reward. Psychostimulant exposure early in life can increase drug use/abuse liability later in life, and MPH+FLX treatment can increase the rewarding properties of cocaine in adulthood. Here, we expand these findings to other psychostimulants by asking whether MPH+ FLX or cocaine exposure would influence behavioral sensitivity to nicotine. Nicotine use is of special interest because it has one of the highest prevalence rates among individuals with mood disorders (Goodman and Capitman 2000; Grant et al. 2004; Leonard et al. 2001).
Materials and methods
Animals
Litters containing C57BL/6J male mice pups with their dams [postnatal day 14 (PD 14) on arrival] (Jackson Laboratory) were used in this study. Mice were housed in clear polypropylene boxes containing wood shavings in an animal colony maintained at 23–25 °C on a 12-h light/dark cycle in which lights were on between 7:00 A.M. and 7:00 P.M. Food and water were provided ad libitum. All procedures were in strict accordance with the guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2003) and approved by the Florida State University Animal Care and Use Committee.
Drugs
MPH, FLX, cocaine hydrochloride, and nicotine hydrogen tartrate were obtained from Sigma-Aldrich (St. Louis, MO). All drugs were dissolved in 0.9 % sterile saline (VEH) and administered in a volume of 4 ml/kg for all drugs. Nicotine solution was adjusted to a pH of 7.4, and the selected dose is expressed as free-base.
Drug treatment
Mice were randomly assigned to the various experimental conditions: drug (VEH, MPH, FLX, MPH+FLX, or cocaine), age of drug exposure (juvenile versus adult), and time of behavioral/biochemical assessment (short-term: 24 h, or long-term: 2 months after the last drug exposure). To avoid oversampling or within litter effects, no more than one pup per litter was assigned to a particular condition (Holson and Pearce 1992; Hughes 1979). That is, mice from the same litter received different drug treatments and remained with their dams until PD24 (weaning) when they were separated by treatment condition into groups of four per cage. Mice received intraperitoneal (i.p.) injections of VEH, MPH (2.0 mg/kg), FLX (5.0 mg/kg), MPH+FLX (2.0 and 5.0 mg/kg, respectively), or cocaine (20 mg/kg) twice daily (4 h apart), from PD20-34 (juveniles) or from PD70-84 (adults) for 15 consecutive days. Juvenile mice weighed between 10 and 15 g while adults weighed between 20 and 25 g. The dose of each drug was selected based on previous reports that have validated these dosing regimens to expected behavioral outcomes and to approximate clinically relevant dosing in humans (Andersen et al. 2002; Bolaños et al. 2003a; Brenhouse et al. 2009; Wargin et al. 1983; Warren et al. 2011). The drug treatment period between PD20-34 was chosen because it parallels childhood in humans (Andersen and Navalta 2004a; Spear 2000). Mice assigned to the short-term biochemical and behavioral testing conditions were tested on PD35, while those assigned to the long-term condition were left undisturbed and tested 2 months after the last drug injection.
Quantitative real-time PCR
Mice were sacrificed either 24 h or 2 months after the last injection of VEH, MPH, FLX, MPH+FLX, or cocaine. Brains were extracted and sliced into 1-mm diameter coronal sections at which time an 18-gauge needle was used to dissect out bilateral VTA punches that were then stored at −80 °C until use. RNA was isolated using Illustra TriplePrep kit (GE Healthcare) according to the manufacturer’s instructions (Valladares et al. 2010). cDNA was then created from these samples using iScript cDNA synthesis kit (Bio-Rad). Quantitative real-time PCRs were performed in duplicates using 96 well PCR plates and RealMasterMix (Eppendorf) with an Eppendorf MasterCycler Realplex2, according to the manufacturer’s instructions. Threshold cycle [C(t)] values were measured using the supplied software and analyzed with the ΔΔC(t) method as previously described (LaPlant et al. 2010; Vialou et al. 2010; Warren et al. 2013). Primer sequences for ERK2 (Mapk1), BDNF (Bdnf), CREB (Creb1), Zif268 (Egr1), cFos (Fos), mTOR (Mtor), and glyceraldehyde-3-phosphate dehydrogenase (Gapdh) are listed in Table 1.
Table 1.
qPCR primers
| Primer sequence |
||
|---|---|---|
| Gene | Forward | Reverse |
| Mapk1 | 5′-GGTTGTTCCCAAATGCTGACT-3′ | 5′-CAACTTCAATCCTCTTGTGAGGG-3′ |
| Creb1 | 5′-AGTGACTGAGGAGCTTGTACCA-3′ | 5′-TGTGGCTGGGCTTGAAC-3′ |
| Bdnf | 5′-GAAGAGCTGCTGGATGAGGAC-3′ | 5′-TTCAGTTGGCCTTTTGATACC-3′ |
| Fos | 5′-AAACCGCATGGAGTGTGTTGTTCC-3′ | 5′-TCAGACCACCTCGACAATGCATGA-3′ |
| Egr1 | 5′-TCGGCTCCTTTCCTCACTCA-3′ | 5′-CTCATAGGGTTGTTCGCTCGG-3′ |
| Mtor | 5′-ACCGGCACACATTTGAAGAAG-3′ | 5′-CTCGTTGAGGATCAGCAAGG-3′ |
| Gapdh | 5′-AGGTCGGTGTGAACGGATTTG-3′ | 5′-TGTAGACCATGTAGTTGAGGTCA-3′ |
Mapk1 Mitogen activated protein kinase 1 (ERK2); Creb1 cAMP response element-binding protein 1 (CREB); Bdnf brain-derived neurotrophic factor (BDNF); Fos FBJ osteosarcoma oncogene (cFos); Egr1 early growth response protein-1 (Zif268); Mtor mammalian target of rapamycin (mTOR); Gapdh glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
Western blotting
Protein from VTA tissue punches was isolated using Illustra TriplePrep kit (GE Healthcare) according to the manufacturer’s instructions and stored at −80 °C until use. Ten micro-grams of protein from each sample was treated with β-mercaptoethanol and subsequently electrophoresed on precast 4–20 % gradient gels (Bio-Rad), as previously described (Iñiguez et al. 2012; Warren et al. 2011). All antibodies were obtained from Cell Signaling (Beverly, Massachusetts). Blots were probed overnight at 4 °C with antibodies against the phosphorylated forms of ERK2, CREB, mTOR, and GAPDH. Separate membranes were probed with antibodies against total CREB, ERK2, mTOR, and GAPDH. All primary antibodies were made to a 1:1,000 dilution. Membranes were washed several times with TBST, and were incubated with peroxidase-labeled goat anti-rabbit IgG (1:10,000; Cell Signaling, Beverly, Massachusetts). Bands were visualized with SuperSignal West Dura substrate (Pierce Biotechnology, Rockford, IL), quantified using ImageJ (NIH), and then normalized to GAPDH.
Place conditioning
Place conditioning for nicotine was performed in a three-compartment apparatus where each compartment differed in floor texture and wall coloring. On the preconditioning day (day 0), mice were allowed to explore the entire apparatus for 15 min to obtain baseline preference to any of the three compartments (length by width by height: side compartments, 35×27×25 cm; middle compartment, 10×27×25 cm). Mice did not show preference for either side compartment (before nicotine exposure). Conditioning trials occurred over eight consecutive days. During conditioning days 2, 4, 6, and 8 mice received saline (4 ml/kg, i.p.) and were confined to one of the side compartment of the apparatus for 15 min. On conditioning days 1, 3, 5, and 7, mice received nicotine (0.05, 0.07, or 0.09 mg/kg) and were confined to the opposite side compartment (drug-paired compartment) for 15 min. On the test day (day 9), mice received saline and were allowed to explore the entire apparatus for 15 min and time spent in the drug-paired compartment was assessed.
Statistical analyses
Data were analyzed using multivariate analysis (MANOVA) followed by Least Significant Difference (LSD) post hoc tests. When appropriate, Student’s t tests were used to determine statistical significance of preplanned comparisons. Data are expressed as the mean±SEM. Statistical significance was defined as p<0.05.
Results
Short- and long-term effects of chronic administration of MPH, FLX, MPH+FLX, or cocaine on ERK-related signaling within the VTA of juvenile C57BL/6J male mice
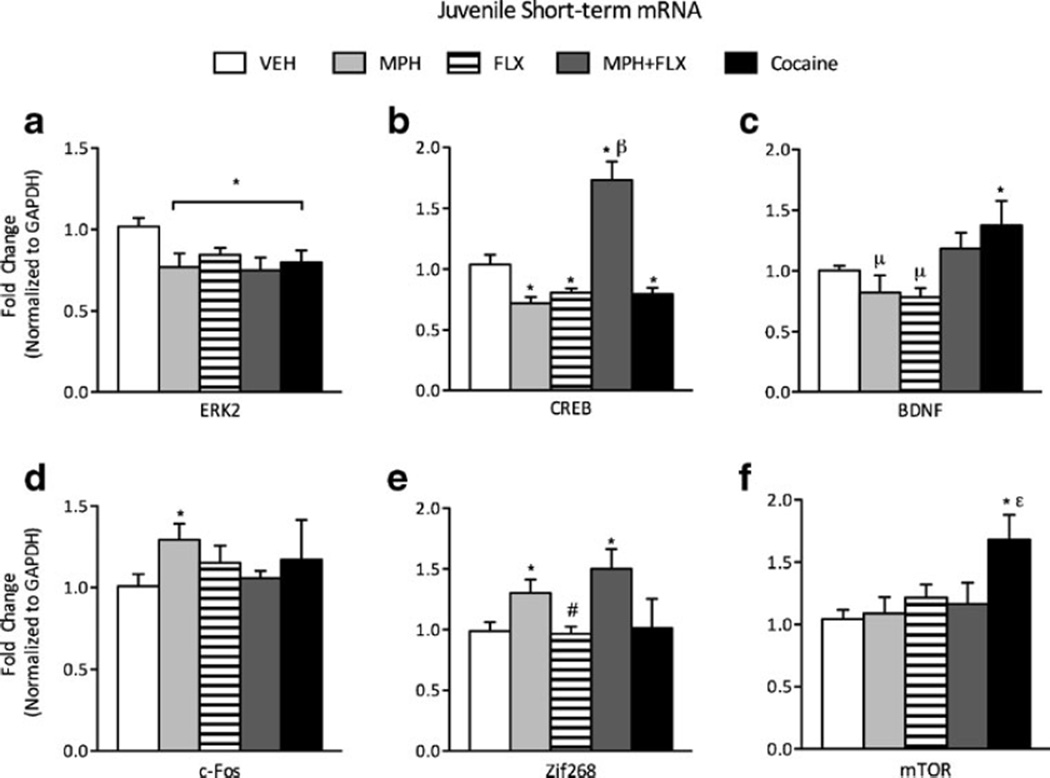
ERK-related gene expression within the VTA was assessed 24 h after exposure to VEH, MPH, FLX, MPH+FLX, or cocaine using qPCR (Fig. 1a-f; N=42; 8–10/group). A one-way MANOVA revealed a significant multivariate main effect of treatment (Wilks’ lambda=0.043, F (24,113)=6.85, p<0.01). Further analysis showed ERK2 (F(4,37) =4.31, p<0.01; Fig. 1a), CREB (F(4,37)=23.94, p<0.01; Fig. 1b), BDNF (F(4,37)=3.99, p<0.05, Fig. 1c), cFos (F(4,37)=1.18, p<0.01; Fig. 1d), Zif268 (F(4,37)=4.17, p<0.01; Fig. 1e), and mTOR (F(4,37)=8.68, p<0.01, Fig. 1f) mRNA varied as a function of juvenile drug exposure 24 h after treatment. MPH treatment had no effect on BDNF or mTOR (p>0.05), but decreased ERK2 and CREB mRNA (p<0.05) while increasing mRNA of cFos and Zif268 (p<0.05) when compared to the VEH-treated controls. FLX decreased ERK2 and CREB (p<0.05, respectively), while having no effect on BDNF, cFos, Zif268, or mTOR mRNA when compared to the VEH-treated controls (p>0.05, respectively). MPH+FLX treatment decreased ERK2, increased CREB, and Zif268 mRNA (p<0.05), but had no effect on BDNF, cFos, or mTOR mRNA (p>0.05, respectively) when compared to the VEH-treated controls. Cocaine treatment decreased ERK2 and CREB mRNA, increased BDNF and mTOR mRNA, but had no effect on cFos or Zif268 mRNA when compared to the VEH-treated controls. Post hoc analyses revealed that MPH+FLX-induced increase in CREB was significantly different when compared to all other treatment groups (p<0.05). MPH- or FLX-alone decreased BDNF while cocaine increased BDNF. MPH alone or MPH+FLX increased Zif268 expression, while FLX alone decreased it (p<0.05, respectively). Lastly, cocaine-induced changes in mTOR were significantly different from all other treatment groups (p<0.05, respectively).
Fig. 1.
Short-term effects of juvenile exposure to VEH, MPH, FLX, MPH+FLX, or cocaine on ERK-related gene expression within the VTA 24 h after the last injection. a ERK2 mRNA was decreased after MPH, FLX, MPH+FLX or cocaine when compared to the VEH-pretreated controls (p<0.05). b CREB mRNA was reduced by MPH, FLX, and cocaine, and increased by MPH+FLX when compared to the VEH-pretreated controls (p<0.05). c BDNF mRNA was increased only after cocaine when compared to the VEH-pretreated controls (p<0.05). d cFos mRNA was increased only by MPH when compared to the VEH-pretreated controls (p<0.05). e Zif268 mRNA was increased by MPH and MPH+FLX when compared to the VEH-pretreated controls (p<0.05). f mTOR was increased only by cocaine when compared to the VEH-pretreated mice (p<0.05). *p<0.05 compared to VEH-pretreated controls. βp<0.05 compared to MPH-, FLX-, and cocaine- pretreated mice p<0.05. #p<0.05 compared to MPH- and MPH+FLX-treated mice. μp<0.05 compared to the MPH+FLX- and the cocaine-treated mice. εp<0.05 compared to MPH-, FLX-, MPH+FLX-, and cocaine-pretreated mice. Data are presented as fold change normalized to GAPDH (mean±SEM)
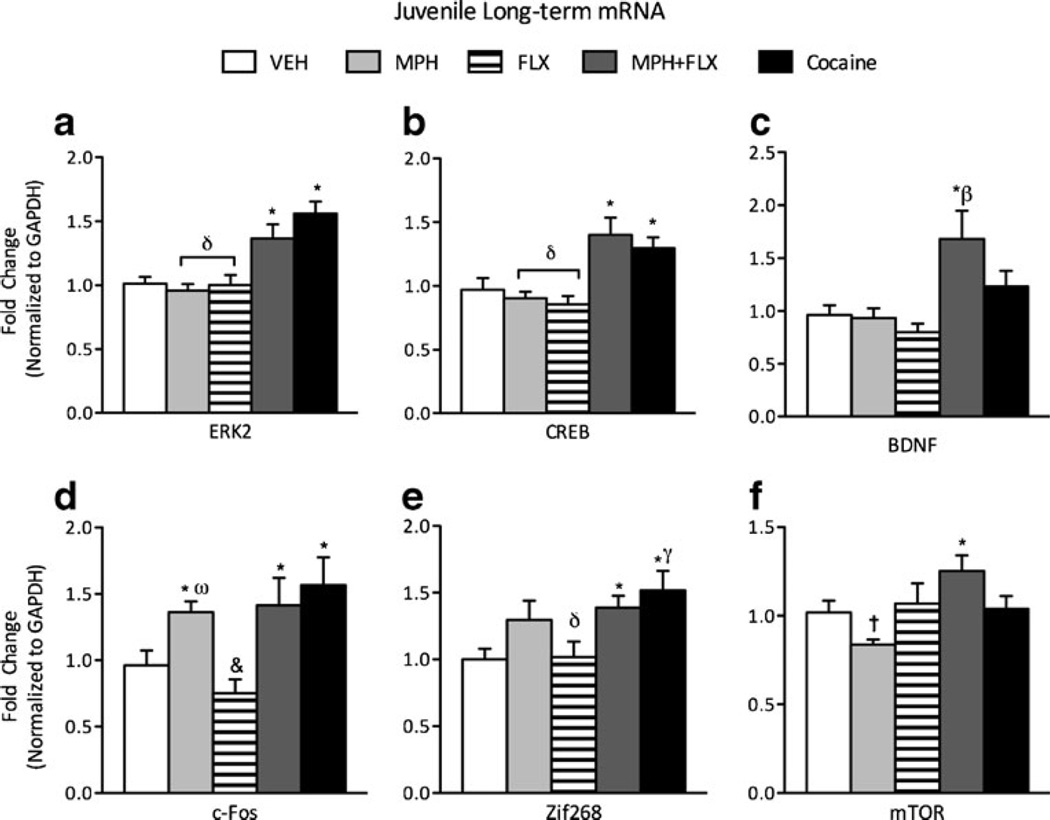
We also measured the effects of juvenile exposure to VEH, MPH, FLX, MPH+FLX and cocaine on gene expression 2 months after the last drug exposure (Fig. 2a-f; N=42; 8–10/ group). A one-way MANOVA revealed a significant multivariate main effect of treatment (Wilks’ lambda=0.112, F(24,113)=4.112, p<0.01). Given that the multivariate test was significant, further analysis was done and we found that ERK2 (F(4,37)=12.89, p<0.01; Fig. 2a), CREB (F(4,37)=8.46, p<0.01; Fig. 2b), BDNF (F(4,37)=4.82, p<0.01; Fig. 2c), cFos (F(4,37)=4.41, p<0.01; Fig. 2d), Zif268 (F(4,37)=5.61, p<0.01; Fig. 2e), and mTOR (F(4,37)=3.55, p<0.05; Fig. 2f) mRNA varied as a function of juvenile drug exposure. More specifically, neither MPH nor FLX alone influenced long-term expression of ERK2, CREB, mTOR, BDNF, or Zif268 mRNA when compared to VEH-treated controls (p>0.05). However, MPH pretreatment did increase cFos mRNA when compared to the VEH-treated controls (p<0.05). On the other hand, MPH+FLX treatment increased expression of ERK2, CREB, cFos, mTOR, BDNF, and Zif268 mRNA when compared to the VEH-treated controls (p<0.05). Similar to MPH+FLX, cocaine pretreatment significantly increased the expression of ERK2, CREB, cFos, and Zif268 mRNA, but had no effect on BDNF or mTOR gene expression when compared to the VEH-treated controls (p<0.05, respectively). Post hoc analyses revealed that MPH+FLX, or cocaine treatment increased ERK2 and CREB mRNAwhen compared to VEH-, MPH-, or FLX-pretreated mice (p<0.05, respectively). MPH+FLX pretreatment significantly increased BDNF expression as compared to all other treatment groups (p<0.05). FLX pretreatment significantly decreased cFos expression when compared to all other treatment groups (p<0.05). Similarly, MPH alone significantly decreased expression of mTOR mRNA when compared to all other treatment groups (p<0.05).
Fig. 2.
Long-term effects of juvenile exposure to VEH, MPH, FLX, MPH+FLX, or cocaine on ERK-related gene expression within the VTA 2 months after the last injection. MPH+FLX and cocaine pretreatment increased a ERK2, b CREB, d cFos, and e Zif268 mRNA when compared to the VEH-pretreated controls (p<0.05). MPH pretreatment increased only d cFos mRNA expression when compared to the VEH-pretreated controls (p<0.05). MPH+FLX pretreatment increased f mTOR and c BDNF mRNA when compared to the VEH-pretreated controls (p<0.05). *p<0.05 compared to VEH-pretreated controls. δp<0.05 compared to MPH+FLX- and cocaine-pretreated mice. βp<0.05 compared to MPH-, FLX- and cocaine-treated mice. ωp<0.05 compared to FLX-pretreated mice. γp<0.05 compared to FLX-pretreated mice. &p<0.05 compared to MPH-, MPH+FLX-, and cocaine-pretreated mice. †p<0.05 compared FLX-, MPH+FLX-, and cocaine-pretreated mice. Data are presented as fold change normalized to GAPDH (mean±SEM)
Short- and long-term effects of chronic administration of MPH, FLX, MPH+FLX, or cocaine on ERK-related signaling within the VTA of adult C57BL/6J mice
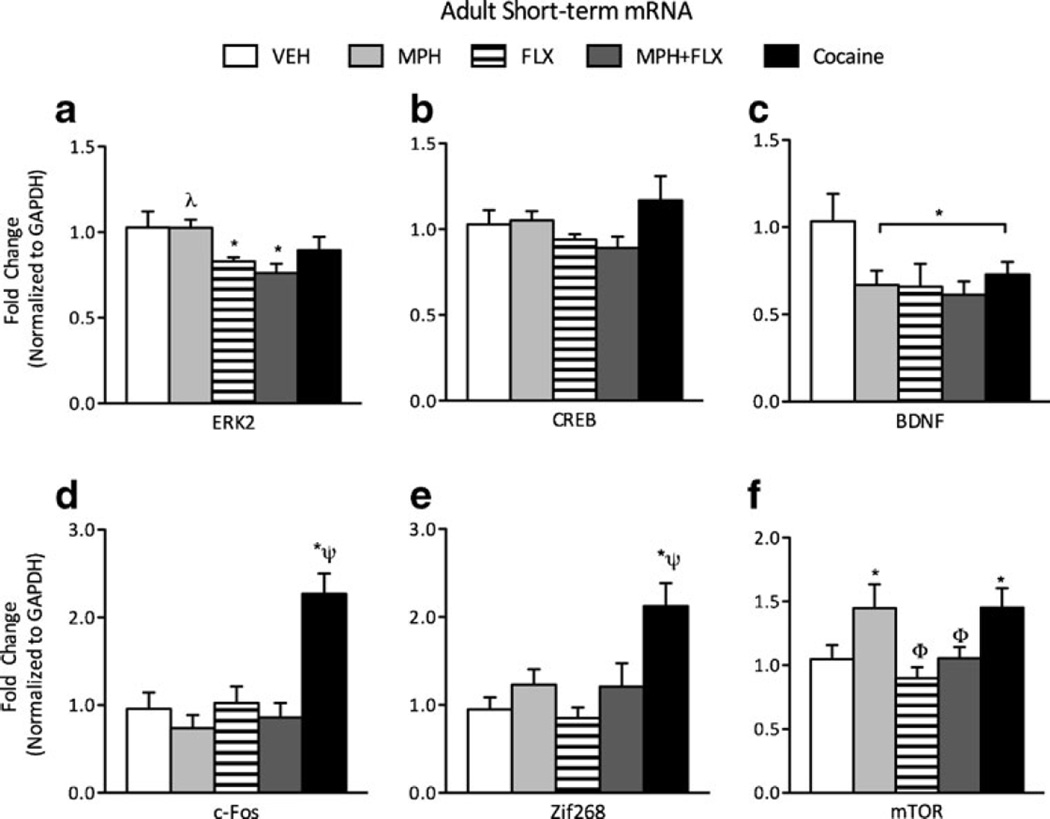
Gene expression was also assessed 24 h after last exposure to MPH, FLX, MPH+FLX or cocaine in a separate group of adult mice to test for age-dependent effects (Fig. 3a-f; N=40; 8/group). A one-way MANOVA revealed a significant multivariate main effect of treatment (Wilks’ lambda=0.136, F(24,106)=3.404, p<0.01). Further, univariate analysis showed that ERK2 (F(4,35)=3.468, p<0.05; Fig. 3a), BDNF (F(4,35)= 2.412, p<0.05; Fig. 3c), cFos (F(4,35) =11.119, p<0.01; Fig. 3d), Zif268 (F(4,35)=6.119, p<0.01; Fig. 3e) and mTOR (F(4,35)=3.788, p<0.05; Fig. 3f) mRNAvaried as a function of drug exposure in adult mice. However, CREB expression was not affected in the adult mice (p>0.05; Fig. 3b). MPH decreased BDNF (p<0.05) and increased mTOR mRNA expression, but had no effect on ERK2, cFos, or Zif268 mRNA expression (p>0.05) when compared to the VEH-treated controls (p<0.05). FLX decreased expression of ERK2 and BDNF mRNA (p<0.05, respectively) without significantly affecting expression of any of the other genes examined (p>0.05). MPH+FLX reduced ERK2 and BDNF mRNA (p<0.05) but did not influence cFos, Zif268, or mTOR mRNA expression when compared to the VEH-treated controls. Cocaine increased cFos, Zif268, and mTOR mRNA, reduced BDNF mRNA (p<0.05, respectively), and had no effect on ERK2 mRNA expression when compared to the VEH-treated controls (p>0.05). Post hoc analyses revealed that cFos and Zif268 were significantly increased by cocaine when compared to all other treatment groups (p<0.05, respectively).
Fig. 3.
Short-term effects of adult exposure to VEH, MPH, FLX, MPH+ FLX, or cocaine on ERK-related gene expression within the VTA 24 h after the last injection. a ERK2 mRNA was decreased by FLX, and MPH+FLX when compared to the VEH-pretreated controls (p<0.05). b CREB mRNA was not affected by drug treatment. c BDNF mRNA was significantly decreased by all drug treatments when compared to the VEH-pretreated controls (p<0.05). Cocaine treatment increased cFos (d) and Zif268 (e) mRNA, while MPH and cocaine treatment increased mTOR mRNA (f). *p<0.05 compared to the VEH-pretreated controls. λp<0.05 compared to FLX and MPH+FLX-pretreated mice. ψp<0.05 compared to all drug treatments. Φp<0.05 compared to MPH- and cocaine-pretreated mice. Data are presented as fold change normalized to GAPDH (mean±SEM)
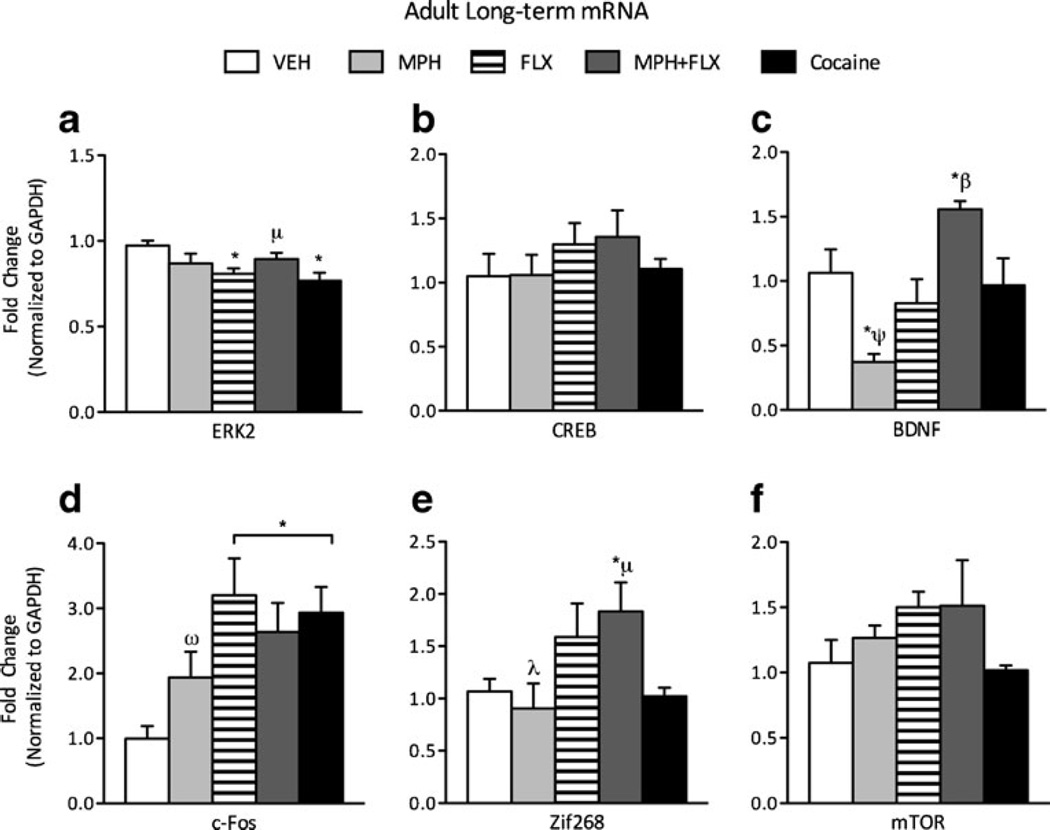
To test for long-lasting effects, gene expression was also assessed 2 months after the last exposure to MPH, FLX, MPH+FLX or cocaine in adult mice (Fig. 4a-f; N=40; 8/group). A one-way MANOVA revealed a significant multivariate main effect of treatment (Wilks’ lambda=0.136, F(24,106)=3.404, p<0.01). Again, univariate test were done for further analysis and revealed that expression of ERK2 (F(4,35) =3.56, p<0.05; Fig. 4a), BDNF (F(4,35) =7.162, p<0.01; Fig. 4c), cFos (F(4,35)=4.548, p<0.01; Fig. 4d), and Zif268 (F(4,35) =3.131, p<0.05; Fig. 4e) mRNA varied as a function of drug treatment. CREB and mTOR mRNA expression did not vary as a function of drug treatment (p>0.05, respectively). MPH decreased BDNF (p<0.05), without affecting ERK2, cFos, or Zif268 mRNAwhen compared to the VEH-treated controls. FLX decreased ERK2 and increased cFos mRNA (p<0.05, respectively), without influencing BDNF or Zif268. MPH+FLX treatment increased BDNF, cFos, and Zif268 expression (p<0.05), without influencing ERK2, when compared to the VEH-treated controls. Cocaine treatment decreased ERK2 (p<0.05) and increased cFos mRNA (p<0.05), but did not alter CREB, BDNF, Zif268, or mTOR mRNA when compared to the VEH-treated controls (p>0.05). Post hoc analysis revealed that MPH+FLX induced changes in ERK 2 expression were significantly higher when compared to cocaine treated mice (p<0.05). The reduction in BDNF after MPH treatment was significantly different when compared to all other pretreatment groups (p<0.05, respectively). Similarly, the MPH+FLX-induced increases in BDNF mRNA expression were significantly different when compared to all other pretreatment groups (p<0.05). MPH-induced expression of cFos was significantly different when compared to all other drug pretreatment groups (p<0.05). Lastly, MPH-induced expression of Zif268 was significantly different when compared to that induced by FLX or MPH+ FLX pretreatment (p<0.05, respectively). MPH+FLX treatment significantly increased Zif268 expression when compared to the cocaine-pretreated mice (p<0.05).
Fig. 4.
Long-term effects of adult exposure to VEH, MPH, FLX, MPH+ FLX, or cocaine on ERK-related gene expression within the VTA 24 h after the last injection. a ERK2 mRNA was decreased by FLX and cocaine when compared to the VEH-pretreated controls. b CREB mRNA was not affected by drug treatment. c BDNF mRNA was significantly decreased by MPH, but significantly increased by MPH+FLX treatment when compared to the VEH-pretreated controls (p<0.05). d cFos and e Zif268 mRNA were increased by MPH+FLX when compared to the VEH-pretreated controls (p<0.05). f mTOR mRNA expression was not affected by drug pretreatment. *p<0.05 compared to VEH-pretreated controls. μp<0.05 compared to cocaine-pretreated mice. ψp<0.05 compared to FLX-, MPH+FLX-, and cocaine-pretreated mice. βp<0.05 compared to MPH-, FLX-, and cocaine-pretreated mice. ωp<0.05 compared to FLX-pretreated mice. λp<0.05 compared to FLX-, and MPH+ FLX-pretreated mice. Data are presented as fold change normalized to GAPDH (mean±SEM)
Short- and long-term effects of juvenile administration of MPH+FLX, or cocaine on protein phosphorylation on ERK-related signaling within the VTA
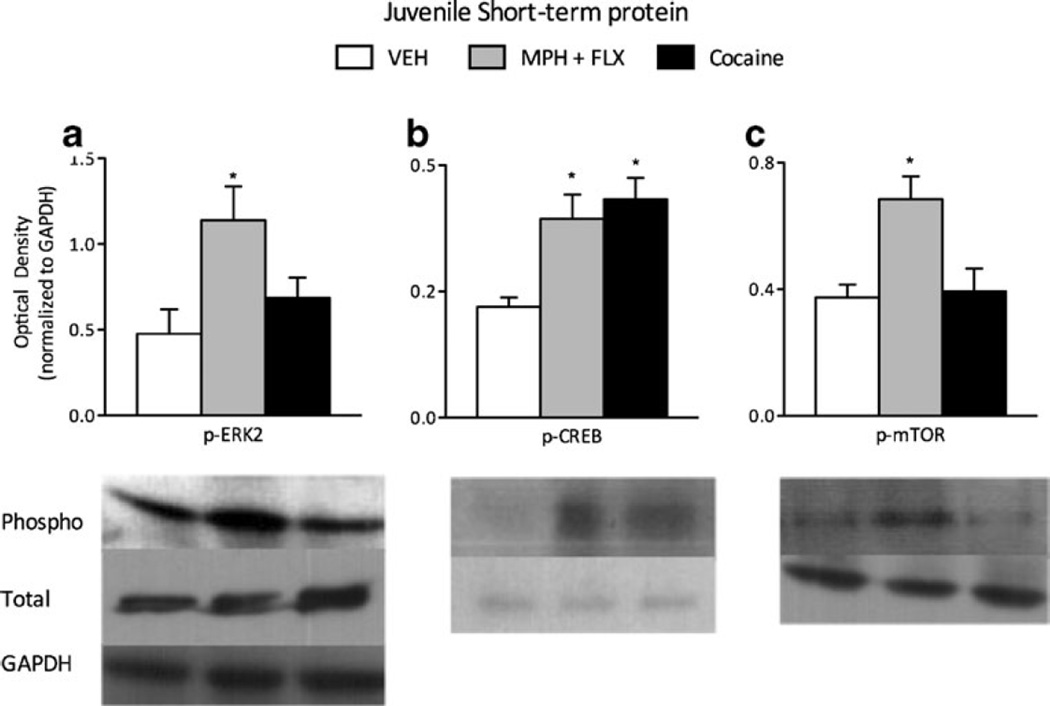
We further assessed the activity of ERK2-related signaling after juvenile VEH, MPH+FLX, or cocaine exposure as inferred from the phosphorylation of ERK2 protein and two downstream targets, CREB and mTOR (Fig. 5a-c; N=24; 6–8/group; all normalized to GAPDH and presented as percent of total protein). Phosphorylation of ERK2 (F(2,21)=19.3, p<0.01; Fig. 5a), CREB (F(2,21)=30.8 p<0.01; Fig. 5b), and mTOR (F(2,21)=26.5, p<0.01; Fig. 5c) within the VTA was influenced by drug treatment. MPH+FLX increased phosphorylation of ERK2, CREB, and mTOR when compared to the VEH-treated controls. Cocaine exposure increased phosphorylation of CREB (p<0.01), but not ERK2 or mTOR. No changes in total levels of ERK2, CREB, mTOR, or GAPDH were detected when compared to the VEH-treated controls (p>0.05, data not shown).
Fig. 5.
Short-term effects of adolescent C57BL/6J mice exposure to VEH, MPH+FLX, or cocaine on protein phosphorylation within the VTA 24 h after the last injection. Pretreatment with MPH+FLX significantly increased a ERK2, b CREB, and c mTOR phosphorylation when compared to the VEH-treated controls (p<0.05). Cocaine exposure significantly increased b CREB protein phosphorylation when compared to the VEH-pretreated mice. *p<0.05 compared to the VEH-pretreated controls. Data are presented as a ratio of total protein normalized to GAPDH (mean±SEM)
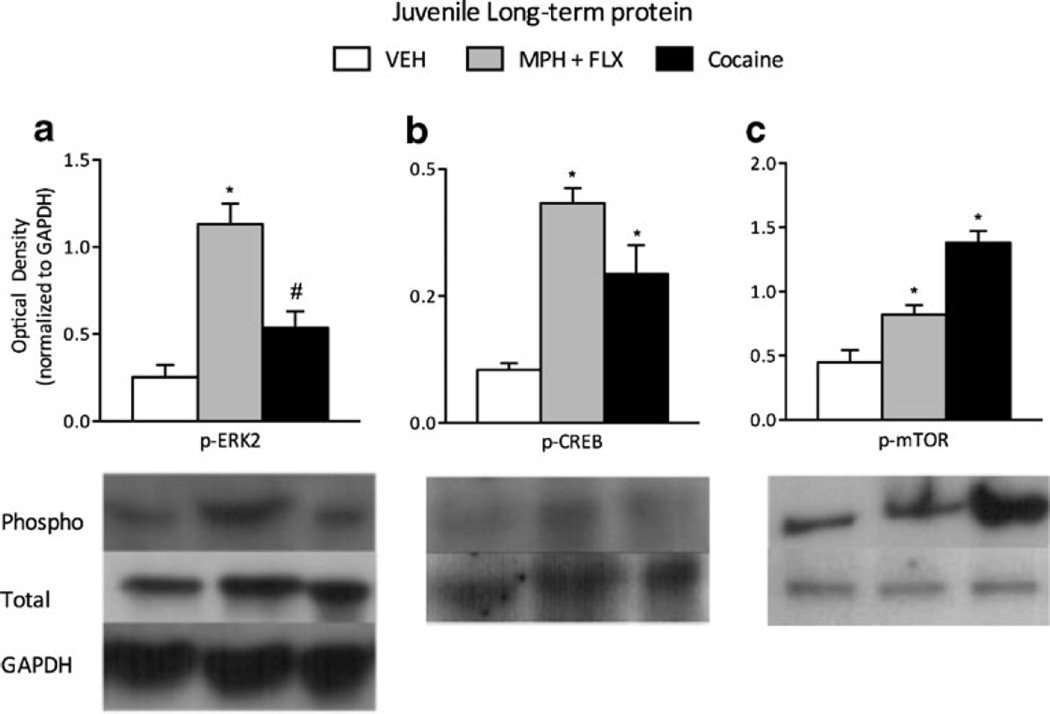
To assess long-lasting changes in intracellular signaling, we also assessed the activity of ERK2-related proteins 2 months after juvenile VEH, MPH+FLX, or cocaine pretreatment (Fig. 6a-c; n=6–8/group). At this time point, phosphorylated ERK2 (F(2,21)=3.2, p<0.05; Fig. 6a) CREB (F(2,21 =5.6 p<0.01; Fig. 6b) and mTOR (F(2,21) =8.2, p<0.01; Fig. 6c) was influenced by drug treatment within the VTA. MPH+FLX increased ERK2, CREB, and mTOR phosphorylation when compared to the VEH-treated controls. Cocaine exposure resulted in a similar pattern of expression as observed in the MPH+FLX-treated groups. Specifically, cocaine induced a trend toward an increased ERK2 activity (p= 0.07) and significantly increased phosphorylation of CREB and mTOR protein (p<0.01, respectively). No changes in total levels of ERK2, CREB, mTOR, or GAPDH protein levels were detected when compared to the VEH-treated controls (p>0.05, data not shown).
Fig. 6.
Long-term effects of adolescent C57BL/6J mice exposure to VEH, MPH+FLX, or cocaine on protein phosphorylation within the VTA 2 months after the last injection. Pretreatment with MPH+FLX resulted in long-lasting activation of a ERK2, b CREB, and c mTOR phosphorylation when compared to the VEH-pretreated controls (p<0.05). In similar fashion, cocaine exposure during adolescence resulted in elevated a ERK2, b CREB and c mTOR protein phosphorylation when compared to the VEH-pretreated controls (p<0.05). *p<0.05 when compared to the VEH-pretreated controls. #p=0.06 when compared to the VEH-pretreated controls. Data are presented as a ratio of total protein normalized to GAPDH (mean±SEM)
Effect of juvenile MPH+FLX or cocaine exposure on nicotine place conditioning
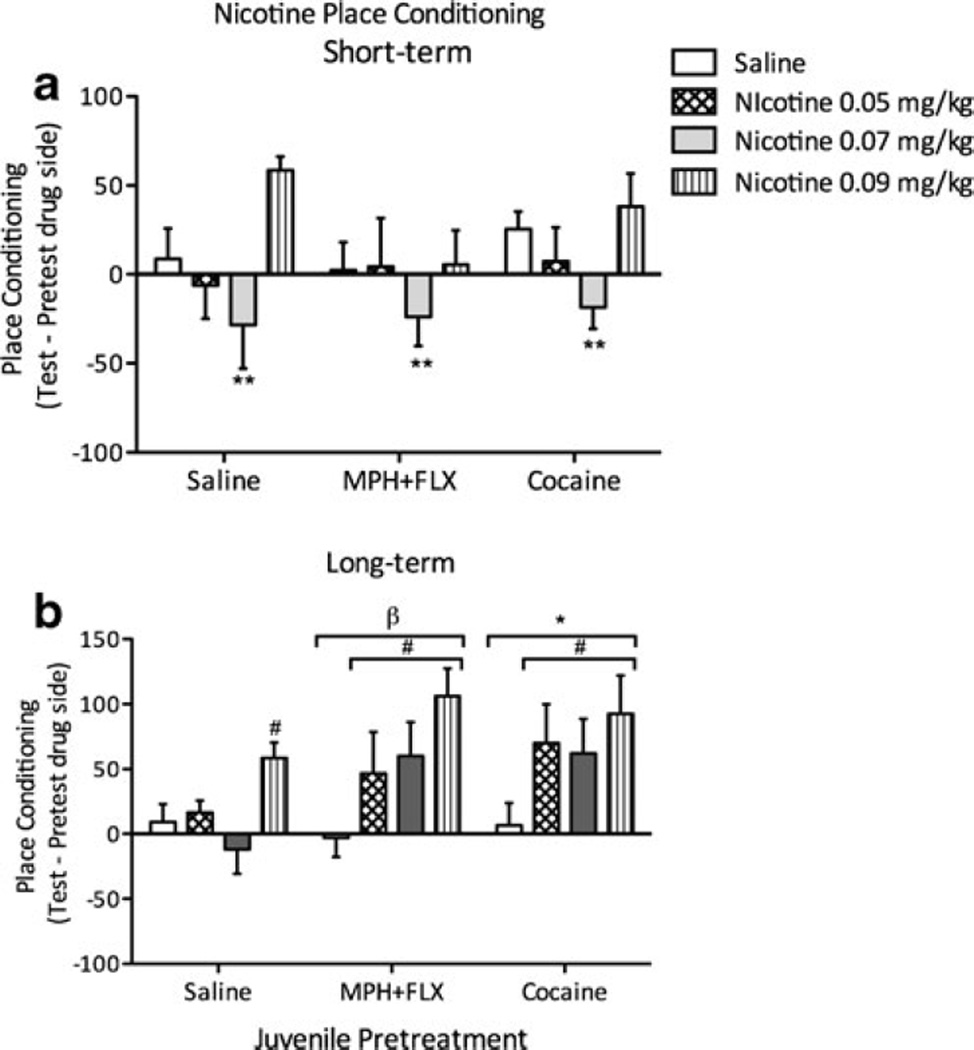
To test for changes in behavioral sensitivity to nicotine reward, place preference conditioning was assessed 24 h (short-term; n=26–30/group) or 2 months (long-term; n=24–26/ group) following juvenile exposure to VEH, MPH+FLX, or cocaine (Fig. 7a - b). No significant effects due to drug pretreatment were observed at any drug dose (p>0.05), nor was there an interaction effect. However, a significant difference between nicotine conditioning doses was observed across all groups (F(3,85)=6.738; p<0.05). Post hoc test revealed that mice conditioned with 0.07 mg/kg nicotine spent significantly less time in the nicotine-paired compartment (p<0.05) when compared to the 0.05 and 0.09 mg/kg doses.
Fig. 7.
Effects of juvenile exposure to VEH, MPH+FLX, or cocaine on nicotine-induced place conditioning. a Short-term (24 h after the last injection): no drug pretreatment induced preference for nicotine. The 0.07 mg/kg nicotine dose induced place aversion regardless of pretreatment condition. b Long-term (2 months after the last injection): MPH+ FLX and cocaine-pretreated mice show an increased preference for the nicotine-paired compartments when compared to the mice receiving the same pretreatment but confined to saline-paired compartments. MPH+ FLX- and cocaine-pretreated mice spent significantly more time in the nicotine-paired compartments when compared to VEH-pretreated controls. *p<0.05 when compared to VEH-pretreated controls. βp<0.05 when compared to VEH-pretreated controls. #p<0.05 when compared to the saline conditioned animals within same drug pretreatment. **p<0.05 when compared to the 0.0, 0.05, and 0.09 nicotine doses within that juvenile drug pretreatment group
When assessing long-term effects of VEH, MPH, FLX, MPH+FLX, or cocaine (Fig. 7b), time spent in the nicotine-paired compartments was significantly influenced by juvenile drug pretreatment (F(2,75)=3.54; p<0.01), and also varied as a function of nicotine dose (F(2,75)=6.707; p<0.01). There was no interaction found between the two variables (F(6,75)=0.938; p<0.05). Cocaine- or MPH+FLX-pretreated mice readily conditioned to the compartments paired with nicotine when compared with the VEH-pretreated mice (p<0.05, respectively).
Interestingly, further analysis indicated that the magnitude of nicotine-induced place conditioning developed by the MPH+FLX- or the cocaine-pretreated mice was not significantly different (p>0.05), indicating that combined MPH+ FLX- or cocaine pretreatment similarly influence adulthood sensitivity to nicotine.
Discussion
This study assessed the short- and long-term neurobiological consequences of chronic administration of MPH and FLX, two drugs frequently prescribed for the management of co-morbid ADHD and MDD in pediatric populations (Safer et al. 2003; Stoll et al. 1996). Here, we demonstrate that chronic treatment with MPH, FLX, MPH+FLX, or cocaine during the juvenile period (PD20-34), but not during adulthood (PD70-84), results in a persistent dysregulation of ERK-related signaling within the VTA. In addition, combined MPH+FLX or cocaine treatment during the juvenile period enhances behavioral sensitivity to the rewarding effects of nicotine in adulthood.
We measured the expression of ERK-driven signaling molecules within the VTA 24 h (short-term) or 2 months (long-term) after juvenile MPH, FLX, MPH+FLX, or cocaine treatment. This approach was taken because these drugs interact with the VTA and the nucleus accumbens (Sekine et al. 2007; Yang et al. 2006), neural circuitry best known for its role in encoding incentive-motivational valence of drugs and natural rewards (Kelley and Berridge 2002; Koob and Le Moal 2008). More recently, this circuit has been implicated in the regulation of mood-related behaviors (Bolaños et al. 2003b; Naranjo et al. 2001; Nestler and Carlezon 2006). Here, we show that ERK2 mRNA was decreased by MPH, FLX, MPH+FLX, and cocaine, whereas CREB mRNA was decreased by either MPH or FLX alone, but surprisingly elevated by combined MPH+ FLX 24 h after treatment. One might expect that decreases in ERK2 expression would accompany decreases in CREB and Zif268 (McClung and Nestler 2003; Turgeon et al. 1997), but MPH+FLX pretreatment induced contrasting effects. Although short-term mRNA levels may not represent a functional relationship between the signaling molecules, it is possible that differences in drug metabolism may account for these discrepancies. Because FLX takes much longer than MPH to metabolize (DeVane 1994; DeVane et al. 2004; Swanson and Volkow 2003), it is possible that these data are confounded by acute MPH withdrawal with persisting FLX effects because increased CREB activity has been shown after chronic psychostimulant exposure (Pliakas et al. 2001; Wallace et al. 2009) and withdrawal (Barr et al. 2002; Barrot et al. 2002; Markou et al. 1998). Decreased ERK2 mRNA after MPH+FLX and cocaine was unexpected because previous reports have shown that similar dosing regimens of MPH+FLX increases ERK2 mRNA expression in juvenile rats (Warren et al. 2011). It is possible that the discrepancy between these findings is due, at least in part, to the different species used between the studies (i.e., rat vs. mice), and/or that age-limits delineating the juvenile period between the two species are not the same. Unfortunately, discrete age boundaries corresponding with different developmental periods across species are lacking (Adriani and Laviola 2004; Andersen and Navalta 2004b; Spear 2000). After 2 months, however, mice are free from both drugs and the relationship between these molecules becomes clearer: MPH+FLX or cocaine treatment resulted in upregulation of ERK2 signaling. This is particularly striking because neither MPH nor FLX induced similar increases when administered alone, suggesting qualitative differences between combined and individual treatments.
To determine whether the effects on gene expression were specific to age of drug exposure, we also treated separate groups of adult mice and assessed for gene expression 24 h or 2 months after the last drug exposure (i.e., matched drug regimen and biochemical assessment as juveniles). Drug treatment during adulthood did not induce consistent patterns of gene expression at either time point of assessment. Our results indicate that the MPH-, FLX-, MPH+FLX- and cocaine-induced effects on gene expression in the VTA may be more prominent after early-life treatment. This postulation is supported by studies demonstrating qualitative and quantitative effects depending on age of drug exposure (Bolaños et al. 1996; Collins and Izenwasser 2002; 2004; Iñiguez et al. 2010b; Iñiguez et al. 2009). The action of these drugs in the adult nervous system is complex, and their effects on the developing nervous system are just beginning to be elucidated (Steiner et al. 2010; Van Waes et al. 2010; Warren et al. 2011), thus adding an extra layer of complexity. Indeed, a more detailed assessment of these phenomena accounting for dose, length of exposure, then discontinuation of drug use and also developmental period at time of exposure are clearly needed (Bolaños et al. 2008; Carlezon and Konradi 2004; Spear 2000; Wiley et al. 2009; Yano and Steiner 2007).
Because increased gene expression does not necessarily result in increased protein levels and tells us little about the activity of these enzymes (Lee et al. 2003; Mehra et al. 2003), we also assessed protein phosphorylation 24 h and 2 months after drug exposure in juvenile mice. We found increased ERK2, CREB, and mTOR phosphorylation within the VTA after MPH+FLX, while the effects induced by cocaine were only apparent for CREB. Given the pharmacodynamic properties of MPH and FLX and that dopamine and serotonin systems interact (Benloucif et al. 1993; Bolaños et al. 2002; De Deurwaerdere et al. 1996), it has been suggested that concurrent use of these drugs may induce cocaine-like effects (Yano and Steiner 2007). In line with this assumption, recent studies show a robust potentiation of immediate early gene transcription and enhanced behavioral reactivity in adult rats receiving acute MPH+FLX treatment, effects similar to those seen after cocaine (Borycz et al. 2008; Steiner et al. 2010; Van Waes et al. 2010). The effects of juvenile MPH+FLX treatment on ERK2, CREB, and mTOR phosphorylation persisted into adulthood (i.e., 2 months after drug exposure). Even more interesting is that after 2 months, cocaine also induced phosphorylation of these proteins. Increased VTA ERK2-CREB activity after MPH+FLX lends support to the notion that concurrent treatment with MPH and FLX may be pharmacodynamically similar to treatment with cocaine, and suggests that combining MPH and FLX early in life may lead to life-long behavioral and biochemical abnormalities (Steiner et al. 2010; Van Waes et al. 2010; Warren et al. 2011; Yano and Steiner 2007).
The functional significance of these biochemical findings still remains unclear. However, chronic exposure to drugs of abuse and stress induce similar changes in ERK-related signaling within the VTA that have been shown to influence behaviors related to both drug reward (Iñiguez et al. 2010a; c; Lu et al. 2009; Ortiz et al. 1995; Russo et al. 2007) and mood disorders (Duman and Monteggia 2006; Iñiguez et al. 2010a; Krishnan et al. 2008; Nestler and Carlezon 2006). Combined MPH+FLX treatment in juvenile rats induces behavioral deficits indicative of increased sensitivity to stress (i.e., anxiety- and depression-like phenotypes) while enhancing sensitivity for the rewarding effects of low doses of cocaine in adulthood (Warren et al. 2011). Given MPH+ FLX-induced increases in ERK2/CREB, molecular adaptations known to increase sensitivity to drugs of abuse (Carlezon et al. 2005; Girault et al. 2007; Iñiguez et al. 2010a; Lu et al. 2009), we assessed the short- and long-term effects of MPH+FLX or cocaine treatment during early life on nicotine reward as measured in the place preference conditioning paradigm. Juvenile mice in the short-term condition showed an aversion to the intermediate dose of nicotine (0.07 mg/kg) suggesting the possibility that these mice were sensitized to the aversive effects of nicotine. The long-term effects were particularly striking: the MPH+FLX- and the cocaine-pretreated mice readily preferred the nicotine-paired compartments. This is interesting because MPH+FLX and cocaine pretreatment induced place preference at a dose that had no effects in the VEH-treated mice (0.07 mg/kg), suggesting that MPH+FLX and cocaine pretreatment during the juvenile period enhances sensitivity to drug reward in adulthood. The lack of place conditioning in the short-term group was surprising given reports indicating that CREB phosphorylation is necessary for acquiring nicotine place preference in adult mice (Brunzell et al. 2009), and that our juvenile mice showed a robust induction of phosphorylated CREB 24 h after MPH+FLX or cocaine treatment. In addition, juvenile rats undergoing the same treatment regimen and behavioral testing show increased sensitivity to the rewarding effects of low doses of cocaine after FLX and MPH+FLX (Warren et al. 2011). This discrepancy may be due to age-dependent responsiveness to nicotine, as adolescent mice (i.e., at the same age tested here) are significantly less sensitive to nicotine when compared to adults (Adriani et al. 2002; Belluzzi et al. 2004; Faraday et al. 2001; Vastola et al. 2002). This is likely the case as our MPH+FLX and cocaine-pretreated mice showed enhanced preference for nicotine as adults. These findings support reports demonstrating that exposure to MPH or FLX during early life leads to long-lasting adaptations in brain reward pathways (Iñiguez et al. 2010b; Mague et al. 2005; Thanos et al. 2007) and suggest that concurrent exposure may enhance this effect (Steiner et al. 2010; Warren et al. 2011).
To summarize, we show here that ERK-related signaling is increased in a similar fashion by both MPH+FLX or cocaine exposure during the juvenile period. Moreover, we demonstrate that MPH+FLX or cocaine treatment results in life-long adaptations manifested in an increased preference for nicotine when tested in adulthood. This data supports the notion that chronic administration of drugs, particularly during early stages of development, may result in lasting dysregulation of second-messenger signaling systems within the VTA. This brain area is not homogeneous and its complexity has long been appreciated (Lammel et al. 2013; Russo and Nestler 2013), with distinct subsets of VTA dopaminergic neurons responding differently to rewarding verses aversive stimuli (Chaudhury et al. 2013; Cohen et al. 2012; Lammel et al. 2011). In addition, there are topographical differences (anterior versus posterior) determining behavioral responding to salient stimuli (Bolaños et al. 2005; Carlezon et al. 2000; Ikemoto 2007; Oades and Halliday 1987; Olson et al. 2005), and additional work assessing the specific contributions of the different subnuclei and neuronal populations within the VTA is much needed. It is also important to note that the findings reported here were derived from “normal animals,” and the same experimental manipulation using an animal model for comorbid ADHD/MDD may yield different results. Furthermore, studies assessing potentially enduring side effects of MPH+FLX in humans are lacking (Daviss 2008b), making interpretative parallels challenging. Our findings necessitate further study into the effects of early-life drug exposure on functional outcomes in adulthood.
Acknowledgments
This work was supported by R01DA026854 from the National Institute on Drug Abuse (NIDA). LF Alcantara was supported by a McKnight Fellowship from the Florida Education Fund, BL Warren by training grant T32MH093311 from the National Institute of Mental Health (NIMH), and SD Iñiguez by a NRSA F31027300 fellowship from NIDA.
Footnotes
Conflict of interest The authors report no conflict of interest.
References
- Adriani W, Laviola G. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behav Pharmacol. 2004;15:341–352. doi: 10.1097/00008877-200409000-00005. [DOI] [PubMed] [Google Scholar]
- Adriani W, Macri S, Pacifici R, Laviola G. Peculiar vulnerability to nicotine oral self- administration in mice during early adolescence. Neuropsychopharmacology. 2002;27:212–224. doi: 10.1016/S0893-133X(02)00295-6. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Navalta CP. Altering the course of neurodevelopment: a framework for understanding the enduring effects of psychotropic drugs. Int J Dev Neurosci. 2004;22:423–440. doi: 10.1016/j.ijdevneu.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Arvanitogiannis A, Pliakas AM, LeBlanc C, Carlezon WA., Jr Altered responsiveness to cocaine in rats exposed to methylphenidate during development. Nat Neurosci. 2002;5:13–14. doi: 10.1038/nn777. [DOI] [PubMed] [Google Scholar]
- Barr AM, Markou A, Phillips AG. A ‘crash’ course on psychostimulant withdrawal as a model of depression. Trends Pharmacol Sci. 2002;23:475–482. doi: 10.1016/s0165-6147(02)02086-2. [DOI] [PubMed] [Google Scholar]
- Barrot M, Olivier JD, Perrotti LI, DiLeone RJ, Berton O, Eisch AJ, Impey S, Storm DR, Neve RL, Yin JC, Zachariou V, Nestler EJ. CREB activity in the nucleus accumbens shell controls gating of behavioral responses to emotional stimuli. Proc Natl Acad Sci USA. 2002;99:11435–11440. doi: 10.1073/pnas.172091899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluzzi JD, Lee AG, Oliff HS, Leslie FM. Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology (Berl) 2004;174:389–395. doi: 10.1007/s00213-003-1758-6. [DOI] [PubMed] [Google Scholar]
- Benloucif S, Keegan MJ, Galloway MP. Serotonin-facilitated dopamine release in vivo: pharmacological characterization. J Pharmacol Exp Ther. 1993;265:373–377. [PubMed] [Google Scholar]
- Bhatara V, Feil M, Hoagwood K, Vitiello B, Zima B. National trends in concomitant psychotropic medication with stimulants in pediatric visits: practice versus knowledge. J Atten Disord. 2004;7:217–226. doi: 10.1177/108705470400700404. [DOI] [PubMed] [Google Scholar]
- Bolaños CA, Nestler EJ. Neurotrophic mechanisms in drug addiction. Neuromolecular Med. 2004;5:69–83. doi: 10.1385/NMM:5:1:069. [DOI] [PubMed] [Google Scholar]
- Bolaños CA, Garmsen GM, Clair MA, McDougall SA. Effects of the kappa-opioid receptor agonist U-50,488 on morphine-induced place preference conditioning in the developing rat. Eur J Pharmacol. 1996;317:1–8. doi: 10.1016/s0014-2999(96)00698-x. [DOI] [PubMed] [Google Scholar]
- Bolaños CA, Trksak GH, Cohen OS, Jackson D. Differential serotonergic inhibition of in vitro striatal [3H]acetylcholine release in prenatally cocaine-exposed male and female rats. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1339–1348. doi: 10.1016/s0278-5846(02)00299-3. [DOI] [PubMed] [Google Scholar]
- Bolaños CA, Barrot M, Berton O, Wallace-Black D, Nestler EJ. Methylphenidate treatment during pre- and periadolescence alters behavioral responses to emotional stimuli at adulthood. Biol Psychiatry. 2003a;54:1317–1329. doi: 10.1016/s0006-3223(03)00570-5. [DOI] [PubMed] [Google Scholar]
- Bolaños CA, Perrotti LI, Edwards S, Eisch AJ, Barrot M, Olson VG, Russell DS, Neve RL, Nestler EJ. Phospholipase Cgamma in distinct regions of the ventral tegmental area differentially modulates mood-related behaviors. J Neurosci. 2003b;23:7569–7576. doi: 10.1523/JNEUROSCI.23-20-07569.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolaños CA, Neve RL, Nestler EJ. Phospholipase C gamma in distinct regions of the ventral tegmental area differentially regulates morphine-induced locomotor activity. Synapse. 2005;56:166–169. doi: 10.1002/syn.20136. [DOI] [PubMed] [Google Scholar]
- Bolaños CA, Willey MD, Maffeo ML, Powers KD, Kinka DW, Grausam KB, Henderson RP. Antidepressant treatment can normalize adult behavioral deficits induced by early-life exposure to methyl-phenidate. Biol Psychiatry. 2008;63:309–316. doi: 10.1016/j.biopsych.2007.06.024. [DOI] [PubMed] [Google Scholar]
- Borycz J, Zapata A, Quiroz C, Volkow ND, Ferre S. 5-HT 1B receptor-mediated serotoninergic modulation of methylphenidate-induced locomotor activation in rats. Neuropsychopharmacology. 2008;33:619–626. doi: 10.1038/sj.npp.1301445. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, Napierata L, Kussmaul L, Leussis M, Andersen SL. Juvenile methylphenidate exposure and factors that influence incentive processing. Dev Neurosci. 2009;31:95–106. doi: 10.1159/000207498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunzell DH, Mineur YS, Neve RL, Picciotto MR. Nucleus accumbens CREB activity is necessary for nicotine conditioned place preference. Neuropsychopharmacology. 2009;34:1993–2001. doi: 10.1038/npp.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Konradi C. Understanding the neurobiological consequences of early exposure to psychotropic drugs: linking behavior with molecules. Neuropharmacology. 2004;47(Suppl 1):47–60. doi: 10.1016/j.neuropharm.2004.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Haile CN, Coppersmith R, Hayashi Y, Malinow R, Neve RL, Nestler EJ. Distinct sites of opiate reward and aversion within the midbrain identified using a herpes simplex virus vector expressing GluR1. J Neurosci. 2000;20:RC62. doi: 10.1523/JNEUROSCI.20-05-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Duman RS, Nestler EJ. The many faces ofCREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo MK, Neve RL, Friedman JM, Russo SJ, Deisseroth K, Nestler EJ, Han MH. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Molina BS, Pelham WE, Applegate B, Dahlke A, Overmyer M, Lahey BB. Very early predictors of adolescent depression and suicide attempts in children with attention-deficit/ hyperactivity disorder. Arch Gen Psychiatr. 2010;67:1044–1051. doi: 10.1001/archgenpsychiatry.2010.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482:85–88. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Cocaine differentially alters behavior and neurochemistry in periadolescent versus adult rats. Brain Res Dev Brain Res. 2002;138:27–34. doi: 10.1016/s0165-3806(02)00471-6. [DOI] [PubMed] [Google Scholar]
- Collins SL, Izenwasser S. Chronic nicotine differentially alters cocaine-induced locomotor activity in adolescent vs. adult male and female rats. Neuropharmacology. 2004;46:349–362. doi: 10.1016/j.neuropharm.2003.09.024. [DOI] [PubMed] [Google Scholar]
- Daviss WB. A review of co-morbid depression in pediatric ADHD: etiology, phenomenology, and treatment. J Child Adolesc Psychopharmacol. 2008;18:565–571. doi: 10.1089/cap.2008.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Deurwaerdere P, Bonhomme N, Lucas G, Le Moal M, Spampinato U. Serotonin enhances striatal dopamine outflow in vivo through dopamine uptake sites. J Neurochem. 1996;66:210–215. doi: 10.1046/j.1471-4159.1996.66010210.x. [DOI] [PubMed] [Google Scholar]
- DeVane CL. Pharmacogenetics and drug metabolism of newer antidepressant agents. J Clin Psychiatry. 1994;55(Suppl):38–45. discussion 46-7. [PubMed] [Google Scholar]
- DeVane CL, Donovan JL, Liston HL, Markowitz JS, Cheng KT, Risch SC, Willard L. Comparative CYP3A4 inhibitory effects of venlafaxine, fluoxetine, sertraline, and nefazodone in healthy volunteers. J Clin Psychopharmacol. 2004;24:4–10. doi: 10.1097/01.jcp.0000104908.75206.26. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Efron D, Hiscock H, Sewell JR, Cranswick NE, Vance AL, Tyl Y, Luk ES. Prescribing of psychotropic medications for children by Australian pediatricians and child psychiatrists. Pediatrics. 2003;111:372–375. doi: 10.1542/peds.111.2.372. [DOI] [PubMed] [Google Scholar]
- Faraday MM, Elliott BM, Grunberg NE. Adult vs. adolescent rats differ in biobehavioral responses to chronic nicotine administration. Pharmacol Biochem Behav. 2001;70:475–489. doi: 10.1016/s0091-3057(01)00642-6. [DOI] [PubMed] [Google Scholar]
- Fishman PA, Stang PE, Hogue SL. Impact of comorbid attention deficit disorder on the direct medical costs of treating adults with depression in managed care. J Clin Psychiatry. 2007;68:248–253. doi: 10.4088/jcp.v68n0210. [DOI] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug- induced plasticity? Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Goodman E, Capitman J. Depressive symptoms and cigarette smoking among teens. Pediatrics. 2000;106:748–755. doi: 10.1542/peds.106.4.748. [DOI] [PubMed] [Google Scholar]
- Grant BF, Hasin DS, Chou SP, Stinson FS, Dawson DA. Nicotine dependence and psychiatric disorders in the United States: results from the national epidemiologic survey on alcohol and related conditions. Arch Gen Psychiatry. 2004;61:1107–1115. doi: 10.1001/archpsyc.61.11.1107. [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Jensen PS, Abikoff H, Blumer JL, Deveaugh-Geiss J, Fisher C, Hoagwood K, Kratochvil CJ, Lahey BB, Laughren T, Leckman J, Petti TA, Pope K, Shaffer D, Vitiello B, Zeanah C. Developing strategies for psychopharmacological studies in preschool children. J Am Acad Child Adolesc Psychiatry. 2003;42:406–414. doi: 10.1097/01.CHI.0000046812.95464.FA. [DOI] [PubMed] [Google Scholar]
- Han DD, Gu HH. Comparison ofthe monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC Pharmacol. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Hughes CW. Outcome of early experience studies as affected by between-litter variance. J Nutr. 1979;109:642–645. doi: 10.1093/jn/109.4.642. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Warren BL, Neve RL, Nestler EJ, Russo SJ, Bolaños-Guzmán CA. Insulin receptor substrate-2 in the ventral teg-mental area regulates behavioral responses to cocaine. Behav Neurosci. 2008;122:1172–1177. doi: 10.1037/a0012893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Warren BL, Parise EM, Alcantara LF, Schuh B, Maffeo ML, Manojlovic Z, Bolaños-Guzmán CA. Nicotine exposure during adolescence induces a depression-like state in adulthood. Neuropsychopharmacology. 2009;34:1609–1624. doi: 10.1038/npp.2008.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Vialou V, Warren BL, Cao JL, Alcantara LF, Davis LC, Manojlovic Z, Neve RL, Russo SJ, Han MH, Nestler EJ, Bolaños-Guzmán CA. Extracellular signal-regulated kinase-2 within the ventral tegmental area regulates responses to stress. J Neurosci. 2010a;30:7652–7663. doi: 10.1523/JNEUROSCI.0951-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Warren BL, Bolaños-Guzmán CA. Short- and long-term functional consequences of fluoxetine exposure during adolescence in male rats. Biol Psychiatry. 2010b;67:1057–1066. doi: 10.1016/j.biopsych.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Warren BL, Neve RL, Russo SJ, Nestler EJ, Bolaños-Guzmán CA. Viral-mediated expression of extracellular signal-regulated kinase-2 in the ventral tegmental area modulates behavioral responses to cocaine. Behav Brain Res. 2010c;214:460–464. doi: 10.1016/j.bbr.2010.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iñiguez SD, Charntikov S, Baella SA, Herbert MS, Bolaños-Guzmán CA, Crawford CA. Post-training cocaine exposure facilitates spatial memory consolidation in C57BL/6 mice. Hippocampus. 2012;22:802–813. doi: 10.1002/hipo.20941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan G, Newcorn JH. Pharmacotherapy for child and adolescent attention-deficit hyperactivity disorder. Pediatr Clin N Am. 2011;58:99–120. doi: 10.1016/j.pcl.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- Koob GF, Nestler EJ. The neurobiology of drug addiction. J Neuropsychiatry Clin Neurosci. 1997;9:482–497. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Mazei-Robison M, Iniguez SD, Ables JL, Vialou V, Berton O, Ghose S, Covington HE, 3rd, Wiley MD, Henderson RP, Neve RL, Eisch AJ, Tamminga CA, Russo SJ, Bolanos CA, Nestler EJ. AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol Psychiatry. 2008;64:691–700. doi: 10.1016/j.biopsych.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Ion DI, Roeper J, Malenka RC. Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron. 2011;70:855–862. doi: 10.1016/j.neuron.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel S, Lim BK, Malenka RC. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 2014;76:351–359. doi: 10.1016/j.neuropharm.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaPlant Q, Vialou V, Covington HE, 3rd, Dumitriu D, Feng J, Warren BL, Maze I, Dietz DM, Watts EL, Iniguez SD, Koo JW, Mouzon E, Renthal W, Hollis F, Wang H, Noonan MA, Ren Y, Eisch AJ, Bolanos CA, Kabbaj M, Xiao G, Neve RL, Hurd YL, Oosting RS, Fan G, Morrison JH, Nestler EJ. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci. 2010;13:1137–1143. doi: 10.1038/nn.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PS, Shaw LB, Choe LH, Mehra A, Hatzimanikatis V, Lee KH. Insights into the relation between mRNA and protein expression patterns: II. Experimental observations in Escherichia coli . Biotech Bioeng. 2003;84:834–841. doi: 10.1002/bit.10841. [DOI] [PubMed] [Google Scholar]
- Leonard S, Adler LE, Benhammou K, Berger R, Breese CR, Drebing C, Gault J, Lee MJ, Logel J, Olincy A, Ross RG, Stevens K, Sullivan B, Vianzon R, Virnich DE, Waldo M, Walton K, Freedman R. Smoking and mental illness. Pharmacol Biochem Behav. 2001;70:561–570. doi: 10.1016/s0091-3057(01)00677-3. [DOI] [PubMed] [Google Scholar]
- Lu L, Wang X, Wu P, Xu C, Zhao M, Morales M, Harvey BK, Hoffer BJ, Shaham Y. Role of ventral tegmental area glial cell line-derived neurotrophic factor in incubation of cocaine craving. Biol Psychiatry. 2009;66:137–145. doi: 10.1016/j.biopsych.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mague SD, Andersen SL, Carlezon WA., Jr Early developmental exposure to methylphenidate reduces cocaine-induced potentiation of brain stimulation reward in rats. Biol Psychiatry. 2005;57:120–125. doi: 10.1016/j.biopsych.2004.10.037. [DOI] [PubMed] [Google Scholar]
- Markou A, Kosten TR, Koob GF. Neurobiological similarities in depression and drug dependence: a self-medication hypothesis. Neuropsychopharmacology. 1998;18:135–174. doi: 10.1016/S0893-133X(97)00113-9. [DOI] [PubMed] [Google Scholar]
- McClung CA, Nestler EJ. Regulation of gene expression and cocaine reward by CREB and DeltaFosB. Nat Neurosci. 2003;6:1208–1215. doi: 10.1038/nn1143. [DOI] [PubMed] [Google Scholar]
- Mehra A, Lee KH, Hatzimanikatis V. Insights into the relation between mRNA and protein expression patterns: I. Theoretical considerations. Biotech Bioeng. 2003;84:822–833. doi: 10.1002/bit.10860. [DOI] [PubMed] [Google Scholar]
- Naranjo CA, Tremblay LK, Busto UE. The role of the brain reward system in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2001;25:781–823. doi: 10.1016/s0278-5846(01)00156-7. [DOI] [PubMed] [Google Scholar]
- National Research Council. Guidelines for the care and use of mammals in neuroscience and behavioral research. Washington, DC: The National Academies Press; 2003. [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Olfson M, Marcus SC, Weissman MM, Jensen PS. National trends in the use of psychotropic medications by children. J Am Acad Child Adolesc Psychiatry. 2002;41:514–521. doi: 10.1097/00004583-200205000-00008. [DOI] [PubMed] [Google Scholar]
- Olson VG, Zabetian CP, Bolanos CA, Edwards S, Barrot M, Eisch AJ, Hughes T, Self DW, Neve RL, Nestler EJ. Regulation of drug reward by cAMP response element-binding protein: evidence for two functionally distinct subregions of the ventral tegmental area. J Neurosci. 2005;25:5553–5562. doi: 10.1523/JNEUROSCI.0345-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz J, Harris HW, Guitart X, Terwilliger RZ, Haycock JW, Nestler EJ. Extracellular signal-regulated protein kinases (ERKs) and ERK kinase (MEK) in brain: regional distribution and regulation by chronic morphine. J Neurosci. 1995;15:1285–1297. doi: 10.1523/JNEUROSCI.15-02-01285.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. J Neurosci. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. Am J Psychiatry. 2007;164:942–948. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Rowles BM, Findling RL. Review of pharmacotherapy options for the treatment of attention-deficit/hyperactivity disorder (ADHD) and ADHD-like symptoms in children and adolescents with developmental disorders. Dev Disabil Res Rev. 2010;16:273–282. doi: 10.1002/ddrr.120. [DOI] [PubMed] [Google Scholar]
- Rushton JL, Whitmire JT. Pediatric stimulant and selective serotonin reuptake inhibitor prescription trends: 1992 to 1998. Arch Pediatr Adolesc Med. 2001;155:560–565. doi: 10.1001/archpedi.155.5.560. [DOI] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci. 2013;14:609–625. doi: 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Bolanos CA, Theobald DE, DeCarolis NA, Renthal W, Kumar A, Winstanley CA, Renthal NE, Wiley MD, Self DW, Russell DS, Neve RL, Eisch AJ, Nestler EJ. IRS2-Akt pathway in mid-brain dopamine neurons regulates behavioral and cellular responses to opiates. Nat Neurosci. 2007;10:93–99. doi: 10.1038/nn1812. [DOI] [PubMed] [Google Scholar]
- Safer DJ. Should selective serotonin reuptake inhibitors be prescribed for children with major depressive and anxiety disorders? Pediatrics. 2006;118:1248–1251. doi: 10.1542/peds.2006-0215. [DOI] [PubMed] [Google Scholar]
- Safer DJ, Zito JM, DosReis S. Concomitant psychotropic medication for youths. Am J Psychiatry. 2003;160:438–449. doi: 10.1176/appi.ajp.160.3.438. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Suzuki K, Ramachandran PV, Blackburn TP, Ashby CR., Jr Acute and repeated administration of fluoxetine, citalopram, and paroxetine significantly alters the activity of midbrain dopamine neurons in rats: an in vivo electrophysiological study. Synapse. 2007;61:72–77. doi: 10.1002/syn.20349. [DOI] [PubMed] [Google Scholar]
- Seymour KE, Chronis-Tuscano A, Halldorsdottir T, Stupica B, Owens K, Sacks T. Emotion regulation mediates the relationship between ADHD and depressive symptoms in youth. J Abnorm Child Psychol. 2012;40:595–606. doi: 10.1007/s10802-011-9593-4. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spencer TJ. ADHD and comorbidity in childhood. J Clin Psychiatry. 2006;67(Suppl 8):27–31. [PubMed] [Google Scholar]
- Steiner H, Van Waes V, Marinelli M. Fluoxetine potentiates methylphenidate-induced gene regulation in addiction-related brain regions: concerns for use of cognitive enhancers? Biol Psychiatry. 2010;67:592–594. doi: 10.1016/j.biopsych.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll AL, Pillay SS, Diamond L, Workum SB, Cole JO. Methylphenidate augmentation of serotonin selective reuptake inhibitors: a case series. J Clin Psychiatry. 1996;57:72–76. [PubMed] [Google Scholar]
- Swanson JM, Volkow ND. Serum and brain concentrations of methylphenidate: implications for use and abuse. Neurosci Biobehav Rev. 2003;27:615–621. doi: 10.1016/j.neubiorev.2003.08.013. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Benveniste H, Wang GJ, Volkow ND. Effects of chronic oral methylphenidate on cocaine self-administration and striatal dopamine D2 receptors in rodents. Pharmacol Biochem Behav. 2007;87:426–433. doi: 10.1016/j.pbb.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Turgeon SM, Pollack AE, Fink JS. Enhanced CREB phosphorylation and changes in c-Fos and FRA expression in striatum accompany amphetamine sensitization. Brain Res. 1997;749:120–126. doi: 10.1016/s0006-8993(96)01316-9. [DOI] [PubMed] [Google Scholar]
- Valladares R, Sankar D, Li N, Williams E, Lai KK, Abdelgeliel AS, Gonzalez CF, Wasserfall CH, Larkin J, Schatz D, Atkinson MA, Triplett EW, Neu J, Lorca GL. Lactobacillus johnsonii N6.2 mitigates the development of type 1 diabetes in BB-DP rats. PLoS One. 2010;5:e10507. doi: 10.1371/journal.pone.0010507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Waes V, Beverley J, Marinelli M, Steiner H. Selective serotonin reuptake inhibitor antidepressants potentiate methylphenidate (Ritalin)-induced gene regulation in the adolescent striatum. Eur J Neurosci. 2010;32:435–447. doi: 10.1111/j.1460-9568.2010.07294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP. Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav. 2002;77:107–114. doi: 10.1016/s0031-9384(02)00818-1. [DOI] [PubMed] [Google Scholar]
- Vialou V, Robison AJ, Laplant QC, Covington HE, 3rd, Dietz DM, Ohnishi YN, Mouzon E, Rush AJ, 3rd, Watts EL, Wallace DL, Iniguez SD, Ohnishi YH, Steiner MA, Warren BL, Krishnan V, Bolanos CA, Neve RL, Ghose S, Berton O, Tamminga CA, Nestler EJ. DeltaFosB in brain reward circuits mediates resilience to stress and antidepressant responses. Nat Neurosci. 2010;13:745–752. doi: 10.1038/nn.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Li TK. Drug addiction: the neurobiology of behaviour gone awry. Nat Rev Neurosci. 2004;5:963–970. doi: 10.1038/nrn1539. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Ding YS, Gatley SJ. Role of dopamine in the therapeutic and reinforcing effects of methylphenidate in humans: results from imaging studies. Eur Neuropsychopharmacol. 2002;12:557–566. doi: 10.1016/s0924-977x(02)00104-9. [DOI] [PubMed] [Google Scholar]
- Wallace DL, Han MH, Graham DL, Green TA, Vialou V, Iñiguez SD, Cao JL, Kirk A, Chakravarty S, Kumar A, Krishnan V, Neve RL, Cooper DC, Bolaños CA, Barrot M, McClung CA, Nestler EJ. CREB regulation of nucleus accumbens excitability mediates social isolation-induced behavioral deficits. Nat Neurosci. 2009;12:200–209. doi: 10.1038/nn.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wargin W, Patrick K, Kilts C, Gualtieri CT, Ellington K, Mueller RA, Kraemer G, Breese GR. Pharmacokinetics of methyl-phenidate in man, rat and monkey. J Pharmacol Exp Ther. 1983;226:382–386. [PubMed] [Google Scholar]
- Warren BL, Iñiguez SD, Alcantara LF, Wright KN, Parise EM, Weakley SK, Bolaños-Guzmán CA. Juvenile administration of concomitant methylphenidate and fluoxetine alters behavioral reactivity to reward- and mood-related stimuli and disrupts ventral tegmental area gene expression in adulthood. J Neurosci. 2011;31:10347–10358. doi: 10.1523/JNEUROSCI.1470-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BL, Vialou VF, Iniguez SD, Alcantara LF, Wright KN, Feng J, Kennedy PJ, Laplant Q, Shen L, Nestler EJ, Bolanos-Guzman CA. Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry. 2013;73:7–14. doi: 10.1016/j.biopsych.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxmonsky J. Assessment and treatment of attention deficit hyperactivity disorder in children with comorbid psychiatric illness. Curr Opin Pediatr. 2003;15:476–482. doi: 10.1097/00008480-200310000-00006. [DOI] [PubMed] [Google Scholar]
- Wigal SB, Chae S, Patel A, Steinberg-Epstein R. Advances in the treatment of attention- deficit/hyperactivity disorder: a guide for pediatric neurologists. Semin Pediatr Neurol. 2010;17:230–236. doi: 10.1016/j.spen.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Wiley MD, Poveromo LB, Antapasis J, Herrera CM, Bolaños Guzmán CA. Kappa-opioid system regulates the long-lasting behavioral adaptations induced by early-life exposure to methylphenidate. Neuropsychopharmacology. 2009;34:1339–1350. doi: 10.1038/npp.2008.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolraich ML. Annotation: the use of psychotropic medications in children: an American view. J Child Psychol Psychiatry. 2003;44:159–168. doi: 10.1111/1469-7610.00110. [DOI] [PubMed] [Google Scholar]
- Yang PB, Swann AC, Dafny N. Chronic methylphenidate modulates locomotor activity and sensory evoked responses in the VTA and NAc of freely behaving rats. Neuropharmacology. 2006;51:546–556. doi: 10.1016/j.neuropharm.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Yano M, Steiner H. Methylphenidate and cocaine: the same effects on gene regulation? Trends Pharmacol Sci. 2007;28:588–596. doi: 10.1016/j.tips.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Zito JM, Derivan AT, Kratochvil CJ, Safer DJ, Fegert JM, Greenhill LL. Off-label psychopharmacologic prescribing for children: history supports close clinical monitoring. Child Adolesc Psychiatry Ment Health. 2008;2:24. doi: 10.1186/1753-2000-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]