Abstract
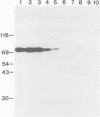
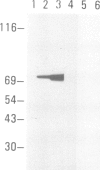
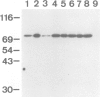
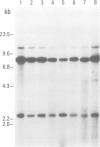
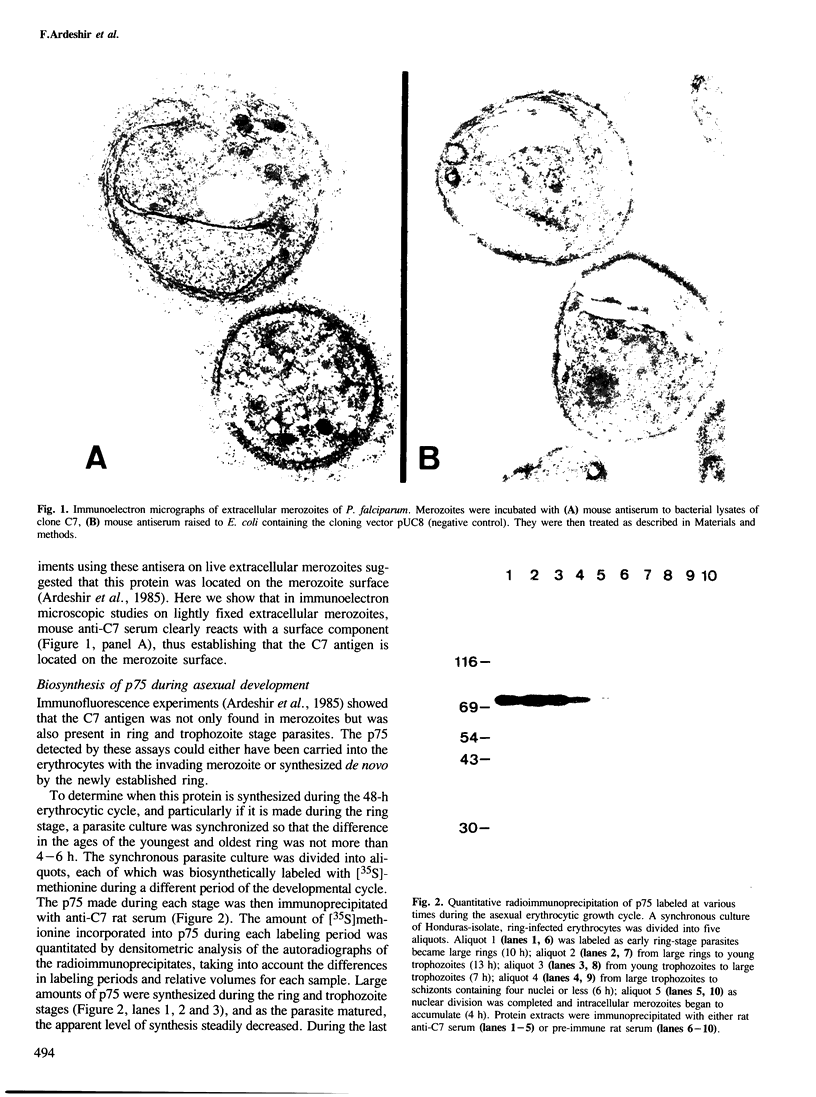
Proteins on the merozoite surface of the human malarial parasite Plasmodium falciparum are targets of the host's immune response. The merozoite surface location of p75, a 75 kd P. falciparum protein, was established by immunoelectron microscopy using antisera raised to the expressed product of a cDNA clone. Immunoprecipitation from protein extracts biosynthetically labeled during different periods of the asexual cycle showed that p75 is made continuously, although ring-stage parasites appear to synthesize larger quantities. p75 is conserved and invariant in size in eight isolates of P. falciparum. The 880 bp cDNA sequence encoding part of p75 reveals one open reading frame containing a repetitive sequence unit of four amino acids. The predicted reading frame is correct since antisera to a synthetic peptide corresponding to the repetitive region recognize p75 in immunoblots. The sequence of p75 is homologous with the sequences of proteins from the ubiquitous, highly conserved family of 70 kd heat-shock proteins, suggesting an important physiological function for p75. The cDNA fragment encoding part of p75 hybridizes with multiple genomic fragments, whose sizes are identical in DNA from nine P. falciparum strains, suggesting that the gene for p75 is well conserved and may be part of a gene family.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ardeshir F., Flint J. E., Reese R. T. Expression of Plasmodium falciparum surface antigens in Escherichia coli. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2518–2522. doi: 10.1073/pnas.82.8.2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M. Xenopus hsp 70 genes are constitutively expressed in injected oocytes. EMBO J. 1984 Nov;3(11):2477–2483. doi: 10.1002/j.1460-2075.1984.tb02159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brison O., Ardeshir F., Stark G. R. General method for cloning amplified DNA by differential screening with genomic probes. Mol Cell Biol. 1982 May;2(5):578–587. doi: 10.1128/mcb.2.5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. V., Culvenor J. G., Crewther P. E., Bianco A. E., Coppel R. L., Saint R. B., Stahl H. D., Kemp D. J., Anders R. F. Localization of the ring-infected erythrocyte surface antigen (RESA) of Plasmodium falciparum in merozoites and ring-infected erythrocytes. J Exp Med. 1985 Aug 1;162(2):774–779. doi: 10.1084/jem.162.2.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell T. G., Welch W. J., Schlossman D. M., Palter K. B., Schlesinger M. J., Rothman J. E. Uncoating ATPase is a member of the 70 kilodalton family of stress proteins. Cell. 1986 Apr 11;45(1):3–13. doi: 10.1016/0092-8674(86)90532-5. [DOI] [PubMed] [Google Scholar]
- Cowman A. F., Saint R. B., Coppel R. L., Brown G. V., Anders R. F., Kemp D. J. Conserved sequences flank variable tandem repeats in two S-antigen genes of Plasmodium falciparum. Cell. 1985 Apr;40(4):775–783. doi: 10.1016/0092-8674(85)90337-x. [DOI] [PubMed] [Google Scholar]
- Craig E. A. The heat shock response. CRC Crit Rev Biochem. 1985;18(3):239–280. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois P., Dedet J. P., Fandeur T., Roussilhon C., Jendoubi M., Pauillac S., Mercereau-Puijalon O., Pereira Da Silva L. Protective immunization of the squirrel monkey against asexual blood stages of Plasmodium falciparum by use of parasite protein fractions. Proc Natl Acad Sci U S A. 1984 Jan;81(1):229–232. doi: 10.1073/pnas.81.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson G. N. Molecular approaches to malaria vaccines. Sci Am. 1985 May;252(5):52–59. doi: 10.1038/scientificamerican0585-52. [DOI] [PubMed] [Google Scholar]
- Goman M., Langsley G., Hyde J. E., Yankovsky N. K., Zolg J. W., Scaife J. G. The establishment of genomic DNA libraries for the human malaria parasite Plasmodium falciparum and identification of individual clones by hybridisation. Mol Biochem Parasitol. 1982 Jun;5(6):391–400. doi: 10.1016/0166-6851(82)90012-3. [DOI] [PubMed] [Google Scholar]
- Guidon P. T., Jr, Hightower L. E. Purification and initial characterization of the 71-kilodalton rat heat-shock protein and its cognate as fatty acid binding proteins. Biochemistry. 1986 Jun 3;25(11):3231–3239. doi: 10.1021/bi00359a023. [DOI] [PubMed] [Google Scholar]
- Hall R., Osland A., Hyde J. E., Simmons D. L., Hope I. A., Scaife J. G. Processing, polymorphism, and biological significance of P190, a major surface antigen of the erythrocytic forms of Plasmodium falciparum. Mol Biochem Parasitol. 1984 Apr;11:61–80. doi: 10.1016/0166-6851(84)90055-0. [DOI] [PubMed] [Google Scholar]
- Heidrich H. G. Plasmodium falciparum antigens as target molecules for a protective immunization against malaria: an up-to-date review. Z Parasitenkd. 1986;72(1):1–11. doi: 10.1007/BF00927730. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R. The three major antigens on the surface of Plasmodium falciparum merozoites are derived from a single high molecular weight precursor. J Exp Med. 1984 Aug 1;160(2):624–629. doi: 10.1084/jem.160.2.624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. F., Stanley H. A., Campbell G. H., Langreth S. G., Reese R. T. Two Plasmodium falciparum merozoite surface polypeptides share epitopes with a single Mr 185 000 parasite glycoprotein. Mol Biochem Parasitol. 1985 Oct;17(1):61–77. doi: 10.1016/0166-6851(85)90128-8. [DOI] [PubMed] [Google Scholar]
- Hunt C., Morimoto R. I. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingolia T. D., Craig E. A., McCarthy B. J. Sequence of three copies of the gene for the major Drosophila heat shock induced protein and their flanking regions. Cell. 1980 Oct;21(3):669–679. doi: 10.1016/0092-8674(80)90430-4. [DOI] [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langreth S. G., Jensen J. B., Reese R. T., Trager W. Fine structure of human malaria in vitro. J Protozool. 1978 Nov;25(4):443–452. doi: 10.1111/j.1550-7408.1978.tb04167.x. [DOI] [PubMed] [Google Scholar]
- Langreth S. G., Reese R. T. Antigenicity of the infected-erythrocyte and merozoite surfaces in Falciparum malaria. J Exp Med. 1979 Nov 1;150(5):1241–1254. doi: 10.1084/jem.150.5.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence F., Robert-Gero M. Induction of heat shock and stress proteins in promastigotes of three Leishmania species. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4414–4417. doi: 10.1073/pnas.82.13.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M. J., Pelham H. R. Involvement of ATP in the nuclear and nucleolar functions of the 70 kd heat shock protein. EMBO J. 1985 Dec 1;4(12):3137–3143. doi: 10.1002/j.1460-2075.1985.tb04056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Mackay M., Goman M., Bone N., Hyde J. E., Scaife J., Certa U., Stunnenberg H., Bujard H. Polymorphism of the precursor for the major surface antigens of Plasmodium falciparum merozoites: studies at the genetic level. EMBO J. 1985 Dec 30;4(13B):3823–3829. doi: 10.1002/j.1460-2075.1985.tb04154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride J. S., Newbold C. I., Anand R. Polymorphism of a high molecular weight schizont antigen of the human malaria parasite Plasmodium falciparum. J Exp Med. 1985 Jan 1;161(1):160–180. doi: 10.1084/jem.161.1.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. An Hsp70-like protein in the ER: identity with the 78 kd glucose-regulated protein and immunoglobulin heavy chain binding protein. Cell. 1986 Jul 18;46(2):291–300. doi: 10.1016/0092-8674(86)90746-4. [DOI] [PubMed] [Google Scholar]
- O'Malley K., Mauron A., Barchas J. D., Kedes L. Constitutively expressed rat mRNA encoding a 70-kilodalton heat-shock-like protein. Mol Cell Biol. 1985 Dec;5(12):3476–3483. doi: 10.1128/mcb.5.12.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese R. T., Langreth S. G., Trager W. Isolation of stages of the human parasite Plasmodium falciparum from culture and from animal blood. Bull World Health Organ. 1979;57 (Suppl 1):53–61. [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J. Heat shock proteins: the search for functions. J Cell Biol. 1986 Aug;103(2):321–325. doi: 10.1083/jcb.103.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staden R. Graphic methods to determine the function of nucleic acid sequences. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 2):521–538. doi: 10.1093/nar/12.1part2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl H. D., Crewther P. E., Anders R. F., Brown G. V., Coppel R. L., Bianco A. E., Mitchell G. F., Kemp D. J. Interspersed blocks of repetitive and charged amino acids in a dominant immunogen of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1985 Jan;82(2):543–547. doi: 10.1073/pnas.82.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley H. A., Howard R. F., Reese R. T. Recognition of a Mr 56K glycoprotein on the surface of Plasmodium falciparum merozoites by mouse monoclonal antibodies. J Immunol. 1985 May;134(5):3439–3444. [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Giannini S. H., Cantor C. R. Heat shock genes: regulatory role for differentiation in parasitic protozoa. Science. 1985 Jun 21;228(4706):1443–1446. doi: 10.1126/science.4012301. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Weber J. L., Hockmeyer W. T. Structure of the circumsporozoite protein gene in 18 strains of Plasmodium falciparum. Mol Biochem Parasitol. 1985 Jun;15(3):305–316. doi: 10.1016/0166-6851(85)90092-1. [DOI] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Rapid purification of mammalian 70,000-dalton stress proteins: affinity of the proteins for nucleotides. Mol Cell Biol. 1985 Jun;5(6):1229–1237. doi: 10.1128/mcb.5.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]