Summary
Optimal management of influenza infection in high-risk groups remains poorly defined. This prospective study compared the efficacy and safety of 2 different systemic neuraminidase inhibitors, peramivir and oseltamivir, in influenza-infected adult outpatients, as well as several inpatients, all of whom were at increased risk for complications.
Keywords: high-risk patient, influenza, neuraminidase inhibitor, oseltamivir, peramivir
Abstract
Background
Clinical studies comparing the different neuraminidase inhibitors for treatment of at-risk patients with influenza have not been performed. To optimize such treatments, we assessed the efficacy and safety of intravenous peramivir compared with oral oseltamivir in treating seasonal influenza A or B virus infection.
Methods
A multicenter, randomized, controlled clinical trial was conducted from December 2012 to May 2014 in high-risk patients infected with seasonal influenza. A total of 92 adult inpatients and outpatients with high risk factors (HRFs) were treated by either a single intravenous infusion of peramivir (600 mg) or oral administration of oseltamivir (75 mg, twice per day for 5 days).
Results
The median times to clinical stability (time to reach <37°C) were 40.0 hours (95% confidence interval [CI] = 23.3–64.5) and 37.8 hours (95% CI = 26.3–45.3) in the peramivir and oseltamivir groups, respectively; these values did not reveal a significant difference. The virus titer and change of mean total symptom scores decreased similarly with both treatments. Results of step-wise regression suggested that virus type was a significantly effective prognostic factor with respect to illness resolution. Adverse events (AEs) with peramivir and oseltamivir occurred in 2.2% (n = 1/46) and 13.0% (n = 6/46) of patients, respectively. The severity of AEs was mild in all cases except 2 patients who showed pneumonia or COPD aggravation; both were in the oseltamivir group.
Conclusions
Intravenous peramivir was effective based on the result of direct comparison with oral oseltamivir. Thus our data show that peramivir is a useful option for the treatment of influenza-infected patients with HRFs.
Influenza virus infection remains a major global health concern. The emergence of novel influenza viruses such as A/H1N1 pdm09 virus (in the 2009 pandemic) has significantly increased hospitalizations and death rates due to lack of immune memory. The avian A/H7N9 virus may follow the same path, although the avian virus currently has a lower potential for human-to-human transmission.
Neuraminidase inhibitors (NAIs) exhibit potent inhibitory activity against neuraminidase (NA), the spike protein of influenza virus. Several recent meta-analyses [1–3] suggest that early treatment (within 48 hours after the onset of illness) with an NAI reduces the risk of hospitalization or death. The morbidity and mortality of influenza infection can be higher, particularly in high-risk populations, which include the elderly and individuals with underlying diseases (respiratory tract diseases, heart diseases, diabetes, immunodeficiency, etc) [4]. Thus, treatment with an NAI is considered essential for high-risk patients. However, this distinction in patient populations is based on an observational study, meaning that high-quality data from randomized controlled studies are lacking.
Peramivir was approved in Japan in 2010 and the compound’s clinical effectiveness, especially rapid fever alleviation [5–8], has been reported since then. A previous Ph3 study (consisting of 42 high-risk patients) demonstrated that high-dose peramivir (600 mg/d, repeating dose accepted) provided significant effectiveness in decreasing the duration of influenza illness and fever alleviation compared with low-dose peramivir (300 mg/d, repeating dose accepted) [9]. In addition, several reports have indicated the efficacies of peramivir for the treatment of critically ill patients who seldom benefit from NAIs [10, 11]. In animal studies, intravenous peramivir has shown robust efficacy in the treatment of lethal influenza and of secondary pneumococcal pneumonia following influenza virus infection [12–14]. Peramivir may have demonstrated efficacy in these studies due to a strong suppressive effect on the initial growth of influenza virus; notably, this compound rapidly reaches high concentrations in the plasma and upper respiratory tract during the early stages of infection [15]. That article suggested that the efficacy of NAIs may be better assessed by measuring viral clearance or alleviation of fever at earlier time points, especially in high-risk patients showing individual differences in immune response [15]. In this context, Kohno et al reported (in a Ph3 study) that peramivir showed significant earlier reduction of these endpoints at day 2, compared with oseltamivir, in patients infected by oseltamivir-resistant virus [16]. Moreover, a significant difference was observed retrospectively in several endpoints, including complications [17–19], mortality [20, 21], length of hospital stay [22], and viral shedding [23], in high-risk or hospitalized patients treated with oseltamivir compared with those not given an NAI.
However, peramivir lacks effectiveness in complicated patients. The single randomized, controlled study of intravenous peramivir in hospitalized patients was terminated for futility and failed to show efficacy [24]. Additionally, observational data from the 2009 pandemic raised concerns regarding serious adverse events in critically ill patients given peramivir [25].
We are unaware of published reports presenting comparative data for the treatment of high-risk outpatients with any pair of different NAIs. Such a study would enable determination of the superiority of either treatment. Considering that the optimal management of high-risk out- and inpatients who developed influenza has not yet been established, we planned and performed a multicenter, randomized, controlled study comparing the efficacy of 2 different systemic NAIs, peramivir and oseltamivir, in the treatment of high-risk outpatients (inpatients accepted) with influenza. We assessed the fever-alleviation time (primary endpoint) and the duration of influenza illness and the virus titer (secondary endpoints). The purpose of this study was to explore the possibility of establishing an optimal regimen for management of influenza in a high-risk patient population.
METHODS
This study was a multicenter, randomized, comparative study performed using the central registration method and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The trial was approved by the Ethics Committee of Nagasaki University. The study was conducted from December 2012 to May 2014. The clinical trial was registered with University Hospital Medical Information Network Clinical Trials as UMIN000009479. For the purposes of our study, high risk factors (HRFs) were defined as the following: age ≥65 years, chronic heart disease, chronic respiratory illness, chronic kidney disease, chronic liver disorder, diabetes mellitus, neurological disorder/neuromuscular disease, hematological disorder, or immunosuppressive conditions accompanied by diseases or requiring treatment. The target number of patients with HRFs was 100 based on the result demonstrating superiority of the high dose compared with the low dose in the previous study of peramivir in high-risk patients [9]. In our study, a total of 92 outpatients and several inpatients aged ≥20 years with influenza A or B virus infection meeting the following inclusion criteria were enrolled: (1) body temperature ≥38°C at hospital visit, (2) initiation of treatment within 48 hours from the onset of influenza illness (as indicated by at least 1 symptom), (3) positive for influenza virus by an influenza rapid diagnostic kit, and (4) having HRFs. We stopped our study early despite not achieving the target enrollment. We were conducting this clinical study for 2 influenza seasons. If we continued for an additional season, 3 seasons would cause larger bias in parallel with an increase in the variety of epidemic influenza virus appearing.
The following 7 influenza symptoms, as defined in the Influenza Symptom Severity [ISS] scale, were adopted: headache, muscle or joint pain, feverishness or chills, and fatigue as general symptoms, and cough, sore throat, and nasal stuffiness as respiratory symptoms. These symptoms were evaluated based on scores of 0–3 (0: no symptom [normal], 1: mild [barely troublesome], 2: moderate [very uncomfortable], 3: severe [intolerable]). Exclusion criteria were pregnancy, women who may become pregnant, breastfeeding women, and patients with pneumonia according to chest X-ray on admission. (Although pregnant women represent an important group at high risk for complications, we excluded this group for safety reasons.)
Using a central registration method, patients were equally randomized to receive peramivir (Rapiacta) or oseltamivir (Tamiflu) according to the respective package inserts. Peramivir was infused intravenously over 15 minutes at 600 mg once (a second infusion at >2 days later, if necessary, was permitted). Oseltamivir was administered orally at 75 mg twice a day for 5 days. All patients were checked at the respective institute for their backgrounds (including the HRFs) at the enrollment time and examined for clinical effects (such as vital signs, influenza symptom severity, complications associated with influenza, and virological examination) on days 1, 2, and 5. Chest X-ray examination and clinical laboratory tests were conducted on day 1 in all patients and on other days if required. Patients evaluated their own influenza symptoms using the ISS, and measured their own body temperature 3 times a day (morning, noon, and at the time of going to bed). It was prespecified to the patients that, if possible, body temperature was not to be assessed within the first 4 hours after taking antipyretics.
Nasopharyngeal swabs collected on days 1, 2, and 5 were used for virus typing, including subtyping, virus titration, and an NA enzyme inhibition assay. These assays and amino-acid sequence analyses were performed by LSI Medience. Infectious viral titers were calculated as log10 50% tissue culture infective doses (TCID50) per milliliter of viral transport medium according to the Spearman-Karber equation. Viral RNAs were not measured. All patients provided written informed consent before participating in the study. Each study was approved by the respective site’s institutional review board before the start of the study.
The primary efficacy endpoint was the fever-alleviation time (time to reach axillary temperature of <37.0°C); in parallel with this endpoint, prognostic factors that might have affected the fever-alleviation time were determined. The secondary endpoints were (1) the duration of influenza illness, (2) the virus titer and identification of virus subtypes, (3) the occurrence of gene mutation in the influenza viruses, (4) incidence of complications associated with influenza infection, and (5) exacerbation of underlying conditions. Viral gene mutation was investigated if a noticeable increase in the half maximal inhibitory concentration (IC50) value for the 4 existing NAIs (oseltamivir, peramivir, zanamivir, and laninamivir) was detected, and the difference in IC50 values was assessed statistically by the Kruskal-Wallis test. Safety of the drugs was evaluated by incidence of adverse events/adverse drug reactions (AEs/ADRs). Severity of the events was graded according to the Division of AIDS table, with grades 1, 2, and ≥ 3 corresponding to mild, moderate, and severe, respectively [26].
The primary efficacy analysis population was the intention-to-treat infected population, which included all of the patients receiving the study drug at least once during the study. The confidence coefficient and the significance levels were to be 0.95 and .05 (2-tailed), respectively. Kaplan-Meier curves were prepared for the fever-alleviation time and for the time to alleviation of influenza symptoms, and thereby a statistical intergroup difference was examined by using the log-rank test. Prognostic factors that might affect the fever-alleviation time were determined by the Cox proportional hazard model. For the estimation of duration of illness, the Wilcoxon rank-sum test was used after calculating the area under the curve of total symptom scores (TSSs) of influenza together with determining key statistics values by group. The key statistics values by group for IC50 values of each drug against a virus were calculated, followed by conducting of the Kruskal-Wallis test. The incidences of complications associated with influenza were calculated and tested by Fisher’s direct probability method.
RESULTS
A total of 92 patients were enrolled from 16 medical hospitals and randomly allocated to 2 groups of equal size; all of the enrolled patients completed the study. All were patients with fever ≥38°C and visited the respective institution within 48 hours after the onset of influenza illness. Table 1 shows backgrounds of the patients who were included in safety and intention-to-treat infected populations. With the exception of the symptom score, the baseline characteristics did not significantly differ between the 2 groups. Table 1 also shows the approximately equal distribution of virus types and subtypes in the 2 groups. The majority of infections were due to influenza A/H3N2 viruses.
Table 1.
Patient Backgrounds and High-Risk Factors at Baseline
| Background Factors | Peramivir Group | Oseltamivir Group | P Value | |||
|---|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |||
| No. of total patients | 46 | … | 46 | … | … | |
| Sex | Male/Female | x21/25 | 45.7/54.3 | 22/24 | 47.8/52.2 | 1.00 |
| Age, y | No. | 46 | 46 | .42 | ||
| Mean ± SD | 72.2 ± 14.1 | 70.1 ± 11.1 | ||||
| Median | 76 | 72 | ||||
| Minimum to maximum | 33 to 92 | 42 to 90 | ||||
| Weight, kg | No. | 45 | 36 | .83 | ||
| Mean ± SD | 55.0 ± 10.4 | 55.6 ± 12.3 | ||||
| Median | 55 | 55.65 | ||||
| Minimum to maximum | 36.0 to 80.5 | 34.2 to 88 | ||||
| Height, cm | No. | 43 | 35 | .25 | ||
| Mean ± SD | 155.3 ± 10.0 | 157.8 ± 8.9 | ||||
| Median | 156 | 158 | ||||
| Minimum to maximum | 135.7 to 174 | 140.6 to 174.1 | ||||
| Hospitalized patients | Inpatient | 7 | 15.2 | 8 | 17.4 | 1.00 |
| Virus type | Type A | 39 | 84.8 | 35 | 76.1 | .43 |
| Type B | 7 | 15.2 | 11 | 23.9 | ||
| A+B | 0 | 0.0 | 0 | 0.0 | ||
| Virus subtype | Type A H1N1 | 0 | 0.0 | 0 | 0.0 | .76 |
| Type A H3N2 | 33 | 71.7 | 28 | 60.9 | ||
| Type B | 7 | 15.2 | 11 | 23.9 | ||
| Type A H1N1 pdm09 | 5 | 10.9 | 6 | 13.0 | ||
| Not detected | 1 | 2.2 | 1 | 2.2 | ||
| Virus titer | No. | 46 | 46 | .496 | ||
| Mean ± SD | 4.20 ± 2.12 | 4.48 ± 1.82 | ||||
| Median | 4.5 | 4.3 | ||||
| Minimum to maximum | <1.5 to 8.5 | <1.5 to 8.5 | ||||
| Smoking | 5 | 10.9 | 8 | 17.4 | .38 | |
| Inoculation with influenza virus vaccine | 27 | 58.7 | 19 | 41.3 | .14 | |
| No. of patients with high-risk factors | Age ≥65 y | 37 | 80.4 | 36 | 78.3 | 1.00 |
| Chronic heart disease | 6 | 13.0 | 8 | 17.4 | .77 | |
| Chronic respiratory illness | 18 | 39.1 | 21 | 45.7 | .67 | |
| Chronic kidney disease | 7 | 15.2 | 4 | 8.7 | .52 | |
| Chronic liver disorder | 8 | 17.4 | 2 | 4.3 | .09 | |
| Diabetes mellitus | 12 | 26.1 | 10 | 21.7 | .81 | |
| Neurological disorder/ neuromuscular disease | 0 | 0.0 | 1 | 2.2 | 1.00 | |
| Hematological disorder | 0 | 0.0 | 0 | 0.0 | … | |
| Immunosuppressive conditions accompanied by diseases or requiring treatment | 5 | 10.9 | 9 | 19.6 | .38 | |
| Underlying disease/ complication | 38 | 82.6 | 40 | 87.0 | .77 | |
| Symptom score | No. | 46 | 45 | .03 | ||
| Mean ± SD | 10.9 ± 2.5 | 9.6 ± 3.1 | ||||
| Median | 10 | 9 | ||||
| Minimum to maximum | 6 to 16 | 4 to 18 | ||||
| ≤14 | 43 | 93.5 | 43 | 93.5 | 1.00 | |
| ≥15 | 3 | 6.5 | 2 | 4.3 | ||
| Not described | 0 | 0.0 | 1 | 2.2 | ||
| Time from onset of influenza to drug dosing, h | N | 46 | 46 | .44 | ||
| Mean ± SD | 28.2 ± 16.1 | 25.8 ± 12.9 | ||||
| Median | 24.7 | 23.1 | ||||
| Minimum to maximum | 2.0 to 73.9 | 2.5 to 49.6 | ||||
Abbreviation: SD, standard deviation.
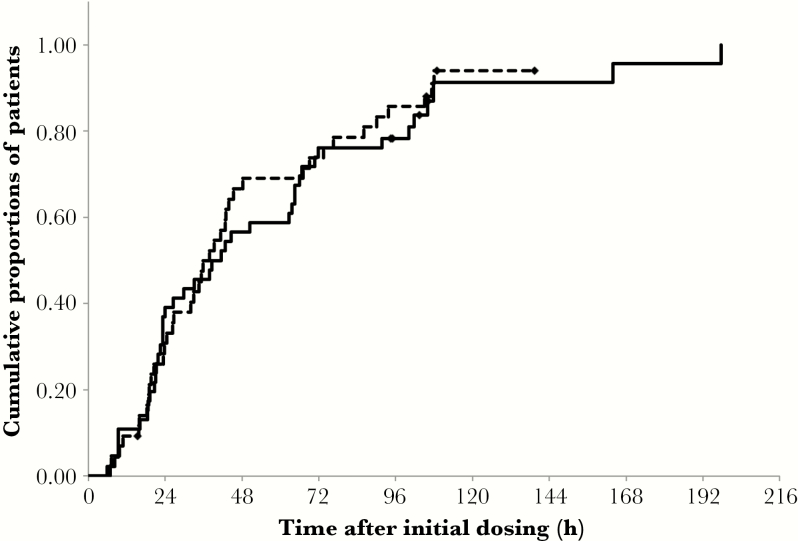
Figure 1 shows a Kaplan-Meier curve for the fever-alleviation time. In this analysis, 3 patients in the oseltamivir group were omitted from the clinical efficacy assessment because patient diaries (including information on body temperature) were not obtained for these subjects. Patients lacking the record of body temperature <37.0°C, irrespective of whether these patients had returned to a normal body temperature, were considered as censored. The respective medians of fever-alleviation time were 40.0 hours (95% confidence interval [CI] = 23.3–64.5) and 37.8 hours (95% CI = 26.3–45.3) in the peramivir and oseltamivir groups, respectively; these values did not exhibit a significant intergroup difference (log-rank test; P = .69; χ2 = 0.156). The Cox hazard analysis of the effect of the 2 NAIs showed that the 95% confidence interval for the difference between the 2 treatments ranged 0.88–2.48, indicating no significant difference between peramivir and oseltamivir. Three of 46 patients in the peramivir-treated group were administered peramivir for 2 days. Notably, this subset of 3 patients included 2 patients that were censored (as described above) and 1 patient who showed an alleviation time of 197.8 hours, a value that was onger than the median alleviation time of 37.8 hours (95% CI = 23.2–62.7) obtained from the remaining 43 patients treated with single-dose peramivir. No persistent virus was observed in the 3 patients administered peramivir for 2 days. Notably, none of the theses 3 patients harbored the H275Y mutation. Among a total of 79 patients who took antipyretics, 37 of 46 (80.4%) were treated with peramivir, and 42 of 46 (91.3%) were treated with oseltamivir. As a result, a total of 3 patients (1 and 2 subjects from peramivir- and oseltamivir-treated groups, respectively) ingested acetaminophen at approximately the same time as the alleviation time; in the other patients that ingested acetaminophen, body temperatures were measured at least 4 hours after the dose of acetaminophen was taken.
Figure 1.
Kaplan-Meier survival curve for the time to fever alleviation. Solid line: peramivir group (n = 46). Dotted line: oseltamivir group (n = 43). ◆ indicates censored case (n = 4 in peramivir group; n = 4 in oseltamivir group). P value for the difference between treatments was .69 (log-rank test).
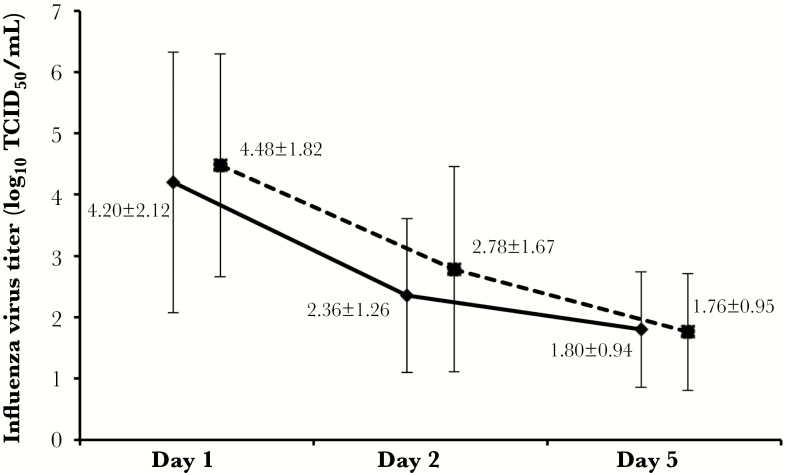
The numbers of days (mean ± SE) required for the disappearance of influenza symptoms were 5.26 ± 0.15 and 5.38 ± 0.16 days for the peramivir and the oseltamivir groups, respectively (log-rank test; P = .65; χ2 value = 0.204). Table 2 shows the change of variation of the TSSs. The scores decreased over time, falling from −2.5 (peramivir) and −1.3 (oseltamivir) on day 2, to −7.1 (peramivir) and −5.9 (oseltamivir) on day 5; significant intergroup differences were not detected for this parameter. The duration of influenza illness was examined by the Wilcoxon rank-sum test. The intergroup difference of TSSs did not achieve significance (P = .051 at day 2). The change in infectious virus titer is shown in Figure 2. The median time for the virus titer to decrease by <101.5 TCID50/mL was approximately 4 days in both groups (Kaplan-Meier method, post hoc analysis); this parameter did not reveal a statistically significant intergroup difference (P = .51; χ2 = 0.436). The decrease of virus titer was examined by virus type and subtype, but no significant intergroup difference was detected (data not shown).
Table 2.
Change of Variation of Total Symptom Scores
| Time | Treatment Group | No. of Subject | Mean ± SD | Median | Minimum | Maximum |
P Value
(Wilcoxon rank sum test) |
|---|---|---|---|---|---|---|---|
| Day 2 | Peramivir | 46 | −2.5 ± 3.5 | −2 | −9 | 6 | .051 |
| Oseltamivir | 45 | −1.3 ± 2.2 | −1 | −5 | 4 | ||
| Day 3 | Peramivir | 46 | −4.2 ± 3.6 | −5 | −12 | 5 | .09 |
| Oseltamivir | 45 | −3.0 ± 3.2 | −4 | −9 | 5 | ||
| Day 4 | Peramivir | 46 | −5.5 ± 3.8 | −6 | −12 | 6 | .22 |
| Oseltamivir | 45 | −4.3 ± 4.2 | −5 | −10 | 6 | ||
| Day 5 | Peramivir | 46 | −7.1 ± 3.6 | −8 | −12 | 5 | .22 |
| Oseltamivir | 45 | −5.9 ± 4.3 | −7 | −13 | 5 |
Variation on each day was calculated using total symptom score on day 1 as standard. Fundamental statistics were calculated by regarding the variation as a continuous quantity. The test was carried out by day without considering multiplicity.
Abbreviation: SD, standard deviation.
Figure 2.
Time course of virus titers in peramivir and oseltamivir dose groups. Solid line: peramivir group. Dotted line: oseltamivir group. The error bars represent standard deviation. Abbreviation: TCID50, 50% tissue culture infective dose.
For persistent viruses sampled from 11 patients whose virus titer did not decrease to <1.5 by day 5, we assessed the IC50 values of the 4 NAIs. The median IC50 values ranged 1.3–6.7 nM; no statistically significant difference was observed among these drugs (Kruskal-Wallis test; P = .19). The persistent viruses associated with prolonged viral shedding were checked for the presence of known NA mutations. Notably, 2 of the 11 strains harbored H275Y mutations: both instances occurred in peramivir-treated patients. Consistent with this observation, these strains showed elevated IC50 values for oseltamivir (250 and 220 nM) and peramivir (18 and 17 nM), respectively. A subanalysis based on the symptoms revealed that there was no delay of healing (fever and duration of influenza illness) when comparing the 11 patients carrying persistent viruses to the remaining 78 patients (data not shown).
Prognostic factors that might affect the fever-alleviation time were examined using the Cox proportional hazard model, to which all of the prognostic factors were inputted. The result of variable selection by the step-wise method confirmed that antiviral agent, virus type, sex, chronic cardiac disease, and chronic liver disorder were possible effective prognostic factors (a significance level of 0.2 to allow a variable into the model and to stay in the model), with respective hazard ratios of 1.461, 0.449, 1.518, 1.579, and 2.091 (Table 3).
Table 3.
Prognostic Factors that May Affect the Fever-Alleviation Time
| Variable | HR (95% CI) | P Value |
|---|---|---|
| Antiviral agent (peramivir/oseltamivir) | 1.461 (0.887–2.408) | .14 |
| Virus type (type A/type B) | 0.449 (0.239–0.843) | .01 |
| Sex (male/female) | 1.518 (0.950–2.425) | .08 |
| Chronic cardiac disease (no/yes) | 1.579 (0.835–2.986) | .16 |
| Chronic liver disorders (no/yes) | 2.091 (0.974–4.487) | .06 |
Results were obtained by using a Cox proportional hazard model that incorporated all of the prognostic factors, with the exception of the 5 factors in the table; these excepted factors instead were examined by variable selection using a stepwise method (a significance level of .2 to allow a variable into the model and to stay in the model).Abbreviations: CI, confidence interval; HR, hazard ratio.
The incidence of complications associated with influenza also was examined as a secondary endpoint. Only 4 of 46 (8.7%) peramivir-treated patients and 6 of 46 (13.0%) oseltamivir-treated patients developed complications associated with influenza; these values did not demonstrate a significant intergroup difference (Fisher’s direct probability test; P = .74). Notably, the exacerbation of underlying conditions and complications did not lead to discontinuation from the study. Hypertension and bronchial asthma gave the most incidences of aggravation (peramivir: n = 3 each; oseltamivir: n = 4 each). The safety of both drugs was examined by monitoring the appearance of AEs/ADRs. The severity of AEs was mild in all cases, with the exception of 1 case each of pneumonia and COPD aggravation. Both of these events occurred in the oseltamivir-treated group and were of moderate severity (Table 4). Adverse drug reactions in the oseltamivir group consisted of 1 case each of hepatic functional abnormality, diarrhea, and decrease in white blood cell count; no ADRs were reported in the peramivir group. The case of pneumonia (in the oseltamivir-treated group) was not thought to be related to the administered medication; this patient recovered on day 10.
Table 4.
Occurrences of Adverse Events (Adverse Drug Reactions), Influenza-Associated Complications, and Exacerbation of Underlying Disease
| Peramivir Group (n = 46) | Oseltamivir Group (n = 46) | ||||
|---|---|---|---|---|---|
| No. of Cases | % | No. of Cases | % | ||
| AEs (ADRs) | Pneumonia | 0 | 0 | 1a (0) | 2.2 |
| Pneumonia/bronchial asthma attack | 1(0) | 2.2 | 0 | 0 | |
| COPD exacerbation /nausea | 0 | 0 | 1a(0) | 2.2 | |
| Liver dysfunction | 0 | 0 | 2 (1) | 4.3 (2.2) | |
| Diarrhea | 0 | 0 | 1 (1) | 2.2 (2.2) | |
| Decrease WBC counts | 0 | 0 | 1 (1) | 2.2 (2.2) | |
| Total | 1 (0) | 2.2 | 6 (3) | 13.0 (8.7) | |
|
Influenza-associated
complications |
Pneumonia | 1 | 2.2 | 2 | 4.3 |
| Bronchitis | 0 | 0 | 1 | 2.2 | |
| Bronchial asthma attack | 2 | 4.3 | 2 | 4.3 | |
| Others | 1 | 2.2 | 1 | 2.2 | |
| Total | 4 | 8.7 | 6 | 13.0 | |
| Exacerbation of underlying diseases | Total | 4 | 8.7 | 7 | 15.2 |
Abbreviations: ADRs, adverse drug reactions; AEs, adverse events; COPD, chronic obstructive pulmonary disease; WBC, white blood cell.
aSeverities of pneumonia and COPD exacerbation were moderate in the indicated case. Severities were mild in all other cases.
DISCUSSION
As described in previous reports [9, 17, 18], duration of influenza illness is apparently longer in influenza patients with >1 HRFs than in otherwise healthy patients [16, 27]. In our study’s investigation of prognostic factors, the step-wise method identified virus type as one of the covariates for fever-alleviation time (Table 3). Vaccination status did not affect treatment outcome, contrary to our expectation. The median durations of influenza illness observed in the peramivir- and oseltamivir-treated groups in our study were approximately 5 days, values that are consistent with those previously reported for high-risk cases treated with oseltamivir [17, 18]. The median fever-alleviation time in our study was approximately 40 hours, a value similar to that reported in other studies [9, 17]. Thus, the results obtained in our study were not notably different from those obtained in other studies with high-risk patients.
In the previous Ph3 study, no significant intergroup difference was observed for any of these endpoints when comparing groups treated with peramivir (intravenous) or oseltamivir (oral). These results contrasted with our expectations, which were based on the fact that the intravenous administration of peramivir yields higher exposure at an earlier stage of infection, possibly leading to more efficient inhibition of the viral NA during the exponential phase of viral replication [15]. It was previously reported that 600 mg (repeating dose accepted) of peramivir showed significantly higher efficacies regarding duration of influenza illness and time to return to normal body temperature compared with 300 mg (repeating dose accepted) in a study with high-risk patients [9]. The medians of the duration of influenza illness and the time to return to normal body temperature with 600 mg were 42.3 hours (90% CI = 30.0–82.7) and 37.6 hours (90% CI = 22.3–46.8), respectively. In our study, which also used a 600-mg dose, medians of the 2 endpoints were 5 days (95% CI = 5–5 days) and 40.0 hours (95% CI = 23.3–64.5), respectively.
Although comparable data were obtained for the fever-alleviation times, the 2 studies resulted in distinctively different median times for duration of influenza illness. This difference in results can be attributed to the different dose regimens used in the 2 studies. Specifically, in the former study, peramivir was administered repeatedly (for >2 days) to 16 of 19 patients, whereas in our study, peramivir was administered only once in almost all of the cases (single dose in 43 of 46 patients; 2 doses in 3 of 46 patients). As a second reason for the difference, the enrollees in the 2 studies exhibited distinct backgrounds. For instance, approximately 26% and 80% of the peramivir-treated groups were aged ≥65 years in the previous high-risk study and in our study, respectively. Moreover, our study enrolled patients with a wider variety and number of HRFs than those enrolled in the former study. The increased median age and larger number of HRFs in our study presumably yielded a larger variance of immune response and clinical presentation. In other words, the sample size for patients with a large variety of HRFs in our study likely was smaller than that for otherwise healthy patients generally required to demonstrate a significant difference in clinical efficacy. A significant difference between 2 drugs may be demonstrated by limiting the number of HRF(s) to the one(s) expected to exhibit smaller variances of response and clinical presentation (eg, diabetes mellitus). Meanwhile, in our study the changes in the TSSs in the peramivir-treated patients tended to be more favorable than those in the oseltamivir-treated patients, especially on day 2 (P ≥ .05) (shown in Table 2), whereas there were no significant differences in efficacy between the 2 drugs. These results indicated that despite such limitations, our data provide some valuable information that can contribute to strategies for the treatment of influenza-infected patients with HRFs.
In conclusion, our results suggest that administration of peramivir as a single (or twice in 2 exceptional cases) 600-mg intravenous dose displayed no significant difference in efficacy compared with oseltamivir administered orally at a dose of 75 mg twice a day for 5 days, which has been already established as a standard treatment. Thus, our data show that peramivir is 1 useful option for the treatment of influenza-infected patients with HRFs.
Acknowledgments
This study was conducted by the multiple sites described below. We thank the representatives of the following institutions for their cooperation in the completion of this study: Internal Medicine, Kawamura Clinic: Sumio Kawamura; Tomonaga Internal Medicine Clinic: Akimitsu Tomonaga; Onitsuka Naika Shokaki Hospital: Yasunori Onitsuka; Department of Internal Medicine, Kouseikai Hospital: Yasumasa Dotsu; Department of Respiratory Organs, Izumikawa Hospital: Kinichi Izumikawa; Department of Respiratory Organs, Isahaya Health Insurance General Hospital: Yuichi Inoue; Department of Respiratory Organs, The Sasebo Municipal Hospital: Yuichi Fukuda; Internal Medicine, Shigeno Hospital: Yoshiteru Shigeno; Department of Internal Medicine, Senju Clinic: Jun Araki; Department of Respiratory Medicine, Hokusho Central Hospital: Yasuhito Higashiyama; Department of Internal Medicine, Nagasaki Harbor Medical Center City Hospital: Naofumi Suyama; Department of Respiratory Medicine, National Hospital Organization Ureshino Medical Center: Eisuke Sasaki; Department of Respiratory Medicine, The Japanese Red Cross Nagasaki Genbaku Hospital: Koji Hashiguchi; Department of Respiratory Medicine, The Japanese Red Cross Nagasaki Genbaku Isahaya Hospital: Kiyoyasu Fukushima; Department of Internal Medicine, Irifune Clinic: Kenji Irifune.
Financial support. This work was supported by Shionogi & Co, Ltd
Potential conflicts of interest. S. K. receives honoraria from Shionogi & Co, Ltd for delivering promotional lectures on infectious diseases and is an adviser to Shionogi & Co, Ltd, which develops anti-influenza drugs. K. Y. declares that he has served as an investigator in a study conducted by Shionogi & Co, Ltd and receives honoraria from Shionogi & Co, Ltd, for delivering promotional lectures. Y. S. is an employee of Shionogi & Co, Ltd. K. I. received a research grant from Shionogi & Co, Ltd. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Muthuri SG, MyIes PR, Venkatesan S et al. Impact of neuraminidase inhibitor treatment on outcomes of public health Importance during the 2009–2010 influenza A(H1N1) pandemic: a systematic review and meta-analysis in hospitalized patients. J Infect Dis. 2013;207:553–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hsu J, Santesso N, Mustafa R et al. Antivirals for treatment of influenza: a systematic review and meta-analysis of observational studies. Ann Intern Med. 2013;7:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muthuri SG, Venkatesan S, Myles PR et al. ; PRIDE Consortium Investigators Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med. 2014;2:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization. WHO Guidelines for Pharmacological Management of Pandemic (H1N1) 2009 Influenza and other Influenza Viruses. Geneva, Switzerland: World Health Organization; 2010; http://www.who.int/csr/resources/publications/swineflu/h1n1_guidelines_pharmaceutical_mngt.pdf [PubMed] [Google Scholar]
- 5. Hikita T, Hikita H, Hikita F et al. Clinical effectiveness of peramivir in comparison with other neuraminidase inhibitors in pediatric influenza patients. Int J Pediatr. 2012;2012:834181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shobugawa Y, Saito R, Sato I et al. Clinical effectiveness of neuraminidase inhibitors—oseltamivir, zanamivir, laninamivir, and peramivir—for treatment of influenza A(H3N2) and A(HINl) pdm09 infection: an observational study in the 2010–2011 influenza season in Japan. J Infect Chemother. 2012;18:858–64. [DOI] [PubMed] [Google Scholar]
- 7. Takemoto Y, Asai T, Ikezoe I et al. Clinical effects of oseltamivir, zanamivir, laninamivir and peramivir on seasonal influenza infection in outpatients in Japan during the winter of 2012–2013. Chemotherapy. 2013;59:373–8. [DOI] [PubMed] [Google Scholar]
- 8. Sugaya N, Sakai-Tagawa Y, Bamba M et al. Comparison between virus shedding and fever duration after treating children with pandemic A H1N1/09 and children with A H3N2 with a neuraminidase inhibitor. Antivir Ther. 2015;20:49–55. [DOI] [PubMed] [Google Scholar]
- 9. Kohno S, Kida H, Mizuguchi M et al. ; S-021812 Clinical Study Group Intravenous peramivir for treatment of influenza A and B virus infection in high-risk patients. Antimicrob Agents Chemother. 2011;55:2803–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nasu T, Ogawa D, Wada J, Makino H. Peramivir for severe influenza infection in a patient with diabetic nephropathy. Am J Respir Crit Care Med. 2010;182:1209–10. [DOI] [PubMed] [Google Scholar]
- 11. Hung S-F, Fung C-P, Perng D-W, Wang F-D. Effects of corticosteroid and neuraminidase inhibitors on survival in patients with respiratory distress induced by influenza virus. J Microbiol Immunol Infect. 2015. 10.1016/j.jmii.2015.08.016 [DOI] [PubMed]
- 12. Boltz DA, Ilyushina NA, Arnold CS et al. Intramuscularly administered neuraminidase inhibitor peramivir is effective against lethal H5N1 influenza virus in mice. Antiviral Res. 2008;80:150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tanaka A, Nakamura S, Seki M et al. The effect of intravenous peramivir, compared with oral oseltamivir, on the outcome of post-influenza pneumococcal pneumonia in mice. Antivir Ther. 2015;20:11–9. [DOI] [PubMed] [Google Scholar]
- 14. Kitano M, Kodama M, Itoh Y et al. Efficacy of repeated intravenous infection of peramivir against influenza A (H1N1) 2009 virus injection in immunosuppressed mice. Antimicrob Agents Chemother. 2013;57:2286–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Saisho Y, Ishibashi T, Fukuyama H et al. Pharmacokinetics and safety of intravenous peramivir, neuraminidase inhibitor of influenza virus, in healthy Japanese subjects. Antivir Ther. 2016; doi:10.3851/IMP3104. [DOI] [PubMed] [Google Scholar]
- 16. Kohno S, Yen MY, Cheong HJ et al. ; S-021812 Clinical Study Group Phase III randomized, double-blind study comparing single-dose intravenous peramivir with oral oseltamivir in patients with seasonal influenza virus infection. Antimicrob Agents Chemother. 2011;55:5267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Singh S, Barghoorn J, Bagdonas A et al. Clinical benefits with oseltamivir in treating influenza in adult populations: results of a pooled and subgroup analysis. Clin Drug Investig. 2003;23:561–9. [DOI] [PubMed] [Google Scholar]
- 18. Dutkowski R. Oseltamivir in seasonal influenza: cumulative experience in low- and high-risk patients. J Antimicrob Chemother. 2010;65:ii11–ii24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patrick M, Sebstian LJ. Influenza infection and COPD. Int J Chron Obstruct Pulmon Dis. 2007;2:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McGeer A, Green KA, Plevneshi A et al. ; Toronto Invasive Bacterial Diseases Network Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin Infect Dis. 2007;45:1568–75. [DOI] [PubMed] [Google Scholar]
- 21. Lee N, Choi KW, Chan PK et al. Outcomes of adults hospitalised with severe influenza. Thorax. 2010;65:510–5. [DOI] [PubMed] [Google Scholar]
- 22. Coffin SE, Leckerman K, Keren R et al. Oseltamivir shortens hospital stays of critically ill children hospitalized with seasonal influenza: a retrospective cohort study. Pediatr Infect Dis J. 2011;30:962–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee N, Chan PK, Hui DS et al. Viral loads and duration of viral shedding in adult patients hospitalized with influenza. J Infect Dis. 2009;200:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Jong MD, Ison MG, Monto AS et al. Evaluation of intravenous peramivir for treatment of influenza in hospitalized patients. Clin Infect Dis. 2014;59:e172–85. [DOI] [PubMed] [Google Scholar]
- 25. Louie JK, Yang S, Yen C et al. Use of intravenous peramivir for treatment of severe influenza A(H1N1)pdm09. PLoS One. 2012;7:e40261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Division of Acquired Immunodeficiency Syndrome, NIAID, NIH. Division of AIDS table for grading the severity of adult and pediatric adverse events. http://www3.niaid.nih.gov/research/resources/DAIDSClinRsrch/PDF/Safety/DAIDSAEGradingTable.pdf. Accessed December 2004. [Google Scholar]
- 27. Kohno S, Kida H, Mizuguchi M, Shimada J; S-021812 Clinical Study Group Efficacy and safety of intravenous peramivir for treatment of seasonal influenza virus infection. Antimicrob Agents Chemother. 2010;54:4568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]