Abstract
Although Helicobacter pylori has a significant impact on the occurrence of severe clinical syndromes, its exact ways of transmission and origin have not been identified. According to the results of some previously published articles, foods with animal origins play a substantial role in the transmission of H. pylori to humans. The present investigation was carried out to study the vacuolating cytotoxin A (vacA) and cytotoxin associated gene A (cagA) genotypes status and antibiotic resistance properties of H. pylori strains recovered from minced-meat and hamburger samples. A total of 150 meat product samples were collected from supermarkets. All samples were cultured and the susceptive colonies were then subjected to nested-PCR, PCR-based genotyping and disk diffusion methods. 11 out of 150 samples (7.33%) were positive for H. pylori. All the isolates were further identified using the nested-PCR assay. Prevalence of H. pylori in hamburger and minced-meat samples was 1.42% and 12.5%, respectively. S1a, m1a and cagA were the most commonly detected genotypes. The most commonly detected combined genotypes in the H. pylori strains of minced-meat were s1am1a (10%), s1am1b (10%) and s2m1a (10%). Helicobacter pylori strains of meat products harbored the highest levels of resistance against ampicillin (90.90%), erythromycin (72.72%), amoxicillin (72.72%), trimethoprim (63.63%), tetracycline (63.63%), and clarithromycin (63.63%). Hamburger and minced-meat samples may be the sources of virulent and resistant strains of H. pylori. Meat products are possible sources of resistant and virulent strains of H. pylori similar to those vacA and cagA genotypes. Using healthy raw materials and observation of personal hygiene can reduce the risk of H. pylori in meat products.
Key Words: Antibiotic resistance pattern, cagA, Helicobacter pylori, Meat products, vacA
Introduction
In spite of the high nutritional value of meat and its products (Valsta et al., 2005 ▶; Pereira and Vicente, 2013 ▶), their production and processing have low hygienic conditions in Iran. Therefore, many kinds of infections and food poisonings can occur due to their consumption.
Helicobacter pylori is a microaerophilic, Gram-negative spiral bacterium which is classically considered as a main cause of duodenal ulcer, peptic ulcer disease, gastric adenocarcinoma, type B gastritis and lymphoma allaround the world (Dunn et al., 1997 ▶; Van Leerdam and Tytgat, 2002 ▶; Kusters et al., 2006 ▶). Human stomach is considered as a main reservoir of H. pylori (Dunn et al., 1997 ▶; Van Leerdam and Tytgat, 2002 ▶; Kusters et al., 2006 ▶) but according to the previous hypothesis and results of some published investigations, foods with animal origins play an important role in the distribution and transmission of H. pylori (Van Duynhoven and Jonge, 2001 ▶; Brown, 2000 ▶; Herrera 2004 ▶). Appropriate circumstances such as pH, activated water (AW), moisture and temperature cause H. pylori to easily survive in foods with animal origin and especially meat and meat products (Brown, 2000 ▶; Herrera, 2004 ▶; Atapoor et al., 2014 ▶).
Genotyping using gold genetic markers like vacuolating cytotoxin A (vacA) and cytotoxin associated gene A (cagA) is an appropriate approach to study the exact correlations between H. pylori isolates from different sources (Momtaz et al., 2012 ▶, 2014; Yahaghi et al., 2014 ▶). VacA gene is polymorphic and containing of mutable signal regions (type s1 or s2) and also mid-regions (type m1 or m2). The s1 type is additionally subtyped into s1a, s1b and s1c and the m1 into m1a and m1b alleles (Momtaz et al., 2012 ▶, 2014; Yahaghi et al., 2014 ▶). Cag pathogenicity island (PAI) has been shown to be complicated in persuading ulceration, inflammation and carcinogenesis (Momtaz et al., 2012 ▶, 2014; Yahaghi et al., 2014 ▶).
Treatment is a precarious point in the epidemiology of H. pylori in humans, animals and food products, since therapeutic options have become somewhat restricted because of the presence of multidrug resistant strains of this bacterium (De Francesco et al., 2010 ▶; Talebi Bezmin Abadi et al., 2010 ▶; Mousavi et al., 2014 ▶). Prevalence of H. pylori resistance against various types of antibiotics were between 5 and 100% (De Francesco et al., 2010 ▶; Talebi Bezmin Abadi et al., 2010 ▶; Mousavi et al., 2014 ▶) which were considerably high.
There were no previously published data on the presence of H. pylori in foods and especially meat products all-around (Brown, 2000 ▶; Van Duynhoven and Jonge, 2001 ▶; Herrera, 2004 ▶; Rahimi and Kheirabadi, 2012 ▶; Atapoor et al., 2014 ▶). Therefore, the present investigation was carried out to genotype and study the antibiotic resistance properties of H. pylori strains isolated from hamburger and minced-meat samples of Iranian supermarkets.
Materials and Methods
Sample collection
From October 2015 to January 2016, a total of 150 meat product samples including hamburger (n=80) and minced-meat (n=70) were collected from supermarkets of various parts of Isfahan province, Iran. Samples (100 mg, in sterile glass containers) were transported to the laboratory at 4°C using ice-packs.
Isolation of H. pylori
Twenty five g of each homogenized sample was added to 225 ml of Wilkins Chalgren anaerobe broth (Oxoid, UK) supplemented with colistin methane sulfonate (30 mg/L), 5% of horse serum (Sigma, St. Louis, MO, USA), nalidixic acid (30 mg/L), vancomycin (10 mg/L), cycloheximide (100 mg/L), and trimethoprim (30 mg/L) (Sigma, St. Louis, MO, USA) and incubated for 7 days at 37°C with shaking under microaerophilic conditions (5% oxygen, 85% nitrogen and 10% CO2) using MART system (Anoxamat, Lichtenvoorde, The Netherlands). Then, 0.1 ml of the enrichment selective broth was plated onto Wilkins Chalgren anaerobe agar (Oxoid, UK) supplemented with 5% of defibrinated horse blood and 30 mg/L colistin methanesulfonate, 100 mg/L cycloheximide, 30 mg/L nalidixic acid, 30 mg/L trimethoprim, and 10 mg/L vancomycin (Sigma, St. Louis, MO, USA) and incubated for 7 days at 37°C under microaerophilic conditions (5% oxygen, 85% nitrogen and 10% CO2) using MART system (Anoxamat, Lichtenvoorde, The Netherlands). For comparison, a reference strain of H. pylori (ATCC 43504) was employed.
Antimicrobial susceptibility testing
The pure cultures of H. pylori were employed for antibiotic susceptibility test. One strain from each H. pylori-positive sample was selected and then subjected to the Kirby-Bauer disc diffusion method using Mueller-Hinton agar (Merck, Germany) supplemented with 5% defibrinated sheep blood and 7% fetal calf serum, according to the Clinical Laboratory Standards Institute (Wayne, 2012 ▶). The antimicrobial resistance of H. pylori was measured against the widely used antibiotics in cases of H. pylori gastric ulcer. The following antimicrobial disks (HiMedia Laboratories, Mumbai, India) were used: ampicillin (10 µg), metronidazole (5 µg), erythromycin (5 µg), clarithromycin (2 µg), amoxicillin (10 µg), tetracycline (30 µg), levofloxacin (5 µg), streptomycin (10 µg), rifampin (30 µg), cefsulodin (30 µg), trimethoprim (25 µg), furazolidone (1 µg) and spiramycin (100 µg). After incubation at 37°C for 48 h in a microaerophilic atmosphere (5% oxygen, 85% nitrogen and 10% CO2) using MART system (Anoxamat, Lichtenvoorde, The Netherlands), the susceptibility of the strains was measured against each antimicrobial agent. Results were construed in accordance with interpretive criteria provided by CLSI (2012) (Wikler, 2006 ▶). The H. pylori ATCC 43504 was used as quality control organisms in the antimicrobial susceptibility determination.
DNA extraction and nested-PCR assay
Typical colonies of H. pylori were further identified using the nested-PCR method. Genomic DNA was extracted from the typical colonies using a DNA extraction kit for cells and tissues (Fermentas, Germany) according to the manufacturer’s instructions and its density was assessed by optic densitometry. The first and second step of PCR was performed based on the method described previously (Yamada et al., 2008 ▶). List of primers and nested-PCR conditions is shown in Table 1.
Table 1.
Oligonucleotide primers and reaction conditions used for confirmation of H. pylori strains of meat products using nested-PCR method
| Target gene | Primer sequence (5´-3´) | Size of product (bp) | Volume of PCR reaction (50 µL) | Annealing temperature | References |
|---|---|---|---|---|---|
| EHC-U | F: CCCTCACGCCATCAGTCCCAAAAA | 417 | 5 µL PCR buffer 10X | 55°C ------------ 120 s | Yamada et al. (2008) |
| EHC-L | R: AAGAAGTCAAAAACGCCCCAAAAC | 5 µL dNTP (Fermentas) | |||
| 3 µL of each primers F & R | |||||
| 0.3 µL Taq DNA polymerase (Fermentas) | |||||
| 5 µL DNA template | |||||
| 31.7 μL distilled water | |||||
| ET-5U | F: GGCAAATCATAAGTCCGCAGAA | 230 | 5 µL PCR buffer 10X | 55°C ------------ 120 s | Yamada et al. (2008) |
| ET-5L | R: TGAGACTTTCCTAGAAGCGGTGTT | 5 µL dNTP (Fermentas) | |||
| 3 µL of each primers F & R | |||||
| 0.3 µL Taq DNA polymerase (Fermentas) | |||||
| 5 µL of first-step PCR product | |||||
| 31.7 µL distilled water |
Genotyping of vacA and cagA genes of H. pylori
Presence of the vacA and cagA alleles was determined using PCR technique. List of primers is shown in Table 2 (Chomvarin et al., 2008 ▶; Mansour et al., 2010 ▶). All PCR reactions were done using the programmable thermal cycler (Eppendorf Co., Germany). All runs included one negative DNA control consisting of PCR grade water and two or more positive controls (26695, J99, SS1, Tx30, 88-23 and 84-183).
Table 2.
Oligonucleotide primers, volume and programs of PCR reactions used for genotyping of vacA and cagA alleles of H. pylori strains of meat products
| Genes | Primer sequence (5´-3´) | Size of product (bp) | Volume of PCR reaction (50 µL) | Annealing temperature | References |
|---|---|---|---|---|---|
| vacA s1a | F: CTCTCGCTTTAGTAGGAGC | 213 | 5 µL PCR buffer 10X | 64°C ------------ 50 s | Chomvarin et al. (2008), Mansour et al. (2010) |
| R: CTGCTTGAATGCGCCAAAC | 1.5 mM Mgcl2 | ||||
| vacA s1b | F: AGCGCCATACCGCAAGAG | 187 | 200 µM dNTP (Fermentas) | ||
| R: CTGCTTGAATGCGCCAAAC | 0.5 µM of each primers F & R | ||||
| vacA s1c | F: CTCTCGCTTTAGTGGGGYT | 213 | 1.25 U Taq DNA polymerase (Fermentas) | ||
| R: CTGCTTGAATGCGCCAAAC | 2.5 µL DNA template | ||||
| vacA s2 | F: GCTAACACGCCAAATGATCC | 199 | |||
| R: CTGCTTGAATGCGCCAAAC | |||||
| vacA m1a | F: GGTCAAAATGCGGTCATGG | 290 | |||
| R: CCATTGGTACCTGTAGAAAC | |||||
| vacA m1b | F: GGCCCCAATGCAGTCATGGA | 291 | |||
| R: GCTGTTAGTGCCTAAAGAAGCAT | |||||
| vacA m2 | F: GGAGCCCCAGGAAACATTG | 352 | |||
| R: CATAACTAGCGCCTTGCA | |||||
| cagA | F: GATAACAGCCAAGCTTTTGAGG | 300 | 5 µL PCR buffer 10X | 56°C ------------ 60 s | Chomvarin et al. (2008), Mansour et al. (2010) |
| R: CTGCAAAAGATTGTTTGGCAGA | 2 mM Mgcl2 | ||||
| 150 µM dNTP (Fermentas) | |||||
| 0.75 µM of each primers F & R | |||||
| 1.5 U Taq DNA polymerase (Fermentas) | |||||
| 3 µL DNA template |
Gel electrophoresis
The PCR amplification products (10 μL) were subjected to electrophoresis in a 2% agarose gel in 1X TBE buffer at 80 V for 30 min, stained with SYBR Green, and images were obtained in a UVIdoc gel documentation system (UK). The PCR products were identified by 100 bp DNA size marker (Fermentas, Germany).
Statistical analysis
Data were transferred to Microsoft Excel spreadsheet (Microsoft Corp., Redmond, WA, USA) for analysis. Using SPSS 16.0 statistical software (SPSS Inc., Chicago, IL, USA), Chi-square test and Fisher’s exact two-tailed test analysis were performed and differences were considered significant at values of P<0.05. Distribution of H. pylori genotypes and antimicrobial resistance properties were statistically analyzed.
Results
Distribution of H. pylori
Table 3 shows the total distribution of H. pylori in the meat product samples. Of 150 samples collected, 11 samples (7.33%) were positive for H. pylori. All the isolates were further identified using the nested-PCR technique (Fig. 1). Prevalence of H. pylori in hamburger and minced-meat samples were 1.42% and 12.5%, respectively. A significant difference for the prevalence of H. pylori was shown between hamburger and minced-meat (P<0.05).
Table 3.
Total prevalence of H. pylori in meat products
| Type of samples | No. samples collected | No. H. pylori in culture (%) | No. H. pylori confirmed in nested-PCR (%) |
|---|---|---|---|
| Hamburger | 70 | 1 (1.42) | 1 (1.42) |
| Minced-meat | 80 | 10 (12.5) | 10 (12.5) |
| Total | 150 | 11 (7.33) | 11 (7.33) |
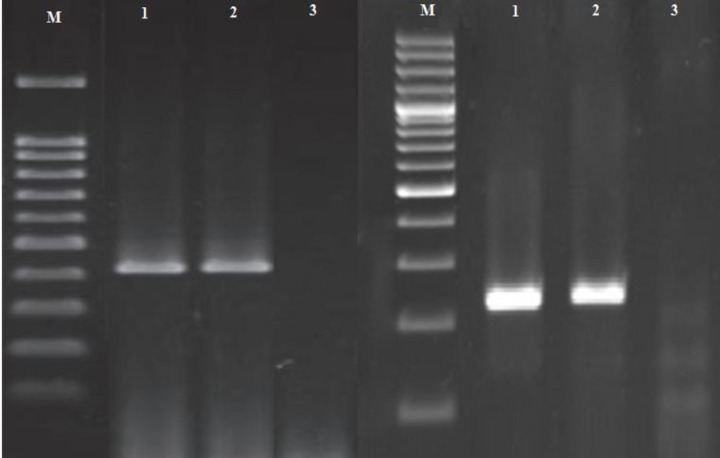
Fig. 1.
Electrophoresis of PCR products in nested-PCR reaction (the left figure is the first PCR and the right figure is the second PCR reactions). M: 100 bp ladder. Line 1: Positive sample, Line 2: Positive control, and Line 3: Negative control
Genotyping pattern of the H. pylori isolates
Table 4 presents the total prevalence of vacA and cagA genotypes in H. pylori strains of meat products. S1a is the only positive allele for the H. pylori strains of hamburger. The most commonly detected genotypes in the H. pylori strains of minced-meat were sla (20%), mla (20%) and cagA (20%). Statistical significant difference was seen between the prevalence of genotypes of types of samples (P<0.05). Table 5 indicates the total prevalence of combined genotypes in the H. pylori strains of meat products. There were no detected combined genotypes in the H. pylori strains of hamburger. The most commonly detected combined genotypes in the H. pylori strains of minced-meat were s1am1a (10%), s1am1b (10%) and s2m1a (10%).
Table 4.
Total prevalence of vacA and cagA genotypes in H. pylori strains of meat products
| Type of samples (No. positive) | Distribution of genotypes (%) |
|||||||
|---|---|---|---|---|---|---|---|---|
|
VacA
|
CagA | |||||||
| S1a | S1b | S1c | S2 | M1a | M1b | M2 | ||
| Hamburger (1) | 1 (100) | - | - | - | - | - | - | - |
| Minced-meat (10) | 2 (20) | 1 (10) | 1 (10) | 1 (10) | 2 (20) | 1 (10) | - | 2 (20) |
| Total (11) | 3 (27.27) | 1 (9.09) | 1 (9.09) | 1 (9.09) | 2 (18.18) | 1 (9.09) | - | 2 (18.18) |
Table 5.
Total prevalence of combined genotypes of H. pylori isolated from meat products
| Type of samples (No. positive) |
Distribution of combined genotypes (%) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| s1am1a | s1am1b | s1am2 | s1bm1a | s1bm1b | s1bm2 | s1cm1a | s1cm1b | s1cm2 | s2m1a | s2m1b | s2m2 | |
| Hamburger (1) | - | - | - | - | - | - | - | - | - | - | - | - |
| Minced-meat (10) | 1 (10) | 1 (10) | - | - | - | - | - | - | - | 1 (10) | - | - |
| Total (11) | 1 (9.09) | 1 (9.09) | - | 1 (9.09) | - | - | - | - | - | 1 (9.09) | - | - |
Antibiotic resistance pattern of H. pylori strains
Table 6 represents the results of the antibiotic resistance pattern of the H. pylori strains of meat products. We found that the H. pylori isolates of meat products harbored the highest levels of resistance against ampicillin (90.90%), erythromycin (72.72%), amoxicillin (72.72%), trimethoprim (63.63%), tetracycline (63.63%) and clarithromycin (63.63%). Statistically significant difference was seen between the types of meat product samples and prevalence of antibiotic resistance (P<0.05).
Table 6.
Antimicrobial resistance properties of H. pylori strains isolated from meat products
| Types of samples (No. positive results) |
Pattern of antibiotic resistance (%) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AM10* | Met5 | ER5 | CLR2 | AMX10 | Tet30 | Lev5 | S10 | RIF30 | Cef30 | TRP25 | FZL1 | Spi100 | |
| Hamburger (1) | 1 (100) | - | 1 (100) | 1 (100) | 1 (100) | 1 (100) | - | - | - | - | 1 (100) | - | - |
| Minced-meat (10) | 9 (90) | 3 (30) | 7 (70) | 6 (60) | 7 (70) | 6 (60) | 5 (50) | 1 (10) | 1 (10) | 1 (10) | 6 (60) | 1 (10) | 1 (10) |
| Total (11) | 10 (90.90) | 3 (27.27) | 8 (72.72) | 7 (63.63) | 8 (72.72) | 7 (63.63) | 5 (45.45) | 1 (9.09) | 1 (9.09) | 1 (9.09) | 7 (63.63) | 1 (9.09) | 1 (9.09) |
AM10: Ampicillin (10 µg), Met5: Metronidazole (5 µg), ER5: Erythromycin (5 µg), CLR2: Clarithromycin (2 µg), AMX10: Amoxicillin (10 µg), Tet30: Tetracycline (30 µg), Lev5: Levofloxacin (5 µg), S10: Streptomycin (10 µg), RIF30: Rifampin (30 µg), Cef30: Cefsulodin (30 µg), TRP25: Trimethoprim (25 µg), FZL1: Furazolidone (1 µg), and Spi100: Spiramycin (100 µg)
Discussion
Results of present investigations revealed that resistant and virulent strains of H. pylori can survive in hamburger and especially minced-meat samples of the Iranian retail markets. It was shown that contaminated hamburger and minced-meat are important vectors for transmission of resistant and virulent strains of H. pylori to humans. We found that 7.33% of all the samples were positive for H. pylori. Although the main reason for considerable prevalence of H. pylori in meat product samples is unknown, the roles of secondary routes of contamination should not be overlooked. High presence of human-based genotypes (s1am1a, s1am1b, s2m1a and …) and also resistance of bacterial isolates against human-based antibiotics (amoxicillin, erythromycin, trimethoprim, clarithromycin and …) can indirectly support the role of cross contaminations of meat product samples. It seems that meat washing, storing, transporting, processing and packaging are the main stages which may increase the prevalence of H. pylori contamination. Transmission of H. pylori strains from the hands and also droplets of saliva of infected staff of factories and also butchers is another reason for high prevalence of H. pylori.
Several investigations have been done in the fields of H. pylori and food contamination allaround the world. Prevalence of H. pylori in vegetable and salad (Yahaghi et al., 2014 ▶), milk (Rahimi and Kheirabadi, 2012 ▶) dairy products (Mousavi et al., 2014 ▶), vegetable (Atapoor et al., 2014 ▶) and restaurant foods (Yahaghi et al., 2014 ▶) of previous investigations was 10.86%, 12.50%, 19.2%, 13.68% and 14%, respectively which were higher than our results. Several studies which have been focused on the prevalence of H. pylori in foods with animal origins (Dore et al., 2001 ▶; Quaglia et al., 2008 ▶; Angelidis et al., 2011 ▶; Rahimi and Kheirabadi, 2012 ▶) indicated that the prevalence of bacteria in these types of foods ranged from 20 to 73%. Mhaskar et al. (2013) ▶ showed that meat consumption (OR: 2.35, 95% CI: 1.30-4.23), eating restaurant food (OR: 3.77, 95% CI: 1.39-10.23) and drinking nonfiltered or non-boiled water (OR: 1.05, 95% CI: 1.01-1.23) were the main risk factors for H. pylori infection which can indirectly support the results of our investigation about considerable prevalence in meat. Webberley et al. (1993) ▶ reported that meat-eater had the highest levels of anti-H. pylori IgG compared to vegans, which may indicate the impact of meat-eating as a risk factor for occurrence of the infection.
VacA and cagA and especially s1a, m1a, s1am1a, s1am1b and s2m1a alleles had a considerable prevalence in the H. pylori isolates of meat products. There were only four previously published data about the genotyping of H. pylori strains of food samples in the literature (Momtaz et al., 2014 ▶; Mousavi et al., 2014 ▶; Yahaghi et al., 2014 ▶; Hemmatinezhad et al., 2016 ▶; Saeidi and Sheikhshahrokh, 2016 ▶). All of these studies were conducted in Iran and were in agreement with our findings. Yahaghi et al. (2014) ▶ showed that the most commonly detected genotypes in the H. pylori strains of vegetable and salad samples were oipA (86.44%), cagA (57.625), vacA s1a (37.28%) and vacA m1a (30.50%). Mousavi et al. (2014) showed that the most frequently detected genes in the H. pylori strains recovered from milk and dairy products were cagA (76.60%) and vacA (75%). Hemmatinezhad et al. (2016) reported that the most frequent genotypes in the H. pylori strains of ready to eat foods were vacA s1a (78.37%), vacA m2 (75.67%), vacA m1a (51.35%), cagA (41.89%), s1am2 (70.27%), s1am1a (39.18%) and m1am2 (31.08%) were the most prevalent detected combined genotypes. Saeidi and Sheikhshahrokh (2016) ▶ revealed that m1as1a (68.52%), m1as1b (60.40%), m1bs1b (55.83%) and m1bs1a (53.29%) were the most regularly detected combined genotypes in the H. pylori strains of foods with animal origins. High prevalence of cagA and vacA s1a, m1a and m2 alleles in the H. pylori strains of human and animal clinical samples and also foods have been reported previously (Nagiyev et al., 2009 ▶; Alikhani et al., 2014 ▶; Havaei et al., 2014 ▶; Momtaz et al., 2014 ▶; Mousavi et al., 2014 ▶; Yahaghi et al., 2014 ▶; Miftahussurur et al., 2015 ▶; Hemmatinezhad et al., 2016 ▶; Saeidi and Sheikhshahrokh, 2016 ▶). Ghorbani et al. (2016) revealed that 20% of all types of food samples were contaminated with H. pylori. They showed that the most commonly detected genotypes were vacA s1a, vacA m1a and vacA m2 and the most commonly determined combined genotypes were s1am2 (45%), s1am1a (40%) and m1am2 (35%), which was similar to our findings, too.
Among various investigations carried on the antibiotic resistance pattern of H. pylori strains of foods, the results of Hemmatinezhad et al. (2016) ▶ (amoxicillin (94.59%), ampicillin (93.24%), metronidazole (89.18%) and tetracycline (72.97%)), Yahaghi et al. (2014) ▶ (metronidazole (77.96%), amoxicillin (67.79%), and ampicillin (61.01%)) and Mousavi et al. (2014) ▶ (ampicillin (84.4%), tetracycline (76.6%), erythromycin (70.5%) and metronidazole (70%)) were similar to our findings. These antibiotics are one of the first choice treatment agents for H. pylori infection and the high prevalence of resistance against these antibiotic is due to their irregular, intense and illegal prescription not only for the cases of H. pylori infections but also for all types of infectious diseases of the digestive tract. The possibility of considering streptomycin, rifampin, cefsulodin, furazolidone and spiramycin antibiotics as an alternative for treatment of H. pylori could be suggested in the cases of infections.
Iranian meat products and especially hamburger and minced-meat harbored resistant strains of H. pylori and their pathogenic genotypes. Contaminated meat products may be the sources of the bacteria which can enter to the human population in a period of time. Diversity of H. pylori genotypes between various types of samples may indicate that there were various sources of contamination. Meat products are commonly cooked before consumption, however, appropriate conditions of processing including time and temperature are not respected well in some factories producing meat products. Cautious prescription of antibiotics and careful health monitoring in slaughterhouses, butchers and meat product factories such as accurate meat inspection, using high quality raw materials and observation of personal hygiene may reduce the risk of transmission of H. pylori to human.
Acknowledgements
The authors would like to thank Dr. A. Akhondzadeh Basti and Dr. F. Safarpoor Dehkordi at the Department of Food Hygiene and Quality Control, College of Veterinary Medicine, University of Tehran, Tehran, Iran and Prof. H. Ghasemian Safaei at the Tropical and Infectious Diseases Research Center, Isfahan University of Medical Sciences, Isfahan, Iran for their important technical and clinical support. This work was supported by the Islamic Azad University, Science and Research Branch, Iran grant number IAU 10662.
Conflict of interest
The authors declare that they have no competing interests.
References
- Alikhani, MY, Arebestani, MR, Khorasani, MS, Majlesi, A, Jaefari, M. Evaluation of Helicobacter pylorivacA and cagA genotypes and correlation with clinical outcome in patients with dyspepsia in Hamadan province, Iran. Iran. Red. Crescent. Med. J. 2014;16:e19173. doi: 10.5812/ircmj.19173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelidis, AS, Tirodimos, I, Bobos, M, Kalamaki, MS, Papageorgiou, DK, Arvanitidou, M. Detection of Helicobacter pylori in raw bovine milk by fluorescence in situ hybridization (FISH) Int. J. Food. Microbiol. 2011;151:252–256. doi: 10.1016/j.ijfoodmicro.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Atapoor, S, Dehkordi, FS, Rahimi, E. Detection of Helicobacter pylori in various types of vegetables and salads. Jundishapur. J. Microbiol. 2014;7:e10013. doi: 10.5812/jjm.10013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, LM. Helicobacter pylori: epidemiology and routes of transmission. Epidemiol. Rev. 2000;22:283–297. doi: 10.1093/oxfordjournals.epirev.a018040. [DOI] [PubMed] [Google Scholar]
- Chomvarin, C, Namwat, W, Chaicumpar, K, Mairiang, P, Sangchan, A, Sripa, B, Tor-Udom, S, Vilaichone, RK. Prevalence of Helicobacter pylorivacA, cagA, cagE, iceA and babA2 genotypes in Thai dyspeptic patients. Int. J. Infect. Dis. 2008;12:30–36. doi: 10.1016/j.ijid.2007.03.012. [DOI] [PubMed] [Google Scholar]
- De Francesco, V, Giorgio, F, Hassan, C, Manes, G, Vannella, L, Panella, C, Ierardi, E, Zullo, A. Worldwide H. pylori antibiotic resistance: a systematic. J. Gastrointestin. Liver Dis. 2010;19:409–414. [PubMed] [Google Scholar]
- Dore, MP, Sepulveda, AR, El-Zimaity, H, Yamaoka, Y, Osato, MS, Mototsugu, K, Nieddu, AM, Realdi, G, Graham, DY. Isolation of Helicobacter pylori from sheep—implications for transmission to humans. Am. J. Gastroenterol. 2001;96:1396–1401. doi: 10.1111/j.1572-0241.2001.03772.x. [DOI] [PubMed] [Google Scholar]
- Dunn, BE, Cohen, H, Blaser, MJ. Helicobacter pylori. Clin. Microbiol. Rev. 1997;10:720–741. doi: 10.1128/cmr.10.4.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghorbani, F, Gheisari, E, Safarpoor Dehkordi, F. Genotyping of vacA alleles of Helicobacter pylori strains recovered from some Iranian food items. Trop. J. Pharm. Res. 2016;15:1631–1636. [Google Scholar]
- Havaei, SA, Mohajeri, P, Khashei, R, Salehi, R, Tavakoli, H. Prevalence of Helicobacter pylorivacA different genotypes in Isfahan, Iran. Adv. Biomed. Res. 2014;3:48. doi: 10.4103/2277-9175.125761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmatinezhad, B, Momtaz, H, Rahimi, E. VacA, cagA, iceA and oipA genotypes status and anti-microbial resistance properties of Helicobacter pylori isolated from various types of ready to eat foods. Ann. Clin. Microbiol. Antimicrob. 2016;15:2. doi: 10.1186/s12941-015-0115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera, AG. Helicobacter pylori and food products: a public health problem. Methods Mol. Biol. 2004;268:297–301. doi: 10.1385/1-59259-766-1:297. [DOI] [PubMed] [Google Scholar]
- Kusters, JG, van Vliet, AH, Kuipers, EJ. Pathogenesis of Helicobacter pylori infection. Clin. Microbiol. Rev. 2006;19:449–490. doi: 10.1128/CMR.00054-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour, KB, Fendri, C, Zribi, M, Masmoudi, A, Labbene, M, Fillali, A, Mami, NB, Najjar, T, Meherzi, A, Sfar, T. Prevalence of Helicobacter pylorivacA, cagA, iceA and oipA genotypes in Tunisian patients. Ann. Clin. Microbiol. Antimicrob. 2010;9:10. doi: 10.1186/1476-0711-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mhaskar, RS, Ricardo, I, Azliyati, A, Laxminarayan, R, Amol, B, Santosh, W, Boo, K. Assessment of risk factors of Helicobacter pylori infection and peptic ulcer disease. J. Glob. Infect. Dis. 2013;5:60–67. doi: 10.4103/0974-777X.112288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miftahussurur, M, Sharma, RP, Shrestha, PK, Suzuki, R, Uchida, T, Yamaoka, Y. Molecular epidemio-logy of Helicobacter pylori infection in Nepal: specific ancestor root. Plos. One. 2015;10:e0134216. doi: 10.1371/journal.pone.0134216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momtaz, H, Dabiri, H, Souod, N, Gholami, M. Study of Helicobacter pylori genotype status in cows, sheep, goats and human beings. BMC. Gastroenterol. 2014;14:61. doi: 10.1186/1471-230X-14-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Momtaz, H, Souod, N, Dabiri, H, Sarshar, M. Study of Helicobacter pylori genotype status in saliva, dental plaques, stool and gastric biopsy samples. World J. Gastroenterol. 2012;18:2105–2111. doi: 10.3748/wjg.v18.i17.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi, S, Dehkordi, FS, Rahimi, E. Virulence factors and antibiotic resistance of Helicobacter pylori isolated from raw milk and unpasteurized dairy products in Iran. J. Venom. Anim. Toxins. Incl. Trop. Dis. 2014;20:51. doi: 10.1186/1678-9199-20-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagiyev, T, Yula, E, Abayli, B, Koksal, F. Prevalence and genotypes of Helicobacter pylori in gastric biopsy specimens from patients with gastroduodenal pathologies in the Cukurova region of Turkey. J. Clin. Microbiol. 2009;47:4150–4153. doi: 10.1128/JCM.00605-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira, PM, Vicente, AF. Meat nutritional composition and nutritive role in the human diet. Meat Sci. 2013;93:586–592. doi: 10.1016/j.meatsci.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Quaglia, N, Dambrosio, A, Normanno, G, Parisi, A, Patrono, R, Ranieri, G, Rella, A, Celano, G. High occurrence of Helicobacter pylori in raw goat, sheep and cow milk inferred by glmM gene: a risk of food-borne infection? Int. J. Food. Microbiol. 2008;124:43–47. doi: 10.1016/j.ijfoodmicro.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Rahimi, E, Kheirabadi, EK. Detection of Helicobacter pylori in bovine, buffalo, camel, ovine, and caprine milk in Iran. Foodborne. Pathog. Dis. 2012;9:453–456. doi: 10.1089/fpd.2011.1060. [DOI] [PubMed] [Google Scholar]
- Saeidi, E, Sheikhshahrokh, A. VacA genotype status of Helicobacter pylori isolated from foods with animal origin. Biomed. Res. Int. 2016;2016:1–6. doi: 10.1155/2016/8701067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talebi Bezmin Abadi, A, Mobarez, AM, Taghvaei, T, Wolfram, L. Antibiotic resistance of Helicobacter pylori in Mazandaran, north of Iran. Helicobacter. 2010;15:505–509. doi: 10.1111/j.1523-5378.2010.00795.x. [DOI] [PubMed] [Google Scholar]
- Valsta, L, Tapanainen, H, Männistö, S. Meat fats in nutrition. Meat Sci. 2005;70:525–530. doi: 10.1016/j.meatsci.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Van Duynhoven, YT, Jonge, RD. Transmission of Helicobacter pylori: a role for food? Bull. World Health Org. 2001;79:455–460. [PMC free article] [PubMed] [Google Scholar]
- Van Leerdam, M, Tytgat, G. Helicobacter pylori infection in peptic ulcer haemorrhage. Aliment Pharmacol Ther. 2002;16(s1):66–78. doi: 10.1046/j.1365-2036.2002.0160s1066.x. [DOI] [PubMed] [Google Scholar]
- Wayne, P. Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial suscep-tibility testing. Twenty-second informational supplement M100-S21. United States: 2012. [Google Scholar]
- Webberley, MJ, Webberley, JM, Newell, DG, Lowe, P, Melikian, V. Seroepidemiology of Helicobacter pylori infection in vegans and meat-eaters. Epidemiol Infect. 1993;108:457–462. doi: 10.1017/s0950268800049967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahaghi, E, Khamesipour, F, Mashayekhi, F, Safarpoor Dehkordi, F, Sakhaei, MH, Masoudimanesh, M, Khameneie, MK. Helicobacter pylori in vegetables and salads: genotyping and antimicrobial resistance properties. Biomed Res Int. 2014 doi: 10.1155/2014/757941. Article ID 75794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, R, Yamaguchi, A, Shibasaki, K. Detection and analysis of Helicobacter pylori DNA in the gastric juice, saliva, and urine by nested PCR. Oral. Sci. Int. 2008;5:24–34. [Google Scholar]