Abstract
Successful reproductive management in buffaloes depends on effective estrus detection. Urinary pheromones identified from natural estrous cycle have been reported to decipher estrus phase. However, its presence has not been analyzed in the urine after synchronization. Thus, our present investigation was to investigate the influence of synchronized estrus urine in bulls and to examine the presence of estrus-specific compounds ascertained in natural estrus in synchronized buffaloes. Mid-stream urine was collected from six synchronized buffaloes during various phases of estrous cycle and volatiles were examined using GC-MS. Sexual provocation in bulls was established by displaying persistent flehmen and frequent mounting towards estrus urine from synchronized animals. Totally forty-two volatile compounds were identified from three phases of estrous cycle, more specifically 4-methyl phenol (p-cresol) and 9-octadecenoic acid (oleic acid) in estrus urine of synchronized animal as similar to natural estrus. Hence, these chemical cues in buffalo urine might be employed as potential marker candidates for the development of an estrus detection aid.
Key Words: CIDR, Flehmen, Mating, 4-methyl phenol, 9-octadecenoic acid
Introduction
Low reproduction potential of buffaloes has been a major concern for decades, which streamed towards a substantial economic loss to developing countries. Protocols have been developed to precisely control the time of ovulation (synchronization) by exogenous hormone administration (De Rensis and López-Gatius, 2007 ▶; Perera, 2011 ▶). However, poorly timed artificial insemination (AI) due to failure in estrus detection would lead to low rate of conception. Pheromones present in the body fluids of female during estrus elicit behavioral changes in males (Halpin, 1986 ▶; Vandenbergh, 1999 ▶; Rekwot, 2001 ▶; Brennan and Keverne, 2004 ▶; Archunan, 2009 ▶). Volatiles such as 4-methyl phenol and 9-octadecenoic acid were identified in urine during natural estrous cycle as estrus-specific (Archunan et al., 2014 ▶). However, no report is available on urinary volatiles during induced estrous cycle. Hence, it is necessary to confirm the presence of estrus-specific compounds in synchronized buffaloes to prove it as a promising candidate for estrus detection.
Therefore, the present investigation was carried out (i) to study the bull’s reproductive behavior towards the urine of synchronized animals and (ii) to examine the urinary volatile compounds in synchronized buffaloes to confirm the presence of estrus-specific compounds ascertained during natural estrus.
Materials and Methods
Animal and synchronization
Six healthy heifer Murrah buffalo (Bubalus bubalis), approximately 20-30 months old, at VCRI, Nammakkal, Tamilnadu, India were recruited for analysis. Animals were housed in sheds and paddocks, and fed with standard diet and water ad libitum. All animals were subjected to synchronization of estrus by controlled internal drug releasing (CIDR) kit (EAZI BREED CIDR 1380 kit, Pfizer limited, India). The vaginal insert, containing 1.38 g of progesterone, was inserted (day 1) into the cervico-vaginal region and 5 ml of PGF2α was injected intramuscularly followed by the removal of kit at the end of the 8th day. On the next day (day 9), 0.75 mg estradiol benzoate was administrated (Tauck et al., 2007).
Sample collection
The phases of estrous cycle were comprehended as pre-estrus (days 1-9), estrus (days 11-12), and post-estrus (days 15-18) based on CIDR treatment protocol. The mid-stream urine samples were collected from synchronized buffaloes by free-catch method in sterilized glass bottles and categorized according to estrous cycle phases (Selvam et al., 2016 ▶). One part of each urine sample was used for behavior assay and another part was filtered through nylon mesh (60-120 µm) immediately after collection and stored as aliquots at -20°C.
Behavioral assay
Urine sample collected during different phase of the estrous cycle from all the six animals was sprayed individually on the genital region of dummy buffaloes and bulls were allowed to sniff it for 15 min. The duration of flehmen behavior and the frequency of mounting exhibited by the bulls in response to the urine samples were recorded (Rajanarayanan and Archunan, 2004 ▶). The genital region was washed cleanly after every assay and a lag-time of 30 min was given between two assays. All experiments were performed in triplicate. Statistical analysis was performed using Microsoft excel and results were recorded in mean±SEM.
Sample preparation for GC-MS
Test urine sample was mixed with DCM (1:1) and filtered through a silica-gel column (60-120 mesh) for 30 min at RT. The filtered extract was reduced to 1/5 of its original volume by cooling with liquid nitrogen. The sample was fractionated and subjected to GC-MS analysis (QP-5050, Schimadzu, Japan) as per Rajanarayanan and Archunan (2011) ▶. The identified estrus-specific compounds were then compared with the standard compounds run under the same conditions, and confirmed on the basis of retention time shown in GC-MS.
Results
Estrus urine from the synchronized animals induced extended flehmen behavior (24.00 ± 1.31 s), whereas the duration of flehmen decreased when exposed to pre-estrus (14.50 ± 0.53 s) and post-estrus (7.64 ± 2.19 s) urine (Fig. 1A). Similarly, highest frequency of mounting behavior was observed with synchronized estrus urine (7.97 ± 0.40), when compared to pre-estrus (2.61 ± 0.61) and post-estrus (1.97 ± 0.44) urine samples (Fig. 1B).
Fig. 1.
Behavioral changes of bull towards female buffalo’s urine during various phases of estrous cycle. Pane A shows the time spent in exhibiting flehmen behavior and panel B shows the frequency of mounting
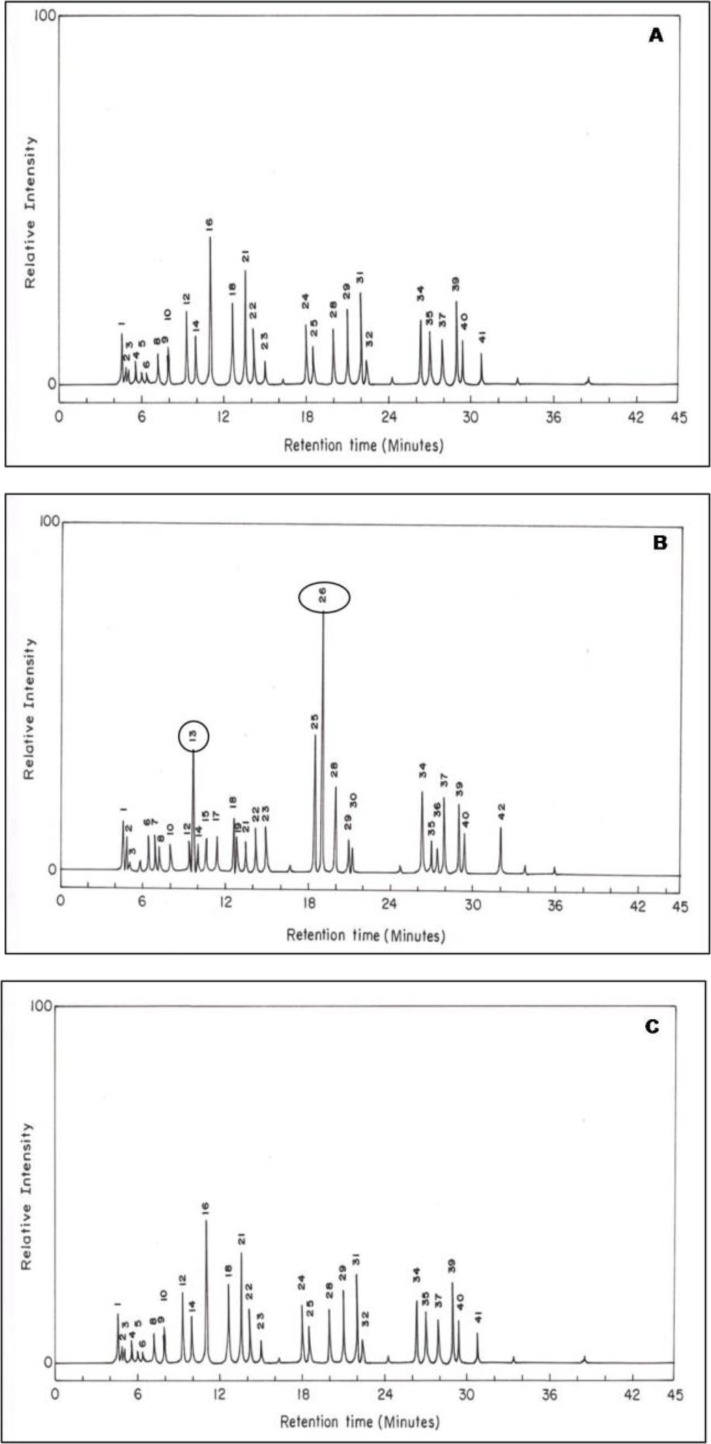
The chromatogram of synchronized buffalo urine exhibited totally 42 different volatile compounds (Figs. 2A-C) and the existence of each volatile, along with its nature, was reported in Table 1 for each phase of synchronized estrous cycle. Certain volatiles such as 1-chloro-1-buten-3-yne, 1,4-dichloromethane, 2-octanone, 2-ethyl-5-methyl-1,2,3-dioxoborolan-4-one, ethyl-2-methylcyclopentane, methylheptane, diisooctylester-1,2, benzene dicarboxylic acid, and 3-methyl-1-pentanol were present in all phases.
Fig. 2.
Representative chromatogram of urine from synchronized animal during various phases of estrous cycle. Panel A: pre-estrus, B: estrus, and C: post-estrus. Peak No. 13 and 26 correspond to p-cresol and oleic acid, respectively
Table 1.
List of compounds identified in the urine of female buffaloes by GC-MS
| Peak No. | Compound name | PE | E | POE | Mol. Wt | Group |
|---|---|---|---|---|---|---|
| 42 | 2-Methyleicosane | √ | √ | × | 296 | Alkane |
| 1 | 1-Chloro-1-buten-3-yne | √ | √ | √ | 86 | Alkyne |
| 2 | 2-Methyl-2-propenamide | × | √ | √ | 85 | Amide |
| 3 | 1,4-Dichloromethane | √ | √ | √ | 146 | Aromatic |
| 4 | 1-Nitro-2-methylpropane | × | × | √ | 101 | Alkane |
| 5 | 2,3-Dihydro-4-methylfuran | × | × | √ | 84 | Heterocyclic |
| 6 | 2-Ethyl-5-methyl-1,2,3-dioxoborolan-4-one | √ | √ | √ | 128 | Aldehyde |
| 7 | Methylpyrrolidine | √ | √ | × | 84 | Alkaloid |
| 8 | 4-Pentanol | × | √ | √ | 86 | Alcohol |
| 9 | 3-Ethyl-2-methylhexane | × | × | √ | 128 | Alkane |
| 10 | 7-Methyl-4-undecane | × | √ | √ | 168 | Alkane |
| 11 | Phenol | √ | × | × | 94 | Phenol |
| 12 | 4-Methyl-3-heptanone | × | √ | √ | 128 | Alkane |
| 13 | 4-Methyl phenol (p-cresol) | × | √ | × | 108 | Phenol |
| 14 | 2-Octanone | √ | √ | √ | 128 | Ketone |
| 15 | 3-Aminoheptane | √ | √ | × | 115 | Alkane |
| 16 | 5-Methyl-2-(methylethyl)-cyclohexanol | √ | × | √ | 156 | Alcohol |
| 17 | 2,3Dimethylheptane | √ | √ | × | 128 | Alkane |
| 18 | 3-Methyl-1-pentanol | √ | √ | √ | 102 | Alcohol |
| 19 | 3-Methyl-1-pentene | √ | √ | × | 84 | Alkene |
| 20 | 3-Propylphenol | √ | × | × | 136 | Phenol |
| 21 | 2-Methyl-n-phenyl-2-propenamide | × | √ | √ | 161 | Amide |
| 22 | Dimethylundecane | × | √ | √ | 184 | Alkane |
| 23 | Decanoic acid | × | √ | √ | 172 | Acid |
| 24 | 1-Chlorotetradecane | × | × | √ | 232 | Alkane |
| 25 | N-N-Bis(2-hydroxyethyl)-dodecanamide | × | √ | √ | 287 | Amide |
| 26 | 9-octadecenoic acid (oleic acid) | × | √ | × | 282 | Acid |
| 27 | Hexadecanol | √ | √ | × | 242 | Alcohol |
| 28 | Tetradecanoic acid | × | √ | √ | 228 | Acid |
| 29 | Nonane | × | √ | √ | 128 | Alkane |
| 30 | 2-Octyldodecan-1-ol | √ | √ | × | 298 | Alcohol |
| 31 | Hexadecanoic acid | × | × | √ | 256 | Acid |
| 32 | Isocyanobutane | × | × | √ | 83 | Alkane |
| 33 | 9-Octadecenal | √ | × | × | 266 | Aldehyde |
| 34 | Diisooctylester-1,2-benzenedicarboxylic acid | √ | √ | √ | 530 | Acid |
| 35 | Ethyl-2-methylcyclopentane | √ | √ | √ | 112 | Alkane |
| 36 | 5-Methyl-4-decene | √ | √ | × | 154 | Alkene |
| 37 | Methylheptane | √ | √ | √ | 114 | Alkane |
| 38 | 3-Methylbutanol | √ | × | × | 86 | Alcohol |
| 39 | 9-Methyl-(z)-5-undecene | × | √ | √ | 168 | Alkene |
| 40 | 1-Tetradecanol | × | √ | √ | 214 | Alcohol |
| 41 | Undecanol | × | × | √ | 172 | Alcohol |
Nevertheless, the compounds viz. methylpyrrolidine, 3-aminoheptane, 2,3-dimethylheptane, 3-methyl-1-pentene, hexadecanol, 2-octyldodecan-1-ol, 5-methyl-4-decene, and 2-methyleicosane were present in pre-estrus and estrus urine; 2-Methyl-2-propenamide, 4-pentanol, 7-methyl-4-undecane, 4-methyl-3-heptanone, 2-methyl-n-phenyl-2-propenamide, N-N-bis(2-hydroxyethyl)- dodecanamide, nonane, decanoic acid, dimethylundecane, tetradecanoic acid, 9-methyl-(z)-5- undecene, and 1-tetradecanol were present in estrus and post-estrus urine; 5-methyl-2-methylethyl)-cyclohexanol was identified in pre-estrus and post-estrus urine.
The volatiles such as phenol, 3-propylphenol, 9-octadecenal, and 3-methylbutanol were found only in pre-estrus urine and 1-nitro-2-methylpropane, 2,3-dihydro-4-methylfuran, 3-ethyl-2-methylhexane, 1-chlorotetradecane, hexadecanoic acid, isocyanobutane, and undecanol were identified only in post-estrus urine. However, 4-methyl phenol and 9-octadecenoic acid were the volatile found only during estrus.
Discussion
The phase of estrous cycle was reconfirmed by adopting gynaeco-clinical and biochemical parameters, and coincided with CIDR based characterization. It is well known that estrus urine generally has behaviorally important chemical signals that provoke bulls to manifest premating behaviors (Rivard and Klemm, 1990 ▶; Rasmussen, 1998 ▶; Rekwot et al., 2001 ▶). Estrus signaling compound from urine was suggested to lead bulls to perform flehmen reaction, and later to a more intense mounting behavior (Garcia et al., 1986 ▶). Flehmen was observed with all the tested samples, but the duration (seconds) varied between estrous cycle phases (Fig. 1A). In concordance with previous study (Rajanarayanan and Archunan, 2004 ▶), intermittent flehmen was observed in response to estrus urine in the present study, which clearly shows the perceptivity of the bull towards the chemical cues released from female buffaloes. Bulls translate the olfactory cues to the brain to recognize an apt-phase for paring-off (Masaki and Otha, 1990 ▶). The high frequency of mounting in response to estrus urine revealed that the volatile chemicals in estrus urine have an important role in mating.
Along with the three reported volatiles in the pre-estrus urine of natural estrous cycle, 3-methylbutanol is additionally present in the pre-estrus urine of synchronized estrous cycle. The estrus-specific volatiles compounds identified in the natural estrous cycle such as p-cresol and oleic acid were also found in the estrus urine of synchronized buffaloes. Cresols have germicidal property and may act as antiseptics, protecting the genitals of both sexes during the sexually active periods. In mares, increased signal of p-cresols during estrus suggested it as an estrus indicating pheromone (Morris et al., 1979 ▶; Mozuraitis et al., 2011 ▶).
The present study demonstrates that synchronized estrus female influences reproductive behavior in the bull and evidenced that pheromones are indeed present in the synchronized estrus urine as it exists in natural estrus. In addition, the present study convincingly shows that the females could be hormonally regulated to exhibit estrus through synchronization process and the characteristic estrus-specific volatile compounds released during normal estrous cycle are also expressed in synchronized buffaloes. Thus, the bulls displayed significant flehmen, and mounting behavior towards the compounds present during estrus urine in synchronized buffaloes. Based on behavioral observations, the present study concludes p-cresol and oleic acid are the two volatile compounds of estrus phase involved in influencing the sexual arousal and reproductive behavior of bull. Therefore, the study provides strong support to proceed further in developing an estrus detection kit with the estrus-specific volatiles.
Acknowledgements
We thank Professor M. A. Akbarsha, director, MGDCA, BDU, for critical evaluation of the manuscript. Assistance rendered by Mr. J. Rayappan, technical assistant, is highly acknowledged. The financial assistant from DBT (BT/PR10247/AAQ/01/364/2007) and DST, New Delhi is acknowledged. The facilities availed from UGC-SAP phase II and DST-FIST level I, Government of India, are also acknowledged.
Conflict of interest
No potential financial or personal conflicts of interest exist between authors.
References
- Archunan, G. Vertebrate pheromones and their bio-logical importance. J. Exp. Zoo. 2009;12:239–241. [Google Scholar]
- Archunan G, Rajanarayanan S, Karthikeyan K. Cattle pheromones. In: Mucignat-Caretta, C, editor. Neurobiology of chemical communication. Chapter 16. Boca Raton (FL): CRC Press; 2014. pp. 461–488. [PubMed] [Google Scholar]
- Brennan, PA, Keverne, EB. Something in the air? New insights into mammalian pheromones. Curr. Biol. 2004;14:81–89. doi: 10.1016/j.cub.2003.12.052. [DOI] [PubMed] [Google Scholar]
- De Rensis, F, López-Gatius, F. Protocols for synchronizing estrus and ovulation in buffalo (Bubalusbubalis): a review. Theriogenology. 2007;6:209–216. doi: 10.1016/j.theriogenology.2006.09.039. [DOI] [PubMed] [Google Scholar]
- Garcia, MC, McDonell, SM, Kenny, RM, Osborne, HG. Bull behavior tests: stimulus cow affects per-formance. Appl. Ani. Behav. Sci. 1986;16:1–10. [Google Scholar]
- Halpin, ZT. Individual odour among mammals origin and functions. Adv. Stu. Behav. 1986;16:39–70. [Google Scholar]
- Masaki J, Otha M. In: Mating behavior of a bull for estrus synchronized cows and a possible involvement of pheromonal factors. Sagara, Y, Seto, K, editors. Carnforth, UK: The Parthenon Publishing Group Limited; 1990. pp. 63–75. [Google Scholar]
- Morris, JA, Khettry, A, Seitz, EW. Antimicrobial activity of aroma chemicals and essential oils. J. Amer. Oil Chem. Soc. 1979;56:595–603. doi: 10.1007/BF02660245. [DOI] [PubMed] [Google Scholar]
- Mozuraitis, R, Buda, V, Borg-Karlson, AK. Optimization of solid phase micro-extraction sampling for analysis of volatile compounds emitted from estrous urine of mares. Z. Naturforsch C. 2010;65:127–133. doi: 10.1515/znc-2010-1-220. [DOI] [PubMed] [Google Scholar]
- Perera, BMAO. Reproductive cycles of buffaloes. Anim. Reprod. Sci. 2011;124:94–99. doi: 10.1016/j.anireprosci.2010.08.022. [DOI] [PubMed] [Google Scholar]
- Rajanarayanan, S, Archunan, G. Occurrence of flehmen in male buffaloes (Bubalusbubalis) with special reference to estrus. Theriogenology. 2004;61:866–871. doi: 10.1016/j.theriogenology.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Rajanarayanan, S, Archunan, G. Identification of urinary sex pheromones in female buffaloes and their influence on bull reproductive behavior. Res. Vet. Sci. 2011;91:301–305. doi: 10.1016/j.rvsc.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Rasmussen, LEL. Chemical communication: an integral part of functional Asian elephant (Elephas maximus) society. Ecoscience. 1998;5:411–429. [Google Scholar]
- Rekwot, PI, Ogwu, DEO, Sekone, VO. The role of pheromone and biostimulation in animal reproduction. Ani. Reprod. Sci. 2001;65:157–170. doi: 10.1016/s0378-4320(00)00223-2. [DOI] [PubMed] [Google Scholar]
- Rivard G, Klemm WR. Sample contact required for complete bull response to oestrous pheromones in cattle. In: MacDonald, DW, Muller-Scharze, D, Natynczuk, SE, editors. Chemical signals in vertebrates. 5th Edn. Oxford: Oxford University Press; 1990. pp. 627–633. [Google Scholar]
- Selvam RM, Onteru SK, Nayan V, Sivakumar M, Singh D, Archunan G. Exploration of luteinizing hormone in Murrah buffalo (Bubalus bubalis) urine: extended surge window opens door for estrus prediction. Gen. Comp. Endocrinol., doi. 2016. http://dx.doi.org/10.1016/j.ygcen.2016.12.002. [DOI] [PubMed]
- Vandenbergh JG. Pheromones in mammals. In: Knobil, E, Neill, JD, editors. Encyclopedia of reproduction. (1st Edn.) . UK: Academic Press; 1999. pp. 764–769. [Google Scholar]