Abstract
Emotion is often understood in terms of a circumscribed set of cortical and subcortical brain regions. I propose, instead, that emotion be understood in terms of large-scale network interactions spanning the entire neuroaxis. I describe multiple anatomical and functional principles of brain organization that lead to the concept of “functionally integrated systems,” cortical-subcortical systems that anchor the organization of emotion in the brain. The proposal is illustrated by describing the cortex-amygdala integrated system and how it intersects with systems involving the ventral striatum/accumbens, septum, hippocampus, hypothalamus, and brainstem. The important role of the thalamus is highlighted, too. Overall, the model clarifies why the impact of emotion is wide ranging, and how emotion is interlocked with perception, cognition, motivation, and action.
Keywords: emotion, brain, networks, amygdala, thalamus, brainstem
Search for the Brain Mechanism of Emotion
Where does emotion reside in the brain? Thinking about the brain basis of emotion has fluctuated between a focus on regions and a focus on circuits. In the 1920–30s, work by Cannon and Bard propelled the hypothalamus to the epicenter of the emotional brain [1]. At the same time, the idea that emotion depends on distributed circuits also was present in early work. In the 1930s, Papez proposed a distributed mechanism responsible for emotion involving, among others, the hypothalamus, hippocampus, anterior thalamus, and cingulate gyrus [2]. The region vs. circuit tension was present in subsequent developments, too, including MacLean’s proposal of the visceral brain or “limbic system” [3] (itself partly based on ideas by Broca in the previous century [4, 5]), and Panksepp’s framework of specialized subcortical circuits for basic emotions [6].
Today, characterizing circuit interactions is believed to be key to unravelling how emotion is organized in the brain. The key question addressed here is as follows: How is emotion instantiated in the brain? To quote Papez [2], what is the “mechanism of emotion?” I propose the concept of cortical-subcortical “functionally integrated systems” as a model of the emotional brain, which is based on large-scale networks and their interactions, and aimed at understanding the brain basis of emotion and interactions between emotion with perception, cognition, motivation, and action. I first outline six principles of brain organization that define the broader context needed for understanding the emotional brain. They are reviewed here not as a short tutorial on large-scale networks, but as concepts leading to the model of functionally integrated systems described subsequently. To anticipate, some of the consequences of the principles are as follows: The brain’s anatomical and functional architecture are highly non-modular; signal distribution and integration are the norm, allowing the confluence of information related to perception, cognition, emotion, motivation, and action; in addition, the functional architecture is comprised of overlapping networks that are highly dynamic and context sensitive.
Principles of Brain Organization
Principle 1: Massive Combinatorial Anatomical Connectivity
Computational analysis of anatomical connectivity demonstrates that both cortical and subcortical brain regions are densely interconnected [7–11]. Rich connectivity is not limited to specific sectors of the brain (say, prefrontal cortex) but instead encompasses all of them (including brainstem and cerebellum). Important anatomical properties include: 1) Massive interconnectivity; 2) High global accessibility [12] (see Glossary); and 3) The existence of a “connectivity core” or “rich-club” of regions marked by especially high levels of connectivity. Focusing on macaque cortical regions, one study described a core set of 17 regions spanning parietal, temporal, and frontal cortex marked by 92% connectivity density (92% of the connections that could exist were present) [12]. By combining multiple sources of data, another study described a core that was distributed across all major brain sectors (all cortical lobes, thalamus, and subcortical regions in the forebrain) [13].
Although progress has been made in elucidating properties of the brain’s large-scale anatomical architecture, computational studies likely underestimate existing connectivity, and provide a limited characterization of the existing organization. This is because, not only is current knowledge of existing pathways (and strength) largely incomplete, but several known properties of connectivity are not explicitly incorporated into computational analyses, which also focus mostly on cortical connectivity. Notably, specific cortical-subcortical anatomical connectional systems (involving long-range and large-scale pathways) have been characterized that are not generally considered, or are only partly incorporated. These include striatal, thalamic, cerebellar, hypothalamic, claustrum, and brainstem circuits, to name some [14]. These connectivity systems have the potential to substantially alter overall architectural properties and to influence information exchange, as discussed next.
Principle 2: Cortical-Subcortical Anatomical Connectional Systems
The entire cortical sheet (with the exception of area V1) projects to the striatum [14]. Studies in the 1970–80s led to the concept of cortico-basal ganglia-thalamo-cortical systems, or cortical-basal ganglia loops for short [15] (Figure 1A). This research attempted to map out how different parts of cortex are connected to different parts of the striatum, forming a series of possibly parallel, functionally segregated circuits. It was found that striatal territories receiving cortical input do not directly reciprocate their connections, which instead return via different parts of the thalamus (after an additional step in the pallidum), thus forming the loop.
Figure 1.
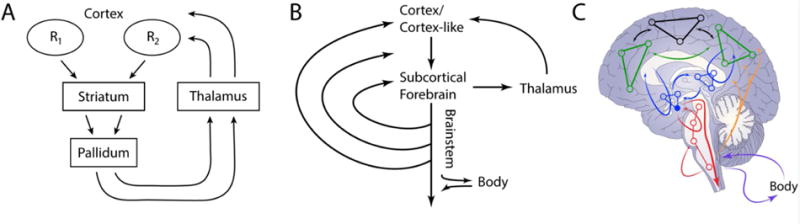
Cortical-subcortical anatomical connectional systems. (A) Cortico-basal ganglia-thalamo-cortical circuits (cortical-basal ganglia loops for short). Each thalamic region projects back to one of the cortical areas that feeds into the circuit, thus completing the “closed loop” portion of the circuit. (B) Multiple regions at the subcortical forebrain are the target of cortical (or cortex-like) projections. Like standard basal ganglia loops, the thalamus provides a route back to cortex (or cortex-like) regions. Notably, subcortical forebrain regions are also connected with caudally situated regions along the brainstem. (C) Network representation of cortical-subcortical connectional systems. Cortical regions, including those with strong connections to subcortical forebrain target regions (green) and other cortical networks (black). Subcortical forebrain regions (blue), including target regions such as the amygdala, striatum, and septum (blue, filled circle). Cortical-subcortical loops (blue lines with arrows returning to cortex) involve the thalamus (not shown). Subcortical forebrain and brainstem regions form loops (red lines with arrows); circuits are also present at the level of the brainstem (red lines with arrows). Ascending systems from the brainstem (orange lines) influence subcortical and cortical processing. Signals to and from the body are also exchanged (fuchsia arrows). Panel A reproduced with permission from [15].
The architecture described for cortical-basal ganglia loops was proposed to be more general and to apply to other structures at the base of the forebrain [16], including the septum and the extended amygdala (involving the central nucleus of the amygdala and a nearby area called the bed nucleus of the stria terminalis [17]). In identifying properties in line with the organization of cortical-basal ganglia loops (neuronal types, thalamic return projections, etc.), it was suggested that the “cortical” projections should be thought of as originating in the cortex-like hippocampus in the case of the septum, and the cortex-like basal and lateral amygdala in the case of the extended amygdala [16]. Another property of the connectional architecture is that regions at the base of the forebrain project along the brainstem, which is extensively connected with subcortical forebrain structures themselves, as well as cortex (Figure 1B) [18].
Therefore, large-scale cortical-subcortical anatomical connectional systems are not confined to the traditional basal ganglia, but involve additional subcortical forebrain structures, too. The importance of these systems is that cortical signaling must be understood in terms of an expanded framework in which cortical and subcortical mechanisms are intimately interrelated (see [19]). To reiterate, a subcortical forebrain target region (for example, ventral striatum or extended amygdala) receives extensive inputs, thus allowing it to be sensitive to a wealth of signals (sensory, cognitive, emotional, motivational, motor) (Figure 2A). From the target region two types of pathway exist: one projects back to cortex (or cortex-like regions) via the thalamus (Figure 2A); another descends along the brainstem (Figure 2B). The descending projections contact regions that: i) have further descending projections; ii) loop back within the brainstem; iii) loop back to the subcortical forebrain; and iv) have extensive (and at times diffuse) connectivity with subcortical and cortical regions (Figure 2C). Notably, the descending pathways contact sites that are components of ascending projection systems involving a broad range of neurotransmitters, including acetylcholine, dopamine, epinephrine, norepinephrine, and serotonin [14].
Figure 2.
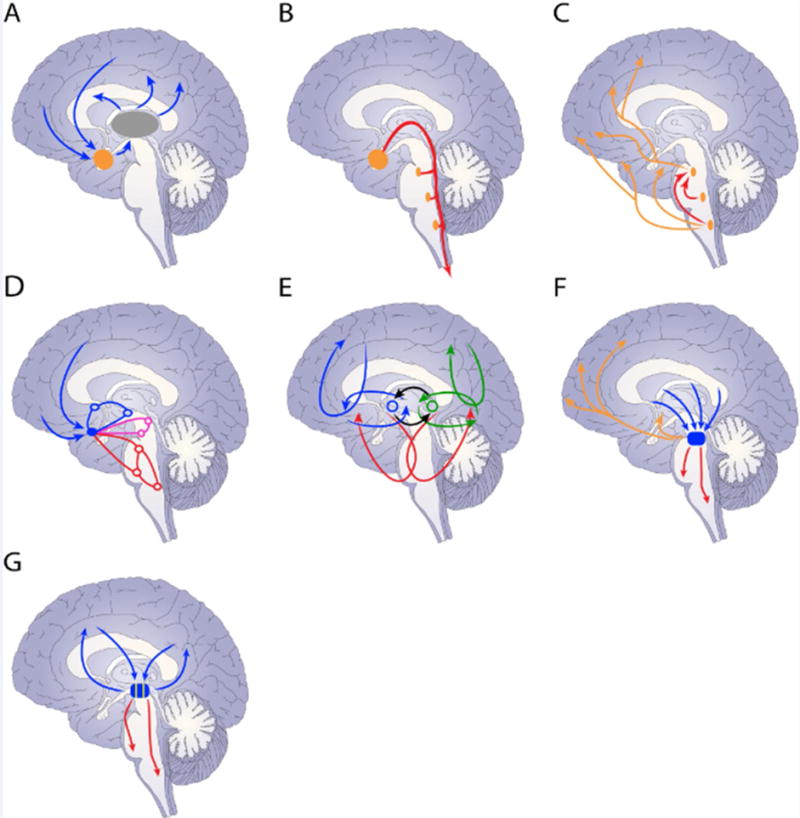
Properties of cortical-subcortical anatomical connectional systems. (A) Target regions (orange) in the subcortical forebrain (thalamus shown in gray). (B) Descending projections along the brainstem. (C) Brainstem connections and diffuse projection systems. (D) A connectional system links multiple levels along the neuroaxis. (E) Connectional systems (in blue and green) interact with each other at the level of the subcortical forebrain (black lines). (F) Multiple systems merge at subcortical convergence hubs. (G) Partial segregation of systems.
Principle 3: High Distributed Functional Connectivity
Understanding brain function requires characterizing how regions are functionally connected, that is, how their signals co-vary. What is important is not simply a region’s anatomical location, but its position in a space of functional relationships to other regions [20–22]. From the perspective of a brain region, at a given time, a region affiliates (or clusters with) with a set of other regions, thereby defining a momentary circuit.
The relationship between structural and functional connectivity is not a simple one. A structure-function dissociation is illustrated by an unusual population of adults without the corpus callosum. Although starkly different structurally relative to controls, individuals without the callosum exhibit similar patterns of functional connectivity (as measured during rest with functional MRI). Thus, relatively spared coordinated activity can emerge in brains with substantially altered structural connectivity [23, 24].
Anatomical architectural features support the efficient communication of information even when strong direct structural connections are not present, and allow functional interactions that vary as a function of context. More broadly, indirect connections may support functional relationships between regions that are not robustly linked by direct pathways ([25]; see also [26]), as exemplified by a study of functional connectivity in macaques [27]. Amygdala functional connectivity was more strongly associated with “communicability” of structural connections, which considered both monosynaptic and polysynaptic links, than with monosynaptic connectivity. Furthermore, selective chemical inactivation of the amygdala degraded functional connectivity among other regions, including between medial prefrontal, orbitofrontal, anterior cingulate, and anterior temporal cortex. Thus, understanding brain circuits requires moving beyond structural connectivity and considering functional relationships.
Principle 4: Overlapping Brain Networks
Both anatomical (e.g., [13]) and functional (e.g., [28]) networks are typically described in terms of disjoint sets, such that each brain region belongs to a single community (a community or cluster comprises a set of closely associated regions). However, the importance of understanding and characterizing overlapping network structure has been discussed across multiple disciplines, as “actual networks are made of interwoven sets of overlapping communities” [29].
Analysis of anatomical/functional data has repeatedly revealed regions with widespread connectivity [7–13, 30], highlighting the limitations of parsing the brain into segregated networks. To assuage this problem, researchers have also described how regions behave as different types of hubs (particularly well-connected regions), so as to capture signal segregation and integration properties [31–33]. Although this approach may be satisfactory in relatively modular systems, given the degree of brain interconnectivity, it is more fruitful to consider networks as inherently overlapping [34, 35].
A promising approach is to describe the brain based on networks (thus highlighting their relative independence), but allowing regions to belong to multiple networks simultaneously [36]. When we applied this approach to functional MRI data during both taskless (“rest”) and task conditions [35], it detected commonly observed communities, such as the task-negative (or default) network. However, the distribution of “membership values” (the extent to which each region participated in each network; 0 and 1 in the extreme case of non-overlapping networks) indicated that nodes participated in multiple networks simultaneously. Distributed participation was even more evident in a community of frontal and parietal regions important for attention and executive control, consistent with a multi-functional role of these regions [32]. Supporting this notion, we found that “membership diversity” (the extent to which regions participated across networks) during rest scans was positively related to functional diversity (which characterizes a region’s involvement in multiple mental functions, and can be assessed by interrogating large imaging databases [37]; see also [38, 39]). Thus, regions that participated in more communities at rest tended to be activated by a wide variety of tasks – that is, they were functionally diverse.
Principle 5: Dynamic Brain Networks
Brain networks are not static but evolve temporally [40, 41]. Although anatomical pathways change across the life span, the dynamics discussed here focuses on the functional connections between regions. Functional connections vary as a function of context, and are altered by cognitive, emotional, and motivational variables. Therefore, network organization must be understood dynamically. Indeed, the growth of methods to describe time-varying functional connectivity has begun to yield novel characterizations of how network organization evolves [42–44].
There are two important ways in which brain networks are dynamic. First, we can consider how specific networks evolve across time. For example, how does the efficiency [45] of the fronto-parietal attentional network evolve as a function of task phase? More generally, it is important to characterize how multiple network properties change with time. Second, networks are not static and fixed collections of brain regions. Networks are suggested to be dynamic coalitions of brain regions that form and dissolve to meet specific computational needs. Accordingly, network descriptions need to specify how groupings of regions evolve temporally. This poses several challenges, as the very notion of a network as a coherent unit is possibly undermined. For instance, at what point does a coalition of regions become something other than, say, the salience network? Conceptualizing networks as inherently overlapping (Principle 4) helps to mitigate this problem. For example, as previously discussed, each node can be considered to be a member of every network with a specific probability-like “membership value” [35], which fluctuates across time.
Implications of the Principles of Brain Organization
Together, the brain’s “mechanism of emotion” should be consistent with the principles outlined above, as well as the principle of anatomical connectivity to and from the body (Box 1). Therefore, the brain basis of emotion involves large-scale cortical-subcortical networks that are distributed and sensitive to bodily signals. The high degree of signal distribution and integration provides a nexus for the intermixing of information related to perception, cognition, emotion, motivation, and action. Importantly, the functional architecture consists of multiple overlapping networks that are highly dynamic and context sensitive. Thus, how a given brain region affiliates with a specific network shifts as a function of task demands and brain state.
BOX 1. Anatomical Connectivity to and from the Body.
The contribution of the body in emotion has been debated since William James suggested its central role in emotional experience [97]. The precise status of the body notwithstanding, emotion is closely linked to bodily states. What is the anatomical connectivity to the body (expression) and from the body (interoception) supporting emotion-related processes?
To the body: hypothalamus and cortex
The hypothalamus illustrates the to-the-body connectivity. This structure is involved in the regulation of endocrine functions, and the generation of autonomic reactions and basic behavioral patterns. To carry out these functions, the hypothalamus works in concert with a multitude of other sites, several of which are located in the brainstem and spinal cord. Preganglionic neurons in the spinal cord represent the final central nervous system output of the autonomic network, and produce bodily changes that maintain homeostasis (preganglionic fibers terminate in various autonomic ganglia). In particular, hypothalamic nuclei such as the paraventricular nucleus are among the few structures that innervate all levels of the sympathetic preganglionic outflow [98].
Connectivity affecting the body also originates in cortex, most notably in specific sectors of the cingulate gyrus ([99]; see also [100]). Descending projections to autonomic regulatory structures have been described, notably to the lateral hypothalamus, periaqueductal gray, parabrachial nucleus, and the nucleus of the solitary tract [14]. This connectivity is consistent with effects of cingulate electrical stimulation on virtually all autonomic processes, as well as many endocrine mechanisms.
From the body: nucleus of the solitary tract and cortex
The general visceral pathways can be divided into four tracts, namely, cardiovascular, pulmonary, respiratory, and gastrointestinal [101], which innervate distinct subnuclei of the nucleus of the solitary tract, a structure that spans the caudal and rostral medulla. Visceral information reaches many other structures, including the parabrachial nucleus (in the pons), periaqueductal gray, the hypothalamus (paraventricular nucleus and lateral area), and amygdala (central nucleus). Most structures receiving these signals from lower structures feedback onto the lower ones.
Cortical regions with notable body-related signals include the medial orbitofrontal cortex, the cingulate gyrus, and the insula [102]. In particular, the physiological condition of the entire body is conveyed to the posterior insular cortex [102], which can be considered interoceptive cortex, much like parts of parietal cortex are somatosensory cortex, for example.
In conclusion, both cortex and subcortex are part of extensive connectional systems that link the body to the brain. In this manner, the entire spectrum of brain signals can affect the body, and vice versa (Figure 1B).
Integrated Functional Systems: Example of the Cortex-Amygdala System
The principles of brain organization outlined above set the stage for the concept of integrated functional systems. The starting point is the notion of a cortical-subcortical anatomical connectional system (Principle 2; Figure 1B and 2A). Here, I describe the cortex-amygdala anatomical connectional system involving the basal, lateral, and extended amygdala. The basal and lateral (basolateral) amygdala are extensively interconnected with cortex [46]. They receive input from the visual, auditory, and somatosensory systems [46]. More broadly, they are connected with temporal, frontal, and insular cortices [46]. Connectivity with frontal cortex is densest for medial and orbital components, but weaker connectivity with lateral prefrontal cortex has been detected, too [47].
The basolateral amygdala is connected with the extended amygdala, thus gaining access to the hypothalamus and brainstem [14]. Extended amygdala outputs course down along the medial forebrain bundle contacting multiple structures along the brainstem [14, 18]. By engaging the brainstem at multiple levels, the extended amygdala exerts widespread effects across the neuroaxis, from the brainstem itself, to midbrain, subcortical forebrain, thalamus, and cortex (Figures 2B–C). The extended amygdala is also interconnected with the thalamus providing a separate source of influence on cortex [14] (Figure 2A).
More broadly, the basolateral amygdala plus extended amygdala anatomical system closely interacts with multiple subcortical regions (Figure 2D), establishing circuits at the level of the subcortical forebrain (with multiple hypothalamic nuclei, subiculum, substantia innominata, etc.), at the level of the midbrain (with periaqueductal gray, ventral tegmental area, substantia nigra, etc.), and at the level of the brainstem (parabrachial nucleus, nucleus of the solitary tract, etc.) [14]. Together, these architectural features allow the amygdala to be influenced by, and influence, a vast array of cortical and subcortical regions.
Interlinking Connectional Systems
An anatomical connectional system can be described as a more or less independent unit, with several of them defining parallel systems (much like the idea of multiple independent cortical-basal ganglia loops [15]). However, cortical-subcortical anatomical systems interact with one another at the level of the subcortical forebrain (Figure 2E).
Let us consider an example. The ventral striatum is a key region of the classical cortico-basal ganglia-thalamo-cortical loops [15]. Importantly, the ventral striatum and basolateral amygdala form a circuit [18], thereby interlinking their respective connectional systems (Figure 3, Key Figure). Whereas the basolateral amygdala projects robustly to the ventral striatum, the ventral striatum projects to the ventral pallidum, which in turn projects to the basolateral amygdala, thereby forming the circuit (Principle 3) [14]. As each connectional system (amygdala anatomical system involving the basal, lateral, and extended amygdala; ventral striatum cortical-basal ganglia loop) is sensitive to a different spectrum of cortical and subcortical influences, the pathways between them expand the influences they receive, allowing them to integrate diverse types of signals. Indeed, recent studies are now characterizing interactions between systems. For example, optical stimulation of a basolateral amygdala pathway to the accumbens facilitates reward seeking [48] and decreases long-term fear [49] (see also [50]). In sum, connectional systems communicate with each other at the level of the subcortical forebrain (Figure 2E), providing a substrate that affords greater integration and potential for context dependence.
Figure 3.
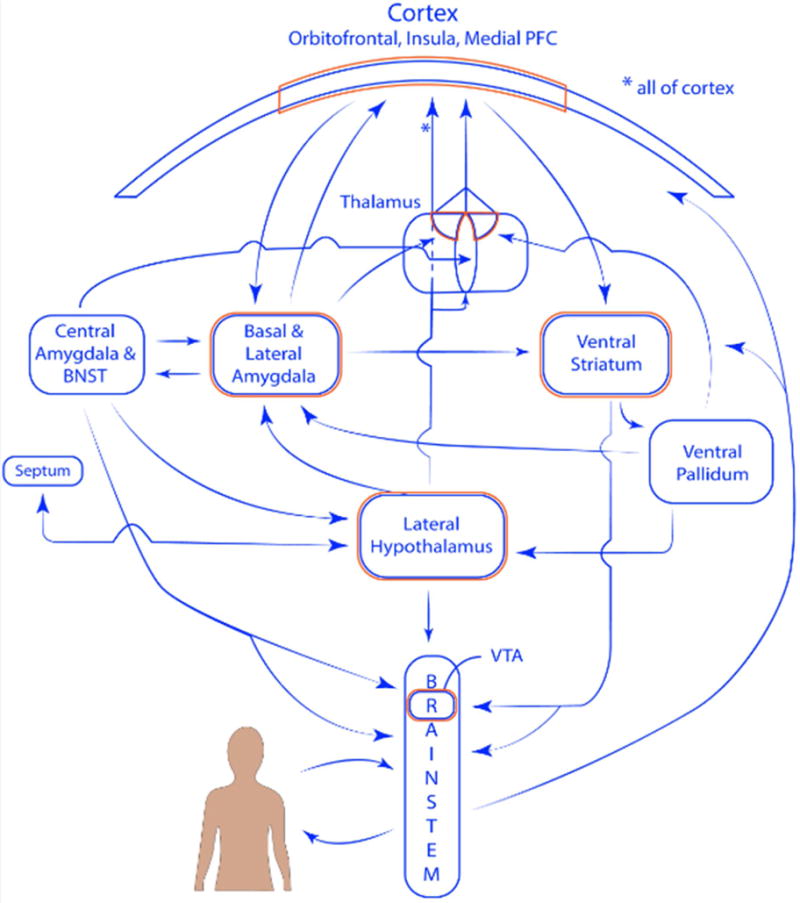
Functioanally integrated systems. Connectivity between the amygdala and ventral striatum systems (only some of the connections are displayed). Regions outlined in orange indicate some of the convergence sites. The representation of cortex at the top is meant to represent all of cortex; the portion with the orange outline indicates the orbitofrontal cortex, insula, and medial prefrontal cortex. Within the thalamus, the ellipse represents midline nuclei, the bilateral sectors represent the mediodorsal nucleus. Projections from the lateral hypothalamus reach all parts of cortex (see asterisks).
Subcortical Convergence Hubs
Anatomical systems come together at subcortical convergence hubs (Figure 2F), too. For example, in the midbrain tegmentum, the pedunculopontine tegmental nucleus receives projections from amygdala, striatum, and septum connectional systems [18, 51]. The pedunculopontine tegmental nucleus projects further downstream to other brainstem structures, and originates an ascending cholinergic projection system, which can then influence processing across subcortical and cortical areas [18, 51]. Signals impinging on convergence regions are not only influenced by different parts of cortex and subcortex, but have been further processed by multiple connectional systems. Thus, convergence onto subcortical hubs further enhances potential signal integration. Other convergence hubs include the lateral hypothalamus (as discussed in the context of Figure 3 below) and possibly the ventral tegmental area.
Targets of connectional systems also converge onto partially segregated zones (Figure 2G), providing the substrate for some signal segregation and relative selectivity. For example, the accumbens and related parts of the ventral striatum project strongly to the ventral tegmental area [18]. In contrast, the extended amygdala appears to connect most robustly with the lateral parts of the substantia nigra (compact part) and adjacent retrorubral field [18] (but see [52]).
From Anatomical to Functional Systems
I call the large-scale cortical-subcortical connectional systems that have the properties summarized in Figure 2 “integrated functional systems,” which include the amygdala, dorsal striatum, ventral striatum, and septum-hippocampus systems. Together, the overall architecture contains a series of spiraling pathways that communicate and integrate signals across different spatial extents (Figure 1C). Although function is always anchored on anatomy, given the principle of functional connectivity, circuit overlap, and dynamics, the systems should be understood at the level of the functional relationships between regions.
The overall architecture is comprised of both “closed” and “open” loops, namely loops that return to the originating regions and loops whose return is less topographically organized. These circuits join cortex with the subcortical forebrain, the subcortical forebrain with the brainstem, and different components of the brainstem [19]. This organization leads to multiple convergence hub regions at all levels of the neuroaxis, including cortex, thalamus, subcortical forebrain, and brainstem (outlined in orange in Figure 3), which play important information processing roles given their relative centrality [53, 54]. Functionally integrated systems involve cortical-subcortical loops, and although loops via the thalamus play a prominent role, the systems span multiple levels of the neuroaxis. For related ideas, see the concept of “macrosystems” [16, 18]. The present proposal, however emphasizes integration and coordination, while others have described specialization and relatively restricted communication between cortical-subcortical connectional systems [18]; for more integrated proposals, see [19, 55].
Figure 3 illustrates general properties of the amygdala and ventral striatum systems, and why the systems are “integrated.” Whereas the two systems have distinct connectional fingerprints, they converge at multiple stages. The architecture is organized around both vertical (say, between subcortical forebrain and brainstem) and horizontal (say, cortex to cortex) interactions (Figure 1C). In all, functionally integrated systems communicate with one another, thereby conferring organisms with greater behavioral flexibility via the integration of multiple signal types (including signals linked to appetitive and aversive processing; see below).
Coordinated Activity, not Top-Down Regulation
Since the study of decerebrate animals in the 1850s, a hierarchical view of brain organization has enjoyed a dominant role in neuroscience [19]. In this view, subcortical structures are outflow stations that influence musculoskeletal and autonomic output. In the overall hierarchical plan, cortical structures regulate subcortical ones thus guaranteeing appropriate behaviors. At first glance, some mechanisms fit this scheme, such as the role of the medial PFC in regulating the amygdala during fear extinction [56].
The architecture of integrated systems (Figures 2–3) stands in stark contrast to this viewpoint. The organization is not hierarchical but heterarchical [57], shifting the problem from one of understanding controller and controlled regions to understanding inter-region coordination dynamics [58, 59], that is, how signals from multiple regions collectively evolve. Understanding the latter is considerably more challenging because, while hierarchical interactions can be cast in terms of one region inhibiting (or exciting) another, coordination requires elucidating how distributed signals jointly bring about behaviors.
Let us consider the example of fear extinction in more detail. A top-down explanation emphasizes the role of the medial PFC in regulating the amygdala. However, considering the PFC as “top” and the amygdala as “down” does not take into account the richness of the existing interactions [60, 61]. Multiple populations within the basolateral amygdala actually project to the medial PFC whose outputs in turn influence amygdala signals [62]; see also [60]) (one study even suggested a “top” role for amygdala “extinction neurons” [62]; see also [63]). The ventral hippocampus projects to multiple medial PFC sites too [64], in addition to the basolateral amygdala; and hippocampal inputs to medial PFC appear to be potentiated during extinction [65]. Furthermore, medial PFC sites receive substantial inputs from mediodorsal thalamus [14], itself a major subcortical-cortical connectivity hub (for and even broader set of interactions, see [61]). More generally, during both fear expression and extinction, signals from the basolateral amygdala are integrated in the medial PFC with signals from multiple sources (including hippocampus and orbitofrontal cortex) to, collectively, determine whether or not to produce a response [60, 61]. In this manner, interactions (including bi-directional ones) between the amygdala, medial PFC, and several other regions, afford greater malleability when responding to threat. Taken together, top-down descriptions need to be expanded to mechanistic explanations in terms of coordination dynamics.
As an additional example, consider the hypothalamus, which is often conceptualized as the “head ganglion” (or “top”) of the autonomic system [66, 67]. But as highlighted in Figure 3, the hypothalamus is deeply embedded within cortical-subcortical systems. In the context of emotion (see next section), it is particularly important given that it interfaces with the amygdala, striatum, and septum systems. Thus, the hypothalamus is not simply a downstream controller. In particular, the hypothalamus projects to cortex via the thalamus (via midline and mediodorsal nuclei) [68]. Additionally, the lateral hypothalamus (also the posterior hypothalamic area) projects to the entire cortical sheet. The hypothalamus thus illustrates again why coordinated activity across large-scale circuits needs to studied.
Implications for Emotion Research
How does the present model relate to other frameworks? According to the “constructionist” approach, emotion is built via a set of domain-general, basic operations (such as core affect generation and body-related attention) [69]. Like the constructionist framework, the present model advocates for a distributed, network-based approach. However, there are important differences between the two models; I will briefly comment on two of them. 1) In the present model, there are no domain-general basic operations. The mind-brain is not built from a set of finite primitives, but from dynamic emergent processes [70]. For example, the search for primitives in perception has produced limited success in understanding visual processing (for dynamic alternatives, see [70]). 2) In the constructionist model, basic operations map onto distributed networks, notably those characterized during resting-state functional MRI. Here, the proposed functionally integrated systems are flexible and dynamic, thus highly context dependent. Consider the cortex-amygdala system, for example. It does not have a core function like “affect generation.” Instead, its particular functional state determines how it will contribute to multiple mental operations, which involve not only arousal, vigilance, and novelty, but also attention, value determination, and decision making more broadly [71].
In another framework, emotions are viewed as “functional states” implemented by neural systems to regulate complex behaviors [72]. In particular, investigators should attempt to disentangle the neural correlates of emotions from all other processing with which they interact [73]. The present framework advocates, in contrast, to focus on interactions because the brain’s structural-functional organization is strongly non-modular.
The functionally integrated systems framework also speaks to current attempts to classify brain states, where distributed patterns of functional MRI activation have been utilized to predict affective dimensions and discrete emotions with high levels of specificity [74]. Interestingly, in one study [75], predictive patterns spanned multiple cortical and subcortical systems, with no single system being necessary or sufficient for predicting affective experience. Furthermore, predictive patterns were not reducible to activity in traditional “emotion-related” regions (say, amygdala) or resting-state networks (say, task-negative network). The present proposal is consistent with these results in that emotional states are highly distributed and should not cleanly map onto standard resting-state networks. However, the model also predicts that brain “signatures” should be highly context-dependent, and not generalize well across tasks and conditions.
Returning to one of the central questions addressed here: Are there specialized circuits in the brain for emotion? In an important sense the answer is “no,” as the very boundary between emotion and the “rest of the brain” is ill defined. But how can the “emotion researcher” proceed then? From the standpoint of studying specific tasks or conditions, multivariate (and distributed) activation fingerprints provide useful summaries of evoked responses or states (Figure 4A). Further insight can be obtained by studying multiple related tasks/conditions, and determining a fingerprint that highlights the relative commonality of activation across regions; for instance, showing that regions RA and RB tend to participate together across some tasks/conditions (Figure 4B). As an additional recommendation, a complementary summary can be generated by characterizing the (multiple) functions of specific circuits of interest, which can be summarized via a “functional diversity profile” [37] (Figure 4C). For example, in the case of the amygdala mentioned above, it would involve arousal, vigilance, novelty, attention, value determination, and decision making, among others.
Figure 4.
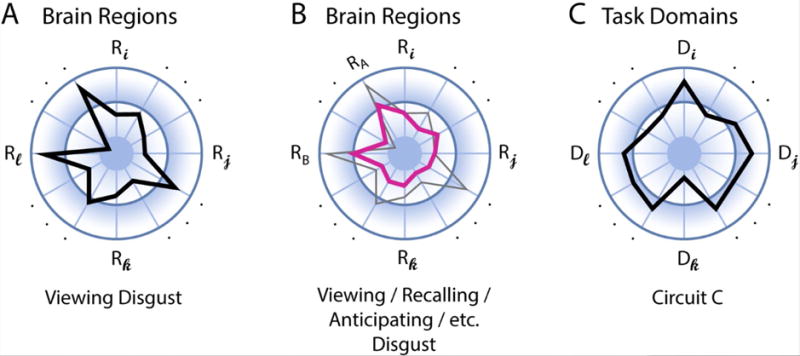
Distributed characterization of structure-function mapping. (A) The polar plot shows the distributed pattern of activation across regions R during an emotion task, such as viewing pictures eliciting disgust. The length of the segments indicates signal strength. (B) Multi-region pattern of activation across tasks. The profile in pink represents the activations that are (relatively) common across tasks (the gray outline is the same profile indicated in panel A). (C) Functional diversity profile of neural circuit of interest. The task domains D represent a set of potential mental functions of interest (spanning perception, cognition, emotion, motivation, and action). For example, this circuit is involved strongly in mental function Di but less so in function Dk.
Finally, the present framework underscores the importance of investigating large-scale circuits and their interactions in clinically oriented studies. To illustrate this point, consider a recent study targeting local central amygdala circuits involved in selecting between active and passive fear responses (flight vs. freeze) [76]. A major finding of the study was that it revealed inhibitory connections between two subpopulations of central amygdala cells linked to the two behaviors. Now, suppose that with a future technology we could identify and target the same cells in humans and modify the balance between the two subpopulations to (hypothetically) ameliorate maladaptive behaviors linked to anxiety disorders. The framework proposed here suggests that, without understanding how the local central amygdala circuit interacts with multiple regions (including hypothalamus, periaqueductal gray, and cortex), it would be unlikely that the treatment would be effective. Overall, a fuller understanding of neural circuit function that is clinically relevant will require the understanding of both local and large-scale circuit interactions, including those across multiple functionally integrated systems.
Functional Integrated Systems and the Emotional Brain
I suggest that to understand the brain organization of emotion, it is necessary to consider general principles of brain organization (Principles 1–5 and Box 1). I extend the concept of cortical-subcortical connectional systems (Principle 2) and develop the idea of functionally integrated systems. I propose that they provide a unifying framework to understand the emotional brain, and how emotion is interlocked with perception, cognition, motivation, and action.
Emotion has been studied from multiple vantage points. Some investigators suggest that emotions are states elicited by rewards and punishers [77], while others focus on how emotions are involved in the conscious/unconscious evaluation of events [78]. Strong evidence also links emotions to the body [79]. Despite varying views (for discussion, see [80]), the brain basis of emotion has centered on a relatively small number of brain structures, including, subcortically, amygdala, hypothalamus, periaqueductal gray, and ventral striatum, and cortically, insula, orbitofrontal cortex, and medial PFC [81, 82].
All of these structures (and several others discussed in the emotion literature) are important hub regions of the functionally integrated systems discussed here. In particular, the integrated systems of the amygdala, ventral striatum, and septum-hippocampus, are intimately linked to both the evaluative and expression dimensions of emotion. For instance, the amygdala has been likened to a “danger detector” and suggested to be part of an “information gathering system” [83]. The close association of the amygdala with the hypothalamus, for example, assures that it influences homeostatic mechanisms and neuroendocrine signaling. The ventral striatum is closely associated with appetitive processing and reward-related mechanisms. The septum, a structure poorly studied in humans, is thought to play important roles related to affect and motivation [61, 84].
As illustrated in Figure 3, not only are many structures of the “emotional brain” chief components of integrated systems but they participate across systems. This overlap is a central feature of brain networks (Principle 4), and is one reason why multiple signal types are present in brain regions. Indeed, signal plurality is the norm in brain regions [85]. For example, the ventral striatum, which is recognized for its role in reward-related processing, also exhibits aversion-related signals [86, 87] (in the case of the midbrain, see [88]); conversely, the bed nucleus of the stria terminalis, which is important during aversive states, is engaged by both aversive and rewarding stimuli [52, 89] (in the case of the amygdala, see also [90, 91]). In particular, the recent growth of neurotechniques will allow the disentangling of two scenarios: interdigitated but separate populations of positive and negative signals, or convergence at the synaptic/neuronal level. While the prevalence of these two types of organization remains to be determined [87, 92], the discovery of multiple sites of convergence illustrates that opportunities for signal integration abound (see [85] and references therein).
Concluding Remarks
Emotions mobilize the body (via autonomic, neuroendocrine, musculoskeletal systems), in part by how functionally integrated systems have access to the hypothalamus and structures in the brainstem and medulla that are linked to the body. Emotions also mobilize brain responses, influencing attention, memory, and decision making. The mobilization of body and brain, which is closely associated with neurotransmitter systems, relies initially on the most robust anatomical pathways of connectional systems. However, the mobilization is rapidly expanded to include vast portions of the brain. This is accomplished due to the general architectural principles of the brain, including combinatorial anatomical connectivity and distributed functional connectivity. These properties, in conjunction with extensive network overlap, assure that events of biological significance lead to the temporal reorganization of network affiliations to meet the demands faced by the organism.
In the proposed framework, the concept of emotion is not adequately summarized by the idea of a biasing mechanism, a metaphor frequently used in the literature. As discussed, descriptions in terms of coordination dynamics have the potential to capture interactions that go beyond simple biasing. In this context, at the spatiotemporal resolution of fMRI, we and others have started to characterize how emotion influences the temporal unfolding of large-scale network organization [93–96]. In a recent study [95], periods of “anxious anticipation” were associated with transient and sustained changes to salience, executive, and task-negative networks in the human brain. Importantly, how the bed nucleus of the stria terminalis and the amygdala participated in network communication (as quantified by the measure of centrality) was altered during anxious states.
In conclusion, the model proposed here helps clarify why some structures are so important for emotion, such as the amygdala: they are important hubs of distributed cortical-subcortical functionally integrated systems. The framework raises several important questions (see Outstanding Questions), too, while describing why the impact of emotion is so wide ranging. It vividly highlights the limitations of pointing to specific structures as the “emotional brain,” or even specific levels of the brain, as in the focus on cortex of some human work and the focus on subcortex in some animal work (see also [80]). In the end, emotion is a large-scale network property of brain function.
Trends.
Anatomical connectivity data are being acquired and collated at a scale that vastly exceeds what was recently feasible, allowing novel formal and quantitative analyses of large-scale circuits.
Large datasets of functional data are being obtained too, providing the basis for robust formal analyses of the functional relationships of signals from diverse brain regions. This mapping of functional relationships complements anatomical information and further informs the anatomical-functional organization of the brain.
Although research on the brain basis of emotion has often focused on particular brain regions, the investigation of associated larger-scale circuits is growing at a considerable pace. This is not only the case in human research with functional MRI, but also with genetic and molecular techniques that afford increasing control and enhanced monitoring of neuronal populations in non-human animals.
Outstanding Questions.
I proposed that cortical-subcortical connectional systems, and especially integrated systems, are central to the understanding of emotion in the brain. What is their role in processes traditionally described as cognitive? Is their importance underappreciated in that case, too?
Do we need novel perspectives on causation in the brain? The architecture proposed here challenges simple models of causation (region A causes activity of region B). Can probabilistic causation where causes change the probability of occurrence of their effects (say, investigated by quantifying the likelihood that a change in activity in one neuronal population affects the activity in another) provide a better approach?
Can the type of architecture described here be investigated without formal/mathematical tools? Given the complexity of the interactions, does neuroscience need to migrate to a model that is closer to that of physics? For example, experimental physicists are not lacking in mathematical sophistication. To some extent, neuroscience has evolved into extremely sophisticated “laboratory techniques” that are at times divorced from formal approaches. How should we train future generations of brain scientists?
What is the role of functional specialization? Given the multitude of circuit interactions of the architectural framework, is there room for the functional specialization often ascribed by researchers to brain regions? Relatedly, can a relative degree of modularity be supported by the distributed interactions of the observed architecture?
Acknowledgments
I’m grateful to the National Institute of Mental Health for research support (MH071589) and Alex Shackman, Srikanth Padmala, and Livia Tomova for feedback on the manuscript. Feedback from the reviewers was also valuable in refining the arguments presented here. I also thank Christian Meyer for assistance with figures and references.
Glossary
- Accessibility
Extent to which information in one part of a system can reach other parts via direct and/or indirect pathways. Accessibility can be measured by using network measures of “efficiency,” which can be local or global
- Centrality
Centrality analysis aims to identify the most important, or central, elements of a system (like the most important person in a social group). Multiple mathematical definitions of centrality have been described in the literature
- Connectional system
Large-scale anatomical pathways interlinking multiple regions, and typically spanning different levels of the neuroaxis. A prototypical example is the concept of cortico-basal ganglia-thalamo-cortical systems
- Coordination dynamics
Seeks to identify and track the temporal evolution of collective states, which reflect emergent properties of the system. The overarching idea is that the function of a complex biological system lies in the interaction between context-sensitive components
- Cortex-like
Cortex is characterized by its layered pattern. Some brain regions have simple lamination and are at times called “old cortex,” like the hippocampus, and here referred to as “cortex like.” The amygdala has both pallial and subpallial components (the “pallium” refers to the dorsal part of the telencephalon); the basolateral amygdala appears to be mostly of pallial origin, and here referred to as “cortex like.”
- Decerebrate
An animal preparation in which the entire cerebral cortex is removed from the brain
- Efficiency
Measure of information transfer between nodes. In networks with high efficiency, information travels via short paths between different parts of the network
- Extended amygdala
The central nucleus of the amygdala and the lateral bed nucleus of the stria terminalis are at times described as the “extended amygdala” given their shared cellular and developmental properties
- Functional connectivity
The extent of coherence between signals of two regions, often indexed by correlating the associated signal time series
- Functional diversity
A multidimensional characterization of a region’s involvement across multiple mental functions or processes
- Heterarchy
Type of organization where elements of a system are at the same “horizontal” level, or where a clear hierarchy is not observed
- Hubs
Brain regions with a high degree of connectivity
- Neuroaxis
Main axis of the central nervous system (roughly vertical in bipedal humans); at times spelled “neuraxis.”
- Subcortical forebrain
Subcortical regions at the base of the telencephalon
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bard P. On emotional expression after decortication with some remarks on certain theoretical views: Part i. Psychol Rev. 1934;41:309–329. [Google Scholar]
- 2.Papez JW. A proposed mechanism of emotion. Arch Neuro Psychiatr. 1937;38:725–743. [Google Scholar]
- 3.MacLean PD. Psychosomatic disease and the ‘visceral brain’: Recent developments bearing on the papez theory of emotion. Psychosom Med. 1949;11:338–353. doi: 10.1097/00006842-194911000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Pessoa L, Hof PR. From paul broca’s great limbic lobe to the limbic system. J Comp Neurol. 2015;523:2495–2500. doi: 10.1002/cne.23840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Broca P. Comparative anatomy of the cerebral convolutions: The great limbic lobe and the limbic fissure in the mammalian series. J Comp Neurol. 2015/1878;523:2501–2554. doi: 10.1002/cne.23856. translation. [DOI] [PubMed] [Google Scholar]
- 6.Panksepp J. Affective neuroscience: The foundations of human and animal emotions. Oxford University Press; 1998. [Google Scholar]
- 7.Sporns O. Networks of the brain. MIT press; 2010. [Google Scholar]
- 8.Oh SW, et al. A mesoscale connectome of the mouse brain. Nature. 2014;508:207–214. doi: 10.1038/nature13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bota M, et al. Architecture of the cerebral cortical association connectome underlying cognition. Proc Natl Acad Sci U S A. 2015;112:E2093–E2101. doi: 10.1073/pnas.1504394112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swanson LW, Lichtman JW. From cajal to connectome and beyond. Annu Rev Neurosci. 2016;39:197–216. doi: 10.1146/annurev-neuro-071714-033954. [DOI] [PubMed] [Google Scholar]
- 11.Hilgetag CC, et al. The primate connectome in context: Principles of connections of the cortical visual system. Neuroimage. 2016;134:685–702. doi: 10.1016/j.neuroimage.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markov NT, et al. Cortical high-density counterstream architectures. Science. 2013;342:1238406. doi: 10.1126/science.1238406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Modha DS, Singh R. Network architecture of the long-distance pathways in the macaque brain. Proc Natl Acad Sci U S A. 2010;107:13485–90. doi: 10.1073/pnas.1008054107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nieuwenhuys R, et al. The human central nervous system. Fourth. Steinkopff: 2008. [Google Scholar]
- 15.Alexander GE, et al. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 16.Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: The striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- 17.Fox AS, et al. Extending the amygdala in theories of threat processing. Trends Neurosci. 2015;38:319–329. doi: 10.1016/j.tins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heimer L, et al. Anatomy of neuropsychiatry: The new anatomy of the basal forebrain and its implications for neuropsychiatric illness. Academic Press; 2007. [Google Scholar]
- 19.Parvizi J. Corticocentric myopia: Old bias in new cognitive sciences. Trends Cogn Sci. 2009;13:354–9. doi: 10.1016/j.tics.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Aertsen A, Preissl H. Dynamics of activity and connectivity in physiological neuronal networks. In: Schuster HG, editor. Non linear dynamics and neuronal networks. VCH; 1991. pp. 281–302. [Google Scholar]
- 21.Friston KJ, et al. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 22.Pessoa L. Understanding brain networks and brain organization. Phys Life Rev. 2014;11:400–435. doi: 10.1016/j.plrev.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tyszka JM, et al. Intact bilateral resting-state networks in the absence of the corpus callosum. J Neurosci. 2011;31:15154–62. doi: 10.1523/JNEUROSCI.1453-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Reilly JX, et al. Causal effect of disconnection lesions on interhemispheric functional connectivity in rhesus monkeys. Proc Natl Acad Sci U S A. 2013;110:13982–7. doi: 10.1073/pnas.1305062110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adachi Y, et al. Functional connectivity between anatomically unconnected areas is shaped by collective network-level effects in the macaque cortex. Cereb Cortex. 2012;22:1586–92. doi: 10.1093/cercor/bhr234. [DOI] [PubMed] [Google Scholar]
- 26.Averbeck BB, Seo M. The statistical neuroanatomy of frontal networks in the macaque. PLoS Comp Biol. 2008;4 doi: 10.1371/journal.pcbi.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grayson DS, et al. The rhesus monkey connectome predicts disrupted functional networks resulting from pharmacogenetic inactivation of the amygdala. Neuron. 2016;91:453–466. doi: 10.1016/j.neuron.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeo BT, et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–65. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Palla G, et al. Uncovering the overlapping community structure of complex networks in nature and society. Nature. 2005;435:814–8. doi: 10.1038/nature03607. [DOI] [PubMed] [Google Scholar]
- 30.Barbas H. Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neurosci Biobehav Rev. 1995;19:449–510. doi: 10.1016/0149-7634(94)00053-4. [DOI] [PubMed] [Google Scholar]
- 31.Guimera R, Nunes Amaral LA. Functional cartography of complex metabolic networks. Nature. 2005;433:895–900. doi: 10.1038/nature03288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cole MW, et al. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci. 2013;16:1348–55. doi: 10.1038/nn.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Power JD, et al. Evidence for hubs in human functional brain networks. Neuron. 2013;79:798–813. doi: 10.1016/j.neuron.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeo BT, et al. Estimates of segregation and overlap of functional connectivity networks in the human cerebral cortex. Neuroimage. 2014;88:212–227. doi: 10.1016/j.neuroimage.2013.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Najafi M, et al. Overlapping communities reveal rich structure in large-scale brain networks during rest and task conditions. Neuroimage. 2016;135:92–106. doi: 10.1016/j.neuroimage.2016.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gopalan P, Blei D. Efficient discovery of overlapping communities in massive networks. Proc Natl Acad Sci U S A. 2013;110:14534–14539. doi: 10.1073/pnas.1221839110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anderson ML, et al. Describing functional diversity of brain regions and brain networks. Neuroimage. 2013;73:50–8. doi: 10.1016/j.neuroimage.2013.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson JL, et al. The functional connectivity of the human caudate: An application of meta-analytic connectivity modeling with behavioral filtering. Neuroimage. 2012;60:117–29. doi: 10.1016/j.neuroimage.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Uddin LQ, et al. Beyond the tripartite cognition-emotion-interoception model of the human insular cortex. J Cogn Neurosci. 2014;26:16–27. doi: 10.1162/jocn_a_00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varela FJ, et al. The brainweb: Phase synchronization and large-scale integration. Nat Rev Neurosci. 2001;2:229–39. doi: 10.1038/35067550. [DOI] [PubMed] [Google Scholar]
- 41.Buzsaki G. Rhythms of the brain. Oxford University Press; 2006. [Google Scholar]
- 42.Bassett DS, et al. Dynamic reconfiguration of human brain networks during learning. Proc Natl Acad Sci U S A. 2011;108:7641–6. doi: 10.1073/pnas.1018985108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hutchison RM, et al. Dynamic functional connectivity: Promise, issues, and interpretations. Neuroimage. 2013;80:360–378. doi: 10.1016/j.neuroimage.2013.05.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Helfrich RF, Knight RT. Oscillatory dynamics of prefrontal cognitive control. Trends Cogn Sci. 2016 doi: 10.1016/j.tics.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Latora V, Marchiori M. Efficient behavior of small-world networks. Phys Rev Lett. 2001;87:198701. doi: 10.1103/PhysRevLett.87.198701. [DOI] [PubMed] [Google Scholar]
- 46.Amaral DG, et al. Anatomical organization of the primate amygdaloid complex. In: Aggleton J, editor. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. Wiley-Liss; 1992. pp. 1–66. [Google Scholar]
- 47.Ghashghaei HT, et al. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–23. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stuber GD, et al. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Correia SS, et al. Amygdala-ventral striatum circuit activation decreases long-term fear. Elife. 2016;5:e12669. doi: 10.7554/eLife.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Britt Jonathan P, et al. Synaptic and behavioral profile of multiple glutamatergic inputs to the nucleus accumbens. Neuron. 2012;76:790–803. doi: 10.1016/j.neuron.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gut NK, Winn P. The pedunculopontine tegmental nucleus—a functional hypothesis from the comparative literature. Mov Disord. 2016 doi: 10.1002/mds.26556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jennings JH, et al. Distinct extended amygdala circuits for divergent motivational states. Nature. 2013;496:224–228. doi: 10.1038/nature12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Freeman LC. A set of measures of centrality based upon betweenness. Sociometry. 1977;40:35–41. [Google Scholar]
- 54.Newman M. Networks: An introduction. Oxford University Press; 2010. [Google Scholar]
- 55.Haber SN. The primate basal ganglia: Parallel and integrative networks. J Chem Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 56.Morgan MA, et al. Extinction of emotional learning: Contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- 57.McCulloch WS. A heterarchy of values determined by the topology of nervous nets. Bull Math Biophys. 1945;7:89–93. doi: 10.1007/BF02478429. [DOI] [PubMed] [Google Scholar]
- 58.Grossberg S. Processing of expected and unexpected events during conditioning and attention: A psychophysiological theory. Psychol Rev. 1982;89:529–572. [PubMed] [Google Scholar]
- 59.Tognoli E, Kelso JS. The metastable brain. Neuron. 2014;81:35–48. doi: 10.1016/j.neuron.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: More than just extinction. Curr Opin Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tovote P, et al. Neuronal circuits for fear and anxiety. Nat Rev Neurosci. 2015;16:317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- 62.Herry C, et al. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–606. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- 63.Do-Monte FH, et al. Revisiting the role of infralimbic cortex in fear extinction with optogenetics. J Neurosci. 2015;35:3607–3615. doi: 10.1523/JNEUROSCI.3137-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct. 2007;212:149–179. doi: 10.1007/s00429-007-0150-4. [DOI] [PubMed] [Google Scholar]
- 65.Hughes S, Garcia R. Reorganization of learning-associated prefrontal synaptic plasticity between the recall of recent and remote fear extinction memory. Learn Mem. 2007;14:520–524. doi: 10.1101/lm.625407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morgane PJ. Historical and modem concepts of hypothalamic organization and function. In: Morgane PJ, J P, editors. Handbook of the hypothalamus: Anatomy of the hypothalamus. Marcel Dekker Inc; pp. 1–64. [Google Scholar]
- 67.Pessoa L. The cognitive-emotional brain: From interactions to integration. MIT Press; 2013. [Google Scholar]
- 68.Risold PY, et al. The structural organization of connections between hypothalamus and cerebral cortex. Brain Res Rev. 1997;24:197–254. doi: 10.1016/s0165-0173(97)00007-6. [DOI] [PubMed] [Google Scholar]
- 69.Lindquist KA, Barrett LF. A functional architecture of the human brain: Emerging insights from the science of emotion. Trends Cogn Sci. 2012;16:533–540. doi: 10.1016/j.tics.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grossberg S. Towards solving the hard problem of consciousness: The varieties of brain resonances and the conscious experiences that they support. Neural Netw. 2017;87:38–95. doi: 10.1016/j.neunet.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 71.Pessoa L. Emotion and cognition and the amygdala: From “what is it?” To “what’s to be done?”. Neuropsychologia. 2010;48:3416–29. doi: 10.1016/j.neuropsychologia.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Anderson DJ, Adolphs R. A framework for studying emotions across species. Cell. 2014;157:187–200. doi: 10.1016/j.cell.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adolphs R. How should neuroscience study emotions? By distinguishing emotion states, concepts, and experiences. Soc Cogn Affect Neurosci. 2016:nsw153. doi: 10.1093/scan/nsw153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kragel PA, LaBar KS. Decoding the nature of emotion in the brain. Trend Cogn Sci. 2016;20:444–455. doi: 10.1016/j.tics.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang LJ, et al. A sensitive and specific neural signature for picture-induced negative affect. PLoS Biol. 2015;13:e1002180. doi: 10.1371/journal.pbio.1002180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fadok JP, et al. A competitive inhibitory circuit for selection of active and passive fear responses. Nature. 2017;542:96–100. doi: 10.1038/nature21047. [DOI] [PubMed] [Google Scholar]
- 77.Rolls ET. Emotion explained. Oxford University Press; 2005. [Google Scholar]
- 78.Sander D, et al. A systems approach to appraisal mechanisms in emotion. Neural Netw. 2005;18:317–52. doi: 10.1016/j.neunet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 79.Damasio AR. The feeling of what happens: Body and emotion in the making of consciousness. Harcourt Brace; 1999. [Google Scholar]
- 80.Adolphs R. How should neuroscience study emotions? By distinguishing emotion states, concepts, and experiences. Soc Cogn Affect Neurosci. 2016:nsw153. doi: 10.1093/scan/nsw153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lindquist KA, et al. The brain basis of positive and negative affect: Evidence from a meta-analysis of the human neuroimaging literature. Cereb Cortex. 2015:bhv001. doi: 10.1093/cercor/bhv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pessoa L. The emotional brain. In: Conn PM, editor. Conn’s translational neuroscience. Elsevier; 2017. pp. 635–656. [Google Scholar]
- 83.Whalen PJ. Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Curr Dir Psychol Sci. 1998;7:177–188. [Google Scholar]
- 84.Gray JA, McNaughton N. The neuropsychology of anxiety: An enquiry into the function of the septo-hippocampal system. Oxford University Press; 2003. [Google Scholar]
- 85.Namburi P, et al. Architectural representation of valence in the limbic system. Neuropsychopharmacology. 2016;41:1697–1715. doi: 10.1038/npp.2015.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reynolds SM, Berridge KC. Emotional environments retune the valence of appetitive versus fearful functions in nucleus accumbens. Nat Neurosci. 2008;11:423–425. doi: 10.1038/nn2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bissonette GB, et al. Impact of appetitive and aversive outcomes on brain responses: Linking the animal and human literatures. Front Syst Neurosci. 2014;8:24. doi: 10.3389/fnsys.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lammel S, et al. Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology. 2014;76:351–359. doi: 10.1016/j.neuropharm.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Waraczynski M. Toward a systems-oriented approach to the role of the extended amygdala in adaptive responding. Neurosci Biobehav Rev. 2016;68:177–194. doi: 10.1016/j.neubiorev.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 90.Paton JJ, et al. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439:865–70. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kim J, et al. Antagonistic negative and positive neurons of the basolateral amygdala. Nat Neurosci. 2016;19:1636–1646. doi: 10.1038/nn.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hayes DJ, et al. A comparison of neural responses to appetitive and aversive stimuli in humans and other mammals. Neurosci Biobehav Rev. 2014;45:350–68. doi: 10.1016/j.neubiorev.2014.06.018. [DOI] [PubMed] [Google Scholar]
- 93.Hermans EJ, et al. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science. 2011;334:1151–3. doi: 10.1126/science.1209603. [DOI] [PubMed] [Google Scholar]
- 94.Hermans EJ, et al. Dynamic adaptation of large-scale brain networks in response to acute stressors. Trends Neurosci. 2014;37:304–314. doi: 10.1016/j.tins.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 95.McMenamin BW, et al. Network organization unfolds over time during periods of anxious anticipation. J Neurosci. 2014;34:11261–11273. doi: 10.1523/JNEUROSCI.1579-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pessoa L, McMenamin BW. Dynamic networks in the emotional brain. Neuroscientist. 2016 doi: 10.1177/1073858416671936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.James W. What is an emotion? Mind. 1884;9:188–205. [Google Scholar]
- 98.Saper CB. The central autonomic nervous system: Conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci. 2002;25:433–469. doi: 10.1146/annurev.neuro.25.032502.111311. [DOI] [PubMed] [Google Scholar]
- 99.Vogt BA, editor. Cingulate neurobiology and disease. Oxford University Press; 2008. [Google Scholar]
- 100.Shackman AJ, et al. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–67. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cameron OG. Visceral sensory neuroscience: Interoception. Oxford University Press; 2002. [Google Scholar]
- 102.Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]


