Abstract
Background and Objective
Soft tissue sarcomas (STS) are a rare and heterogeneous group of malignant tumors that are often treated through surgical resection. Current intraoperative margin assessment methods are limited and highlight the need for an improved approach with respect to time and specificity. Here we investigate the potential of near-infrared Raman spectroscopy for the intraoperative differentiation of STS from surrounding normal tissue.
Materials and Methods
In vivo Raman measurements at 785 nm excitation were intraoperatively acquired from subjects undergoing STS resection using a probe based spectroscopy system. A multivariate classification algorithm was developed in order to automatically identify spectral features that can be used to differentiate STS from the surrounding normal muscle and fat. The classification algorithm was subsequently tested using leave-one-subject-out cross-validation.
Results
With the exclusion of well-differentiated liposarcomas, the algorithm was able to classify STS from the surrounding normal muscle and fat with a sensitivity and specificity of 89.5% and 96.4%, respectively.
Conclusion
These results suggest that single point near-infrared Raman spectroscopy could be utilized as a rapid and non-destructive surgical guidance tool for identifying abnormal tissue margins in need of further excision.
Keywords: near-infrared Raman spectroscopy, soft tissue sarcomas, surgical guidance
INTRODUCTION
Soft tissue sarcomas (STS) are a rare and heterogeneous group of malignant tumors that derive from mesenchymal progenitors for a variety of tissue types including muscle, fat, cartilage, blood vessels, and nerves. As such, there are over 50 different subtypes of STS, each with their own name and prognosis [1]. According to the 2016 American Cancer Society report, there will be an estimated 12,310 new cases of STS and 4,990 related deaths this year in the United States alone with undifferentiated pleomorphic sarcomas and well-differentiated liposarcomas as the most common STS subtypes in adults [2]. By the numbers, STS is a rare disease that accounts for less than 1% of all new cancers each year, making it a relatively difficult disease to study in vivo. Regardless of its prevalence, STS poses an interesting problem in that since 1974, the overall 5-year survival rate following treatment has generally lingered around 60% while treatments methods have not dramatically changed [3,4].
Despite the large heterogeneity of STS subtypes, the mainstay of local treatment is to completely excise the tumor with a wide margin of normal tissue. During this procedure, the surgeon must ensure that all malignant cells have been removed from the tumor bed while avoiding unnecessary damage to the surrounding muscle, blood vessels, nerves, and bone. Incomplete tumor resection has been shown to be a primary cause of local recurrence, which can often lead to increases in patient morbidity and healthcare costs [5–8]. For patients with residual STS, additional therapies such as postoperative radiation and/or re-excision are often required.
Current methods used for surgical guidance and margin assessment are limited and highlight the need for an improved approach that can ensure complete tumor resections in real time. While techniques such as magnetic resonance imaging can provide a general evaluation of the tumor’s size and location during the preoperative phase of the procedure, critical margin evaluation during surgery is often accomplished through frozen section biopsies. This process can take upwards of 30–60 minutes and is prone to sampling errors, especially when assessing the large tumor beds commonly encountered in these cases [9]. The ultimate evaluation of margin status occurs postoperatively when the resected tumor has been serially sectioned and analyzed using full-range histopathology techniques. However, this may take several days to over a week for processing and can restrict treatment options should a positive margin be found.
In terms of current research methods for tumor margin evaluation, optical techniques have been a popular area of study due to their potential to provide quick, automated, and non-destructive assessment of tissue health. However, due to the rare incidence of STSs, a large majority of these studies have been confined to in vitro measurements and animal models. Certain groups have used techniques such as optical coherence tomography in order to study tissue morphology [10] while others have investigated the use of extrinsic contrast agents for delineating tumor margins [11,12].
We recently reported the first in vivo study of STS within human subjects using autofluorescence spectroscopy [13]. By quantitatively measuring the light emissions from an illuminated sample, the study was able to differentiate between normal and cancerous tissue with a sensitivity and specificity of 88.3% and 95.6%, respectively. Although sensitive to changes in bulk tissue properties based on emission intensities, the technique itself is limited in its ability to differentiate specific tissue types due to the broad and relatively featureless profiles of the measured autofluorescence spectra.
Raman spectroscopy, on the other hand, is an optical modality that is well suited for characterizing the distinct biomolecular composition of a tissue sample. When a sample is irradiated with light, the majority of the photons will be scattered elastically with the same amount of input energy, however, a small fraction is scattered inelastically, resulting in an energy loss from the incident light [14]. These energy shifts constitute a sample’s Raman spectrum and are specific to the vibrational modes for a variety of different chemical bonds and functional groups. Since many biological molecules have distinguishably unique Raman spectra, the individual composition of a sample can be determined solely from the “biochemical fingerprint” of its Raman spectrum.
One particularly relevant biochemical change for cancer cells is an increase in the nucleic acid content associated with increased proliferation and genetic instability. This along with others such as changes in glycogen and collagen can all be detected by Raman spectroscopy [15,16]. Several research groups have exploited Raman spectroscopy for disease diagnosis in many organs, including the cervix, [17,18] bladder, prostate, [19] lung, [20] skin, [21,22], and GI tract [23–25]. In terms of sarcoma research, groups have been able to identify spectral features related to levels of polyunsaturation and tryptophan that could be used to differentiate between liposarcoma, rhabdomyosarcoma, Ewing sarcoma, neuroblastoma, and non-Hodgkin’s lymphoma [26,27]. These studies, however, were conducted with ex vivo tissue samples and did not include comparisons to normal tissue.
Towards the goal of developing an optical system for the intraoperative assessment of tumor margins during STS excision, we evaluate the potential of near-infrared Raman spectroscopy for the automated differentiation of in vivo STS from the surrounding normal muscle and fat commonly encountered within a surgical tumor bed.
MATERIALS AND METHODS
The portable Raman spectroscopy system used in this study consists of a custom-designed fiberoptic probe (EmVision LLC, Loxahatchee, FL) connected to a HoloSpec f/1.8i imaging spectrograph (Kaiser Optical Systems Inc., Ann Arbor, MI) equipped with a back-illuminated thermoelectrically cooled deep depletion charge coupled device (CCD) (Princeton Instruments, Trenton, NJ, SPEC-10:256BR). The hand held probe itself is approximately 15 cm in length with a diameter of about 6 mm and was designed to be as intuitive to grip as an office pen. At the tip of the probe is a single 400 μm diameter excitation fiber surrounded by seven 300 μm diameter collection fibers with inline long pass filtering to minimize interference signals from the probe itself. The tip is approximately 1 mm in diameter and covers a tissue area of about 0.79 mm2. A 785 nm diode laser (Innovative Photonics Solutions, NJ) was used as an excitation source and output power at the probe’s tip was maintained at 80 mW. These parameters in combination with the probe’s excitation and collection fiber geometry provides an effective depth interrogation of about 700 μm in to the tissue. Each measurement was collected within a wavenumber range of 550–1800 cm−1 and a spectral resolution of 3.5 cm−1 after binning. Wavenumber calibration was accomplished using standards based on acetaminophen, naphthalene, and a neon argon light source. Wavelength-dependent intensity response was calibrated using a secondary reflective standard to a NIST-certified quartz-tungsten-halogen lamp (Oriel Instruments, Irvine, CA, Model 63355).
After the STS mass was excised from the body, all ambient light within the operating room was turned off and/or covered. The attending surgeon would then acquire measurements by placing the tip of the fiberoptic probe in direct contact with the regions of interest. From the tumor bed, distal measurements were acquired from control normal muscle and fat as visually identified by the surgeon. Afterwards, a small incision was made on the excised tumor and measurements were taken from the interior of the tumor before it was sent for post-operative histology. Five measurements at 3 seconds exposures were acquired from each tissue location with multiple sets of measurements collected at different locations in order to mitigate the effects of possible sampling errors.
All post-processing was performed in MATLAB (Natick, MA, 2014b). Each Raman spectrum was noise smoothed using a Savitzky–Golay filter and fit to a 7th order polynomial to remove background fluorescence [28]. To account for overall intensity variability, each spectrum was normalized to its mean spectral intensity across all wavenumbers.
Forty-two adult subjects undergoing STS excision were recruited under approval of the Vanderbilt University Institutional Review Board (#120813). Subject eligibility was determined during preoperative evaluation by the surgical oncologists participating in the study, and informed written consent was obtained from each subject prior to measurements. Out of the 42 subjects enrolled, 28 were confirmed to have malignant tumors through postoperative histology. Outlier detection was performed within each tissue class in order to exclude measurements with aberrant spectra due to stray light, detector oversaturation, or insufficient contact of the probe to the sample. This was automatically performed through principal component analysis in which data that exceeded a preset Mahalanobis distance within the first five principal components were identified as outliers and excluded from further analysis [29,30]. After outlier detection, the following was retained for analysis: normal fat from 34 subjects, normal muscle from 27 subjects, and tumor measurements from 21 subjects. At least one category of tissue was measured from each of the 42 enrolled subjects. A detailed listing of tissue types, number of subjects, and number of spectroscopic measurements following outlier detection can be seen in Table 1.
TABLE 1.
Recruitment Table Following Outlier Detection
| Tissue type | Subjects | Measurements |
|---|---|---|
| Fat | 34 | 187 |
| Muscle | 27 | 171 |
| Well-differentiated liposarcoma | 7 | 98 |
| Undifferentiated liposarcoma | 5 | 44 |
| Pleomorphic liposarcoma | 2 | 24 |
| Myxofibrosarcoma | 2 | 25 |
| Epithelioid sclerosing fibrosarcoma | 2 | 20 |
| Well-differentiated liposarcoma with myxoid change | 1 | 10 |
| Dedifferentiated liposarcoma | 1 | 15 |
| Pleomorphic rhabdomyosarcoma | 1 | 14 |
Spectral feature reduction, predictive classifier training, and cross-validation was accomplished using a multivariate discrimination algorithm known as sparse multinomial logistic regression (SMLR). As a probabilistic multiclass model based on a Bayesian machine-learning framework of statistical pattern recognition, SMLR’s iterative algorithm allows for the reduction of high dimensional Raman data into a small subset of spectral basis features that are weighted according to their ability to successfully perform classification during training [31]. Theoretically, this should allow for a more robust and generalized model of tissue type discrimination. In order to avoid bias, each model was trained and tested using leave-one-subject-out cross-validation where a decision threshold was placed at a minimum of 50% probability of class membership. A range of input SMLR parameters were tested to achieve the best classification while maximizing the sparsity of spectral features. These include re-centering and rescaling each feature to have zero mean and unit variance, a direct kernel, no added bias term, a Laplacian prior, sparsity control of 0.1, and a non-component update rule. As the study was not powered for subtype differentiation, spectra from all STS subtypes were averaged to create a single diseased category during cross-validation.
A quantitative metric known as feature importance (FI) was utilized in order to assess the relative merit of the spectral features identified by SMLR for classification [32]. The value for FI, which ranges from 0 to 1, was defined as the product of the feature’s cross-validation frequency percentage and its scaled weight. This helps to intuitively emphasize spectral features that are both heavily weighted for decisions and chosen consistently during the model’s training.
RESULTS
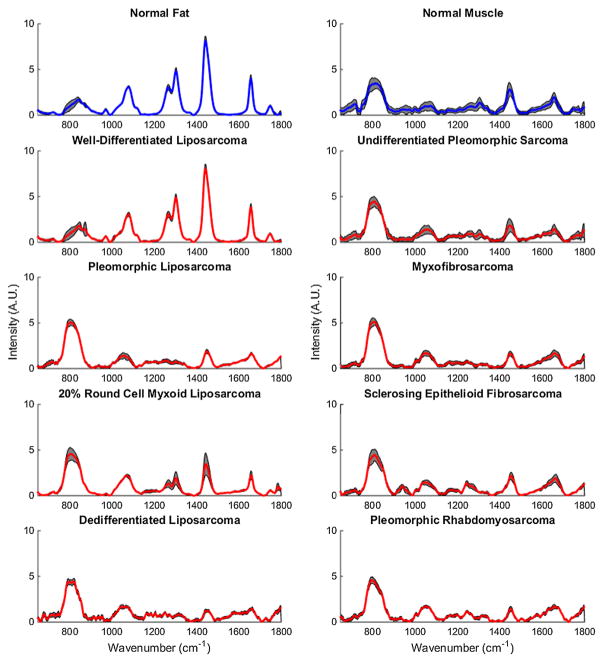
Average normalized Raman spectra for each tissue type measured in this study can be seen in Figure 1. For visual comparison, STS that are lipomatous in origin are arranged on the left, while STS that are non-lipomatous in origin are arranged on the right.
Fig. 1.
Average normalized Raman spectra for each tissue type. Normal tissues are plotted in blue while STS are in plotted red. Gray bands indicate standard deviation. For visual comparison, STS that are lipomatous in origin are arranged on the left while STS that are non-lipomatous in origin are arranged on the right.
Data from each labeled spectrum was subsequently used to develop a model for tissue type classification using SMLR and tested using leave-one-subject-out cross-validation. Since muscle and fat are the two most predominant normal tissues encountered within an STS tumor bed, there was a strong clinical motivation to test the SMLR based classification algorithm’s performance at discriminating between normal muscle, normal fat, and STS. The performance from all individual tests can be seen in the confusion matrices in Tables 2 and 3 where each row indicates a true category and each column indicates a model predicted category. Correctly classified measurements are highlighted in each table’s diagonal. As seen in Table 2, SMLR’s initial sensitivity and specificity for classifying STS from the surrounding normal muscle and fat were 59.6% and 91.69%, respectively. Upon further analysis of the results, it was apparent that 84 of the 98 well-differentiated liposarcoma measurements were misclassified as normal fat. When this specific subtype was excluded from analysis (Table 3), SMLR was able to classify STS from the surrounding normal muscle and fat with a sensitivity and specificity of 89.5% and 96.4%, respectively.
TABLE 2.
SMLR Normal Fat Versus Normal Muscle Versus STS Including Well-Differentiated Liposarcomas
| Prediction
|
Performance
|
||||
|---|---|---|---|---|---|
| Fat | Muscle | STS | Sensitivity (%) | Specificity (%) | |
| Actual | |||||
| Fat | 168 | 0 | 19 | 89.8 | 77.9 |
| Muscle | 3 | 157 | 11 | 91.8 | 94.9 |
| STS | 84 | 17 | 149 | 59.6 | 91.6 |
TABLE 3.
SMLR Normal Fat Versus Normal Muscle Versus STS Excluding Well-Differentiated Liposarcomas
| Prediction | Performance | ||||
|---|---|---|---|---|---|
| Fat | Muscle | STS | Sensitivity (%) | Specificity (%) | |
| Actual | |||||
| Fat | 187 | 0 | 0 | 100 | 100 |
| Muscle | 0 | 158 | 13 | 92.4 | 95.3 |
| STS | 0 | 16 | 136 | 89.5 | 96.4 |
As part of the training process, SMLR was able to minimize the large dimensionality of each Raman spectrum into a reduced set of basis features for improved generalization during classification. After preprocessing, each measurement’s spectrum originally consisted of 357 features associated with the Raman intensity at each wavenumber. When well-differentiated liposarcomas were included, the number of basis features required to differentiate normal fat, normal muscle, and STS was 177, 156, and 206, respectively. With the exclusion of well-differentiated liposarcomas, the number of basis features required were 107, 166, and 160, respectively.
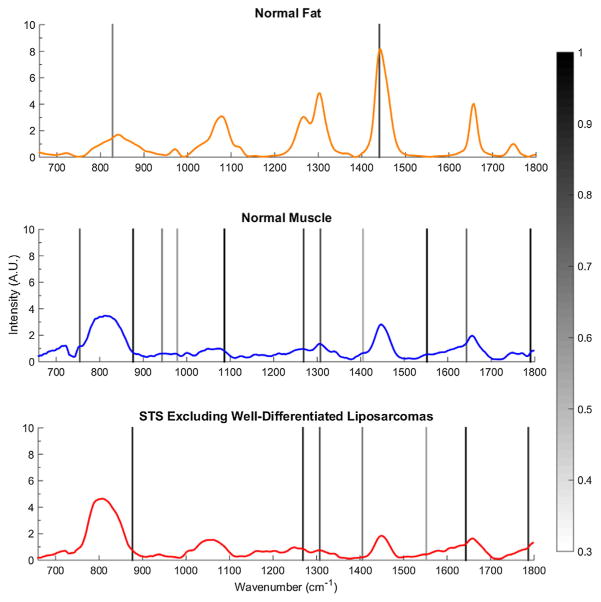
A plot of averaged spectra with encoded regions of significant importance (FI > 0.30) can be seen in Figure 2. These features along with their quantitative importance and wavenumber assignments can be seen in Table 4 where the bands at 876, 1,265, 1,307, 1,405, 1,643, and 1,786 cm−1 were ascribed the highest importance for differentiating STS from normal muscle and fat.
Fig. 2.
Average spectra of normal fat, normal muscle, and STSs excluding well-differentiated liposarcomas. Significant spectral features identified by SMLR for classification are highlighted with graded bands indicating their importance greater than a 0.30 threshold value.
TABLE 4.
Key SMLR Spectral Bands, Feature Importance, and Assignment
| Raman shift (cm−1) | Feature importance | Tissue type | Assignment |
|---|---|---|---|
| 754 | 0.65 | Muscle | Tryptophan [33] |
| 827 | 0.50 | Fat | Tyrosine [15] |
| 876 | 0.95/0.83 | Muscle/STS | Hydroxyproline, tryptophan [33,34] |
| 943 | 0.49 | Muscle | — |
| 978 | 0.40 | Muscle | Phosphorylated proteins and nucleic acids [15] |
| 1,086 | 0.98 | Muscle | Phenylalanine, phospholipids [17] |
| 1,265 | 0.75/0.85 | Muscle/STS | Amide III (C–N) in protein, =C–H in phospholipids [17] |
| 1,307 | 0.67/0.67 | Muscle/STS | CH2 in lipids [34] |
| 1,405 | 0.35/0.52 | Muscle/STS | CH3 bending in protein [15] |
| 1,440 | 0.74 | Fat | CH2 in lipids [34] |
| 1,552 | 0.96/0.37 | Muscle/STS | Tryptophan [17] |
| 1,643 | 0.60/1.00 | Muscle/STS | Amide I C=O in protein [17], C=C in lipids |
| 1,786 | 0.67/0.78 | Muscle/STS | — |
DISCUSSION
To the best of our knowledge, this is the first ever near-infrared Raman spectroscopy study of STS within human subjects. From our measurements, we were able to develop a multivariate classification algorithm that can automatically identify spectral features that could be used to differentiate STS from the surrounding normal tissue. Raman spectroscopy is a feature-rich optical modality that has often been compared to “biological fingerprinting,” and this abundance of information can be seen in the average normalized Raman spectra in Figure 1. For example, qualitative observation of normal fat spectra shows distinct narrow peaks at 1,080, 1,265, 1,304, 1,440, and 1,657 cm−1 due to the vibrational modes of the C–C, C–O, =C–H, CH2, CH3, and C=C groups in lipids [17]. These peaks are not as prominent within many of the normal muscle and STS subtypes, which instead have a stronger, although broader, band between 750 and 850 cm−1 that encompasses a range of wave-numbers often associated with amino acids and nucleotides [15,33,35]. In addition, close similarities can be observed among many of the STS subtypes except for well-differentiated liposarcomas, which instead share strong spectral similarities to normal fat.
From the results presented in Table 2, it can be seen that the algorithm performs with high sensitivity for classifying normal tissue but much lower sensitivity for classifying STS. Upon further analysis, it was apparent that this disparity was due to one specific STS subtype, well-differentiated liposarcomas, which prior research has shown to have histological similarities to normal fat despite atypia [36]. In addition, these tumors are often heterogeneously distributed among large regions of normal adipose tissue, which may contribute to spatial sampling errors during measurements. Correspondingly, the Raman spectra of this STS subtype share close similarities to those of normal fat. As one of the most commonly diagnosed STS subtypes [2], it constituted a large majority of our STS data set, which subsequently lowered the sensitivity and increased the number of basis features required for differentiating STS from normal fat. With the exclusion of well-differentiated liposarcomas from the study, SMLR was able to perform with a significant increase in sensitivity and decrease in required basis features as shown in Table 3. This level of performance despite some subtypes having only a single subject count and strict cross-validation method suggests that these remaining STS subtypes may share common characteristic features.
From a clinical standpoint, the inability to differentiate well-differentiated liposarcomas from the surrounding normal fat has a negligible effect on surgical outcome as this particular subtype is one of the lowest grade sarcomas with the least metastatic potential [37]. Standard treatment using preoperative MRI scans, marginal surgical excision, and post-operative radiation often have 5-year disease specific survival rates close to 95% [37,38]. However, well-differentiated liposarcomas still pose a considerable risk as they can further recur or progress into a much more aggressive de-differentiated state. Histologically, de-differentiated liposarcoma is defined as a biphasic neoplasm with one component of atypical lipomatous tumor and a second component of high grade tumor such as pleomorphic sarcoma [39]. Interestingly, this progression can be observed from the Raman measurements of lipomatous tumors presented in Figure 2, in which the well-differentiated liposarcomas begin with nearly identical spectra to normal fat. For subjects with later progression of de-differentiation such as the 20% round cell myxoid liposarcoma, the strong characteristic lipid peaks between 1,080 and 1,657 cm−1 can be seen to significantly decrease as the peaks between 720 and 880 cm−1 increase, possibly due to increased amino acid and nucleotide content associated with increased proliferation and genetic instability. Once in a de-differentiated state, the tumor has a qualitatively similar spectrum to that of the pleomorphic sarcomas.
As far as we are aware, the only other study of Raman spectroscopy in liposarcomas was carried out by Manoharan et al. in which they were able to observe a significant peak intensity increase at 1667 cm−1 (C=C stretching) compared to 1,442 cm−1 (C–H bending) in their ex vivo liposarcoma samples compared to normal fat [26]. The increase of this ratio as the sample’s mitosis rate increased suggested that more aggressive liposarcomas could be identified by their levels of polyunsaturation. This was further validated by other groups studying liposarcomas with various metastatic potential using nuclear magnetic resonance spectroscopy [40,41]. The results from our study also follow a similar trend with the highest polyunsaturation increase in the dedifferentiated liposarcomas and lowest in the well-differentiated liposarcomas. However, the differences we observed and identified through SMLR went beyond a ratio of those two peaks, suggesting that there may be many other biochemical markers for STS and their progression.
In an effort to develop a robust model for differentiating the various tissue types based on their quantitative spectral differences, SMLR was also utilized to iteratively reduce the data into a smaller set of basis features essential for classification. With the exclusion of well-differentiated liposarcomas, the spectral features that were ascribed the highest importance for classifying STS were the bands at 1,265, 1,307, and 1,405 cm−1 along with peak shoulders at 876, 1,643, and 1,786 cm−1. All of these distinguishing features were also used by SMLR to classify normal muscle to varying extent. The most important feature, the band at 1,643 cm−1 (FI = 1.0), is a shoulder of the peak often attributed to Amide I peptide bonds, and may be indicative of a major change in protein secondary structure within the malignant tumors [17]. This is further validated by the importance of the 1,265 cm−1 peak (FI = 0.85) attributed to Amide III peptide bonds, which is significantly decreased in the STS compared to normal muscle. Despite these changes in protein confirmation, STS had higher intensity spectra from 750 to 876 cm−1 (FI = 0.83), which is a region dominated by signals from various amino acids and nucleotides. By using an SMLR based multivariate analysis approach, we were able to computationally identify differences with a robust algorithm that is not susceptible to subjectivity and overfitting regarding FI.
In terms of clinical impact, the results of this preliminary study suggests that an optical device based on Raman spectroscopy could allow for intraoperative tumor margin assessment. However, there are limitations that must be addressed before this technique can be translated into clinical use. As stated in the introduction, STS is a rare disease that accounts for less than 1% of all new cancers each year. This along with its vast heterogeneity in subtypes, makes it a relatively difficult disease to study in vivo. Yet despite the limited number of STS subtypes recruited for this study, the subtypes that that were measured constitute the ones most commonly encountered in the operating room [2]. With the exclusion of well-differentiated liposarcomas, common spectral features across various STS subtypes were found that could potentially be exploited for future margin assessment studies where there is much ambiguity in terms of tissue type composition. These findings could later be utilized in other Raman modalities such as spatially offset Raman spectroscopy, which has been demonstrated to perform depth sectioned measurements down to 8 mm [42]. Unlike the frozen section biopsies currently used in the operating room which may take 30–60 minutes, Raman spectroscopy could be utilized to non-destructively evaluate a single margin within seconds. The strength of Raman spectroscopy is its inherent biochemical specificity which can allow clinicians to rapidly and automatically identify abnormalities within margins that may require further excision or further adjuvant therapies. This would significantly improve the management of STS with respect to time, patient outcome, and healthcare costs.
CONCLUSION
The results of this study suggests that near-infrared Raman spectroscopy could be used to differentiate in vivo STS from the surrounding normal muscle and fat during surgeries. This was accomplished by developing a SMLR based classification algorithm that was then tested using leave-one-subject out cross-validation. With the exclusion of well-differentiated liposarcomas that have strong spectral similarities to normal fat, the algorithm was able to perform with a sensitivity and specificity of 89.5% and 96.4%, respectively when classifying the remaining STS subtypes from normal muscle and fat. As part of the training process, the algorithm was able to reduce the high dimensional Raman spectra into a smaller set of basis features associated with significant differences in protein secondary structure and amino acid content that could be used to detect the presence of residual STS within a tumor bed.
Acknowledgments
Contract grant sponsor: National Cancer Institute of the National Institutes of Health; Contract grant number: 1F31CA200358; Contract grant sponsor: Orthopaedic Research and Education Foundation/Musculoskeletal Tumor Society Clinical Research Grant in Orthopaedic Oncology; Contract grant sponsor: Vanderbilt Orthopaedic Institute; Contract grant sponsor: Vanderbilt Medical Scholars Fellowship.
Footnotes
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and none were reported.
References
- 1.Demetri GD, Antonia S, Benjamin RS, Bui MM, Casper ES, Conrad EU, 3rd, DeLaney TF, Ganjoo KN, Heslin MJ, Hutchinson RJ, Kane JM, Letson GD, McGarry SV, O’Donnell RJ, Paz IB, Pfeifer JD, Pollock RE, Randall RL, Riedel RF, Schupak KD, Schwartz HS, Thornton K, von Mehren M, Wayne J. Soft tissue sarcoma. J Natl Compr Canc Netw. 2010;8(6):630–674. doi: 10.6004/jnccn.2010.0049. [DOI] [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer facts & figures 2016. Atlanta: American Cancer Society; 2016. [Google Scholar]
- 3.Atean I, Pointreau Y, Rosset P, Garaud P, De-Pinieux G, Calais G. Prognostic factors of extremity soft tissue sarcoma in adults. A single institutional analysis. Cancer Radiother. 2012;16(8):661–666. doi: 10.1016/j.canrad.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 4.Abraham JA, Baldini EH, Butrynski JE. Management of adult soft-tissue sarcoma of the extremities and trunk. Expert Rev Anticancer Ther. 2010;10(2):233–248. doi: 10.1586/era.09.193. [DOI] [PubMed] [Google Scholar]
- 5.Atean I, Pointreau Y, Rosset P, Garaud P, De-Pinieux G, Calais G. Prognostic factors of extremity soft tissue sarcoma in adults. A single institutional analysis. Cancer Radiother. 2012;16(8):661–666. doi: 10.1016/j.canrad.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 6.Blakely ML, Spurbeck WW, Pappo AS, Pratt CB, Rodriguez-Galindo C, Santana VM, Merchant TE, Prichard M, Rao BN. The impact of margin of resection on outcome in pediatric nonrhabdomyosarcoma soft tissue sarcoma. J Pediatr Surg. 1999;34(5):672–675. doi: 10.1016/s0022-3468(99)90353-6. [DOI] [PubMed] [Google Scholar]
- 7.Gronchi A, Casali PG, Mariani L, Miceli R, Fiore M, Lo Vullo S, Bertulli R, Collini P, Lozza L, Olmi P, Rosai J. Status of surgical margins and prognosis in adult soft tissue sarcomas of the extremities: A series of patients treated at a single institution. J Clin Oncol. 2005;23(1):96–104. doi: 10.1200/JCO.2005.04.160. [DOI] [PubMed] [Google Scholar]
- 8.Alamanda VK, Crosby SN, Archer KR, Song Y, Schwartz HS, Holt GE. Predictors and clinical significance of local recurrence in extremity soft tissue sarcoma. Acta Oncol. 2013;52(4):793–802. doi: 10.3109/0284186X.2012.711953. [DOI] [PubMed] [Google Scholar]
- 9.Shives TC. Biopsy of soft-tissue tumors. Clin Orthop Relat Res. 1993;289:32–35. [PubMed] [Google Scholar]
- 10.Carbajal EF, Baranov SA, Manne VG, Young ED, Lazar AJ, Lev DC, Pollock RE, Larin KV. Revealing retroperitoneal liposarcoma morphology using optical coherence tomography. J Biomed Opt. 2011;16(2):020502. doi: 10.1117/1.3541789. [DOI] [PubMed] [Google Scholar]
- 11.Eward WC, Mito JK, Eward CA, Carter JE, Ferrer JM, Kirsch DG, Brigman BE. A novel imaging system permits real-time in vivo tumor bed assessment after resection of naturally occurring sarcomas in dogs. Clin Orthop Relat Res. 2013;471(3):834–842. doi: 10.1007/s11999-012-2560-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demos SG, Gandour-Edwards R, Ramsamooj R, White R. Near-infrared autofluorescence imaging for detection of cancer. J Biomed Opt. 2004;9(3):587–592. doi: 10.1117/1.1688812. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen JQ, Gowani Z, O’Connor M, Pence I, Nguyen TQ, Holt G, Mahadevan-Jansen A. Near-infrared autofluorescence spectroscopy of in vivo soft tissue sarcomas. Opt Lett. 2015;40(23):5498–5501. doi: 10.1364/OL.40.005498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith E, Dent G. Modern Raman spectroscopy—A practical approach. Chichester, West Sussex, England: John Wiley & Sons; 2005. p. 224. [Google Scholar]
- 15.Mahadevan-Jansen A, Richards-Kortum RR. Raman spectroscopy for the detection of cancers and precancers. J Biomed Opt. 1996;1(1):31–70. doi: 10.1117/12.227815. [DOI] [PubMed] [Google Scholar]
- 16.Mahadevan-Jansen A, Patil C, Pence I. Raman spectroscopy: From benchtop to bedside. In: Vo-Dinh T, editor. Biomedical photonics handbook. 2. Boca Raton, FL: CRC Press; 2014. pp. 759–802. [Google Scholar]
- 17.Mahadevan-Jansen A, Mitchell MF, Ramanujam N, Malpica A, Thomsen S, Utzinger U, Richards-Kortum R. Near-infrared Raman spectroscopy for in vitro detection of cervical precancers. Photochem Photobiol. 1998;68(1):123–132. doi: 10.1562/0031-8655(1998)068<0123:nirsfv>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Mahadevan-Jansen A, Mitchell MF, Ramanujam N, Utzinger U, Richards-Kortum R. Development of a fiber optic probe to measure NIR Raman spectra of cervical tissue in vivo. Photochem Photobiol. 1998;68(3):427–431. [PubMed] [Google Scholar]
- 19.Crow P, Molckovsky A, Stone N, Uff J, Wilson B, WongKee-Song LM. Assessment of fiberoptic near-infrared raman spectroscopy for diagnosis of bladder and prostate cancer. Urology. 2005;65(6):1126–1130. doi: 10.1016/j.urology.2004.12.058. [DOI] [PubMed] [Google Scholar]
- 20.Huang Z, McWilliams A, Lui H, McLean DI, Lam S, Zeng H. Near-infrared Raman spectroscopy for optical diagnosis of lung cancer. Int J Cancer. 2003;107(6):1047–1052. doi: 10.1002/ijc.11500. [DOI] [PubMed] [Google Scholar]
- 21.Lieber CA, Majumder SK, Ellis DL, Billheimer DD, Mahadevan-Jansen A. In vivo nonmelanoma skin cancer diagnosis using Raman microspectroscopy. Lasers Surg Med. 2008;40(7):461–467. doi: 10.1002/lsm.20653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sigurdsson S, Philipsen PA, Hansen LK, Larsen J, Gniadecka M, Wulf HC. Detection of skin cancer by classification of Raman spectra. IEEE Trans Biomed Eng. 2004;51(10):1784–1793. doi: 10.1109/TBME.2004.831538. [DOI] [PubMed] [Google Scholar]
- 23.Shetty G, Kendall C, Shepherd N, Stone N, Barr H. Raman spectroscopy: Elucidation of biochemical changes in carcinogenesis of oesophagus. Br J Cancer. 2006;94(10):1460–1464. doi: 10.1038/sj.bjc.6603102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shim MG, Song LM, Marcon NE, Wilson BC. In vivo near-infrared Raman spectroscopy: Demonstration of feasibility during clinical gastrointestinal endoscopy. Photochem Photobiol. 2000;72(1):146–150. doi: 10.1562/0031-8655(2000)072<0146:IVNIRS>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 25.Molckovsky A, Song LM, Shim MG, Marcon NE, Wilson BC. Diagnostic potential of near-infrared Raman spectroscopy in the colon: Differentiating adenomatous from hyperplastic polyps. Gastrointestinal Endosc. 2003;57(3):396–402. doi: 10.1067/mge.2003.105. [DOI] [PubMed] [Google Scholar]
- 26.Manoharan R, Wang Y, Boustany NN, Brennan JF, Iii, Baraga JJ, Dasari RR, Van Dam J, Singer S, Feld MS. Raman spectroscopy for cancer detection instrument development and tissue diagnosis. Proc. SPIE 2328, Biomedical Optoelectronic Devices and Systems II; December 22, 1994; [DOI] [Google Scholar]
- 27.Kast R, Rabah R, Wills H, Poulik J, Auner GW, Klein MD. Differentiation of small round blue cell tumors using Raman spectroscopy. J Pediatr Surg. 2010;45(6):1110–1114. doi: 10.1016/j.jpedsurg.2010.02.072. [DOI] [PubMed] [Google Scholar]
- 28.Lieber CA, Mahadevan-Jansen A. Automated method for subtraction of fluorescence from biological Raman spectra. Appl Spectrosc. 2003;57(11):1363–1367. doi: 10.1366/000370203322554518. [DOI] [PubMed] [Google Scholar]
- 29.De Groot P, Postma G, Melssen W, Buydens L, Deckert V, Zenobi R. Application of principal component analysis to detect outliers and spectral deviations in near-field surface-enhanced Raman spectra. Anal Chim Acta. 2001;446(1):71–83. [Google Scholar]
- 30.Jackson DA, Chen Y. Robust principal component analysis and outlier detection with ecological data. Environmetrics. 2004;15(2):129–139. [Google Scholar]
- 31.Krishnapuram B, Carin L, Figueiredo MA, Hartemink AJ. Sparse multinomial logistic regression: Fast algorithms and generalization bounds. IEEE Trans Pattern Anal Mach Intell. 2005;27(6):957–968. doi: 10.1109/TPAMI.2005.127. [DOI] [PubMed] [Google Scholar]
- 32.Patil CA, Pence IJ, Lieber CA, Mahadevan-Jansen A. 1064 nm dispersive Raman spectroscopy of tissues with strong near-infrared autofluorescence. Opt Lett. 2014;39(2):303–306. doi: 10.1364/OL.39.000303. [DOI] [PubMed] [Google Scholar]
- 33.Erckens RJ, Motamedi M, March WF, Wicksted JP. Raman spectroscopy for non-invasive characterization of ocular tissue: Potential for detection of biological molecules. J Raman Spectrosc. 1997;28(5):293–299. [Google Scholar]
- 34.Frank CJ, McCreery RL, Redd DC. Raman spectroscopy of normal and diseased human breast tissues. Anal Chem. 1995;67(5):777–783. doi: 10.1021/ac00101a001. [DOI] [PubMed] [Google Scholar]
- 35.Schrader B, Keller S, Löchte T, Fendel S, Moore D, Simon A, Sawatzki J. NIR FT Raman spectroscopy in medical diagnosis. J Mol Struct. 1995;348:293–296. [Google Scholar]
- 36.Evans HL, Soule EH, Winkelmann RK. Atypical lipoma, atypical intramuscular lipoma, and well differentiated retroperitoneal liposarcoma: A reappraisal of 30 cases formerly classified as well differentiated liposarcoma. Cancer. 1979;43(2):574–584. doi: 10.1002/1097-0142(197902)43:2<574::aid-cncr2820430226>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 37.Crago AM, Singer S. Clinical and molecular approaches to well differentiated and dedifferentiated liposarcoma. Curr Opin Oncol. 2011;23(4):373–378. doi: 10.1097/CCO.0b013e32834796e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dalal KM, Kattan MW, Antonescu CR, Brennan MF, Singer S. Subtype specific prognostic nomogram for patients with primary liposarcoma of the retroperitoneum, extremity, or trunk. Ann Surg. 2006;244(3):381–391. doi: 10.1097/01.sla.0000234795.98607.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goldblum JR, Weiss SW, Folpe AL. Enzinger and Weiss’s soft tissue tumors. Philadelphia, PA: Elsevier Health Sciences; 2013. [Google Scholar]
- 40.Millis K, Weybright P, Campbell N, Fletcher JA, Fletcher CD, Cory DG, Singer S. Classification of human liposarcoma and lipoma using ex vivo proton NMR spectroscopy. Magn Reson Med. 1999;41(2):257–267. doi: 10.1002/(sici)1522-2594(199902)41:2<257::aid-mrm8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 41.Singer S, Millis K, Souza K, Fletcher C. Correlation of lipid content and composition with liposarcoma histology and grade. Ann Surg Oncol. 1997;4(7):557–563. doi: 10.1007/BF02305536. [DOI] [PubMed] [Google Scholar]
- 42.Matousek P, Stone N. Development of deep subsurface Raman spectroscopy for medical diagnosis and disease monitoring. Chem Soc Rev. 2016;45(7):1794–1802. doi: 10.1039/c5cs00466g. [DOI] [PubMed] [Google Scholar]