Abstract
BACKGROUND
Imatinib is the current standard frontline therapy for chronic myelogenous leukemia (CML). In the majority of patients, imatinib induces a complete cytogenetic response (CCyR); however, complete molecular responses are infrequent. The Bcr-Abl fusion creates a unique sequence of amino acids that could constitute a target for immunomodulation.
METHODS
A mixture of heteroclitic and native peptides derived from both b3a2 and b2a2 sequences was used to vaccinate patients with CML in CCyR who were receiving imatinib therapy and who had stable Bcr-Abl transcript levels.
RESULTS
Ten patients were enrolled, all with b2a2 transcripts (including 2 patients who had coexpression of b2a2 and b3a2). Patients had received imatinib for a median of 62 months. Three of 10 patients achieved 1-log reduction in Bcr-Abl transcript levels, including the 2 patients who had received previous interferon therapy, and 3 other patients achieved a major molecular response. The vaccine was tolerated well, and there were no grade ≥3 adverse events. Vaccination did not affect the leukocyte profiles in peripheral blood except for regulatory T cells, which were down-regulated briefly during the late stage of vaccination in patients who achieved approximately 1-log reduction in Bcr-Abl transcript levels.
CONCLUSIONS
The current data suggested that vaccination-related transient disruption of immune tolerance may contribute to the reduction in Bcr-Abl transcripts. Clinically, this Bcr-Abl peptide vaccine may transiently improve the molecular response in a subset of patients with CML.
Keywords: peptide, vaccine, tyrosine kinase inhibitor, imatinib, chronic myeloid leukemia
Chronic myeloid leukemia (CML) is a clonal disease characterized by a reciprocal translocation resulting in a chimeric BCR-ABL gene.1,2 This fusion gene most commonly transcribes into an 8.5-kb messenger RNA (mRNA), which generates a chimeric protein with tyrosine kinase activity (p210 BCR-ABL).3 The breakpoint in the ABL gene usually occurs 5′ of exon 2 of ABL (a2), whereas the breakpoint locations within BCR vary. In most instances, this occurs either between exons b2 (e13) and b3 (e14) (the b2a2 transcript) or between exons b3 (e14) and b4 (e15) (the b3a2 transcript).4 The b3a2 rearrangement reportedly is more prevalent, representing approximately 60% of all patients.5 Upon translation of the chimeric gene, a new amino acid (lysine in b3a2 and glutamic acid in b2a2) is created at the fusion site. This chimeric p210 protein provides a potential target for immunologic approach, because the p210 protein is expressed only on the CML cells.
Imatinib is standard therapy for patients with CML. In the International Randomized Study of Interferon and STI571 (IRIS) trial, 40% of patients who received frontline imatinib achieved a major molecular response (MMR) at 12 months, although only 4% of patients achieved a complete molecular response (CMR) after a median follow-up of 19 months.6 Although the molecular response rate may continue to improve over time in some patients, others achieve a plateau at which transcript levels remain stable, and a third group of patients may have increasing transcript levels, which may cause an eventual relapse.7 The ability of imatinib to induce a complete cytogenetic response (CCyR) in most patients, albeit with few CMRs, provides an ideal opportunity to treat patients with a vaccine strategy in a minimal residual disease state.
Fusion peptides from the junctional sequences product of the BCR-ABL fusion have the ability to bind several human leukocyte antigen (HLA) class I and II molecules and to elicit peptide-specific T-cell responses.8–11 Such an approach engendered interest in developing this peptide as a possible strategy to induce tumor-specific immune responses in patients who had received treatment with imatinib. Native junction peptides, which are administered in most trials to patients who have CML and minimal residual disease, have induced a specific immune response.12–15 To increase the immunogenicity of native peptides, synthetic peptides can be generated through selective mutations in their HLA-binding sequences (heteroclitic peptides).16–19
We conducted a pilot trial to evaluate the immunogenicity and antileukemic effects of vaccination with CML breakpoint heteroclitic peptides as measured by a decrease in BCR-ABL transcripts. We also investigated the effect of vaccination on T-lymphocyte and B-lymphocyte subsets, natural killer (NK) cells, and dendritic cells by flow cytometry as well as the proliferation of T lymphocytes.
MATERIALS AND METHODS
Patients
Patients aged ≥18 years who had Philadelphia chromosome-positive, chronic phase CML were eligible if they had received imatinib for at least 12 months and had been on a stable imatinib dose for ≥6 months before starting vaccination. There were no restrictions for HLA phenotype, and patients with either b3a2 or b2a2 transcripts were eligible. All patients were required to be in CCyR but without an MMR and had to have BCR-ABL transcript levels ≤0.5 log lower than the lowest value obtained in the previous 6 months. To establish the baseline values for reverse transcriptase-polymerase chain reaction (RT-PCR) analysis for BCR-ABL, peripheral blood samples were drawn 1 month before the first vaccination, 15 days before the first vaccination, and on the day of the first vaccination. The average of these results was used as the baseline transcript level. An MMR was defined as BCR-ABL/ ABL transcripts ≤0.05%. The protocol was approved by the M. D. Anderson Cancer Center Institutional Review Board, and all patients provided written informed consent.
Peptides
The peptides were synthesized using standard technologies with 9-fluorenylmethoxycarbonyl (Fmoc) solid-phase synthesis and were purified by high-performance liquid chromatography. All patients were screened to determine the breakpoint junction that they expressed. The choice of peptide vaccine administered depended on the breakpoint expressed by each patient; patients who coexpressed both breakpoints were vaccinated with the b3a2 cocktail (Table 1). Patients who had the b3a2 breakpoint were vaccinated with a cocktail of 5 peptides, including 2 heteroclitic peptides that differed from native peptides by 2 amino acids and 3 peptides that had the native sequence. Two of the native sequences were 9 amino acids long and had binding affinities for HLA-A3 and HLA-B8, respectively, similar to those used in previously reported trials. The third peptide with the native sequence, which was 24 amino acids long, was included because it could bind many different HLA-DR types and could be processed with other peptides that may bind HLA class I or II types. Patients who had the b2a2 breakpoint received a vaccine that contained a cocktail of 2 peptides, 1 synthetic, heteroclitic b2a2 peptide and 1 peptide that was 23 amino acids long and was chosen using the same considerations that were used for the long b3a2 peptide described above. The peptide products were provided by Breakthrough Therapeutics (Greenwich, Conn). Each vaccine was packaged as a frozen, sterile liquid containing 100 µg of each of the 3 peptides in 0.5 mL phosphate-buffered saline and was stored at −70°C. The material was manufactured under good manufacturing practice (GMP) conditions at American Peptide, Inc. (Sunnyvale, Calif). The peptide product was dispensed in vials and was stored frozen under GMP conditions at BioServ Corporation (San Diego, Calif).
Table 1.
Amino Acid Sequences
| Amino Acid Sequence | HLA Binding | Characteristics |
|---|---|---|
| B3A2 transcript vaccine | ||
| IVHSATGFKQSSKALQRPVASDFE | Class II | Native/long |
| KQSSKALQR | A3 | Native |
| GFKQSSKAL | B8 | Native |
| KLLQRPVAV | A0201 | Heteroclitic |
| YLKALQRPV | A0201 | Heteroclitic |
| B2A2 transcript vaccine | ||
| VHSIPLTINKEEALQRPVASDFE | Class II | Native/long |
| YLINKEEAL | A0201 | Heteroclitic |
HLA indicates human leukemic antigen.
Treatment
The patients continued receiving imatinib at the same dose they had been receiving for the previous 6 months. Dose escalations were not allowed while patients were on the study, and decreases in imatinib dose or treatment interruptions for imatinib toxicity were allowed for imatinib-related adverse events. All patients received 70 µg of granulocyte-macrophage colony-stimulating factor (GM-CSF) (sargramostim) subcutaneously at the same anatomic site of vaccination 2 days before vaccination (Day −2) and on the day of vaccination (Day 0) for each administration. For each vaccination, patients received 100 µg of each of the peptides subcutaneously mixed with the adjuvant montanide. Both GM-CSF and montanide have been used in several clinical cancer vaccine trials to enhance immunologic response.20–22 Thus, patients who had the b3a2 transcript received the vaccine that contained the 5 corresponding peptides (total peptide dose, 500 µg) with adjuvant, and patients who had the b2a2 transcript received the vaccine that contained the 2 corresponding peptides (total peptide dose, 200 µg) with adjuvant (Table 1). Patients who had both b3a2 and b2a2 transcripts received the b3a2 cocktail.14,15 Vaccines were given every 2 weeks ×4 (Weeks 0, 2, 4, and 6), then on Week 9, and monthly for an additional 10 months thereafter, for a total of 15 vaccinations over a 12-month period.
Enumeration of Leukocyte Subsets by Flow Cytometry
Immunophenotyping was performed on whole blood samples at baseline and 3 months, 6 months, 9 months, and 12 months after vaccination by using 4-color flow-cytometric analysis to determine the changes in the percentages of T-cell subsets, including T-regulatory (TR) cells, B cells, NK cells, natural killer T (NKT) cells, and dendritic cells. Aliquots of fresh whole blood were reacted with fluorochrome-conjugated mouse monoclonal antibodies to detect total T cells (CD3), helper-T cells (CD4), suppressor/cytotoxic-T cells (CD8), B cells (CD19), and NK cells (CD56/16). For enumeration of regulatory TR cells, frozen peripheral blood mononuclear cells (PBMCs) were thawed and were assessed for the surface expression of CD3, CD4, and CD25(high) and for the intracellular expression of transcription factor forkhead box P3 (FoxP3) using a monoclonal antibody.23
An anchor population of lymphocytes was identified by gating on cells that reacted with peridinin chlorophyll protein-conjugated anti-CD4 with low side-scatter. By using this reaction, we determined the percentage of CD4-positive T cells that reacted with phycoerythrin-conjugated anti-CD25 and fluorescein isothiocyanate-labeled anti-Foxp3 to define TR cells. In addition, using the allophycocyanin channel, we assessed the expression of programmed death-1 (PD-1), a member of the cytotoxic T-lymphocyte (CTL) antigen 4 (CTLA4) family, and glucocorticoid-induced tumor necrosis factor (TNF) receptor (GITR), a member of the TNF receptor family that is expressed preferentially at high levels on TR cells. Finally, subsets of myeloid dendritic cells (mDC/DC1) and plasmacytoid dendritic cells (pDC/DC2) were enumerated as reported previously.24 Staining intensity was recorded as a measure of fluorescence intensity using a FACSCalibur flow cytometer, and the data were analyzed with Cell-Quest Pro (BD Biosciences, San Jose, Calif).
The FoxP3 antibody was purchased from eBio-science (San Diego, Calif), GITR was purchased from R&D Systems (Minneapolis, Minn), blood dendritic cell antigen 1 (BDCA-1) and BDCA-2 were purchased from Miltenyi Biotec (Gladbach, Germany), and all other antibodies were purchased from BD Biosciences. Whole blood and PBMC samples from 8 control individuals who, based on self-report, were in good health and were not on any immunomodulatory medication were obtained for the laboratory studies.
Proliferation of CD4-positive T cells
The proliferation of CD4-positive T cells was measured before the first vaccination, 2 weeks after the fifth vaccination, and 2 weeks after the last vaccination. CD4-positive T cells were isolated from PBMCs by positive selection using immunomagnetic beads coated with anti-CD4 antibody and passage through a magnetic field in an Auto-MACS cell separator (Miltenyi Biotec, Auburn, Calif). To determine CD4-positive T-cell proliferation, 105 CD4-positive T cells per well were dispensed into quadruplicate wells of a 96-well microtiter plate and incubated with a mixture of vaccine peptides at a concentration of 20 µg/mL, and the microtiter plate was incubated at 37° C for 4 days in a humidity-controlled, 5% CO2 atmosphere. On Day 4, 1 µCi of 3H-thymidine in 20 µL of tissue culture media was added to each well of the microtiter plate, and the plate was incubated for an additional 24 hours. At the end of this period, the cells were harvested onto membrane filters, and the amount of 3H-thymidine that was incorporated into DNA synthesis was measured using a TopCount NXT scintillation and luminescence counter (Packard Instrument Company, Inc., Brookfield, Ill).
Response Assessment
RT-PCR for BCR-ABL was performed in peripheral blood every 3 months as described previously.25 Cytogenetic analysis in bone marrow metaphases was done every 6 months. A molecular response was defined as a 1-log reduction of BCR-ABL transcripts or the achievement of undetectable transcripts. Toxicities were graded according to the Common Terminology Criteria, version 3.0 (National Cancer Institute).
RESULTS
Ten patients were treated, and all 10 completed the study protocol with 15 vaccinations (Table 2). The median patient age was 45 years (range, 28–63 years), and patients had been on imatinib for a median of 62 months (range, 34–70 months). The daily dose of imatinib at the start of vaccination was 800 mg in 2 patients, 600 mg in 5 patients, 400 mg in 2 patients, and 300 mg in 1 patient. Other therapies before imatinib included interferon alpha (IFN) (n = 2), cytarabine (n = 2), homoharringtonine (n = 1), and hydroxyurea (n = 8). All patients were in CCyR at the start of the trial, and the median BCR-ABL/ ABL ratio was 0.238%. During the course of therapy, none of the 10 patients required dose adjustments of imatinib.
Table 2.
Patient Characteristics
| HLA Type | BCR-ABL/ABL Ratio, % | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Age, y | Sex | Transcript Type |
A | B | C | DRB1 | DQB1 | Time on Imatinib, mo |
Imatinib Dose, mg |
Previous Therapies |
Average Baseline |
3 mo |
6 mo |
9 mo |
12 mo |
15 mo |
18 mo |
21 mo |
24 mo |
| 1 | 45 | Woman | b2a2 | 1, 30 | 7,53 | 6, 7 | 9, 15 | 2,6 | 64 | 600 | Hydroxyurea | 0.33 | 0.22 | 0.38 | 0.21 | 0.1 | 0.175 | 0.2 | 0.15 | |
| 2 | 47 | Man | b2a2 | 29, 66 | 44, 44 | 16, 4 | 16, 14 | 6,6 | 58 | 800 | Interferon, hydroxyurea, cytarabine | 0.19 | 0.04 | 0.02 | 0.02 | 0.09 | 0 | 0.025 | ||
| 3 | 32 | Woman | b2a2 | 2, 24 | 13, 38 | 3, 7 | 8, 12 | 4,3 | 35 | 800 | Hydroxyurea | 2.19 | 1.83 | 0.65 | 1.84 | 0.81 | 0.08 | 0.125 | 0.4 | 0.325 |
| 4 | 28 | Man | Both | 2, 3 | 15, 27 | 3, 1 | 4, 1 | 3,3 | 55 | 600 | Hydroxyurea | 0.13 | 0.15 | NA | 0.2 | 0.2 | NA | 0.025 | ||
| 5 | 44 | Man | b2a2 | 2, 32 | 18, 18 | 7, 7 | 11, 11 | 3,3 | 60 | 400 | None | 2.74 | 13.4 | 11.6 | 13.4 | 21.05 | ||||
| 6 | 31 | Woman | Both | 3, 26 | 51, 51 | 1, 15 | 7, 14 | 2,5 | 67 | 600 | Interferon, hydroxyurea, homoharringtonine, cytarabine | 0.083 | 0.26 | 0.14 | 0.28 | 0.24 | 0.001 | 0.025 | ||
| 7 | 52 | Man | b2a2 | 2, 3 | 39, 62 | 3, 7 | 1, 8 | 4,5 | 63 | 600 | Hydroxyurea | 0.286 | 0.25 | 0.13 | 0.43 | 0.24 | 0.15 | 0.2 | ||
| 8 | 63 | Man | b2a2 | 1, 2 | 8,44 | 7, 5 | 3, 1 | 2,6 | 65 | 300 | Hydroxyurea | 1.665 | 0.73 | 1.74 | 0.78 | 0.725 | 0.925 | 1.9 | ||
| 9 | 40 | Man | b2a2 | 1, 24 | 40, 40 | 15, 15 | 15, 15 | 6,6 | 36 | 600 | None | 0.12 | 0.16 | 0.11 | 0.02 | 0.05 | 0.125 | |||
| 10 | 51 | Woman | b2a2 | 2, 3 | 15, 35 | 3, 4 | 4, 7 | 3,2 | 70 | 400 | Hydroxyurea | 0.085 | 0.04 | 0.1 | 0.03 | 0.025 | ||||
BCR indicates breakpoint cluster region; ABL, v-abl Abelson murine viral oncogene; HLA, human leukocyte antigen.
Three patients, including the 2 who had received previous IFN therapy, achieved at least a 1-log reduction in BCR-ABL transcript levels in at least 1 measurement (Table 2). The 1-log reduction in BCR-ABL transcript levels was achieved in all 3 patients after all vaccinations had been completed 15 months after the start of vaccination; although, in all 3 patients, this response was transient. Five patients achieved an MMR (which was sustained in 4 patients at the last follow-up). In addition, 1 of the 3 patients who had a 1-log reduction in BCR-ABL transcripts transiently achieved a CMR (ie, undetectable transcripts with a level of detection ≥4.5 logs) 15 months after the start of vaccination; however, 3 months later, he had detectable transcripts although still with MMR. Both patients who had coexpression of b3a2 and b2a2 achieved an MMR, but the improvement represented a ≥1-log reduction in BCR-ABL transcript levels for only 1 of them. One patient had a sustained rise in BCR-ABL transcript levels throughout vaccination and lost CCyR by the time of the last vaccination (10% Philadelphia chromosome-positive). This patient was switched to dasatinib and eventually achieved MMR.
Toxicities
Vaccination was well tolerated. The toxicities observed included grade 1 or 2 injection site reaction (n = 9); fatigue (n = 4); skin rash (n = 3); diarrhea (n = 3); bone pain (n = 2); nausea (n = 2); and sinus drainage, headache, leg pain, dizziness, tachycardia, myalgias, scalp itchiness, and burning sensation in extremities (n = 1 each). No hematologic toxicity and no grade 3 or 4 adverse events were noted. None of the patients required imatinib dose reductions or treatment interruptions during the treatment period.
Immunophenotype
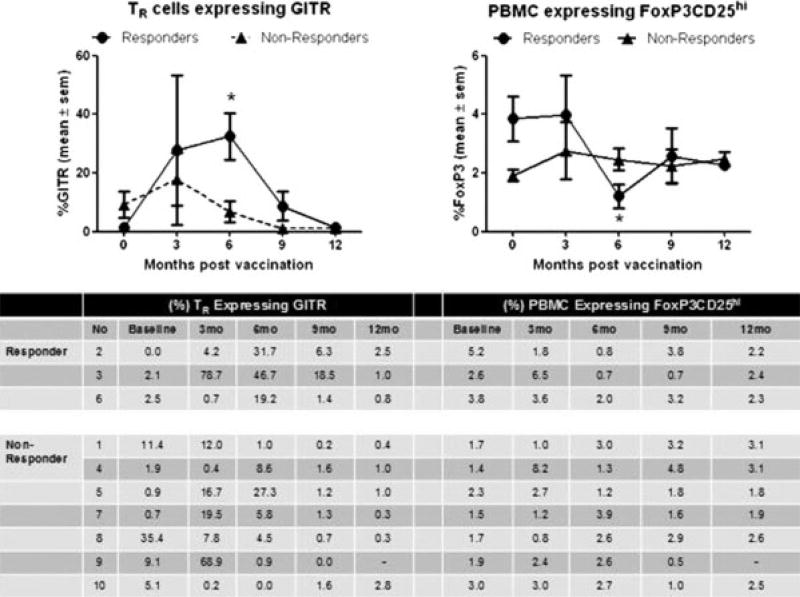
We analyzed the differences in lymphocyte populations between responders and nonresponders within the constraints of the small sample size. Because FoxP3 TR cells could be responsible for maintaining peripheral immune tolerance to BCR-ABL leukemic cells, TR cells were enumerated before and at designated points after vaccination. Patients who achieved a 1-log reduction in BCR-ABL transcripts (responders) had a reduction in their percentage of TR cells accompanied by a gradual increase in the percentage of TR cells expressing GITR that peaked at 6 months after vaccination (Fig. 1). At 6 months, the median GITR expression by CD4-positive/CD25-positive/ Foxp3-positive TR cells was significantly higher in responders than in nonresponders (31.7% vs 4.5% of TR cells, respectively; P = .033; Mann-Whitney U test). At baseline, CD4-positive/CD25-positive/Foxp3-positive TR cells represented 3.7% of PBMCs in responders but only 1.7% of PBMCs in nonresponders (P = .033). However, concurrent with the increased GITR expression observed at 6 months, the responder TR percentage dropped significantly to 1% (P = .009). The only other difference in leukocyte immunophenotype between responders and nonresponders was a decrease in CD4-positive T cells in nonresponders from baseline to 9 months after vaccination (Fig. 2). No additional significant differences between responders and nonresponders were observed in the percentages of any other leukocyte subsets, including neutrophils, lymphocytes, monocytes, CD8 T cells, central memory CD8 T cells, CD4 T cells, B cells, NK cells, NKT cells, pDC/DC2, mDC/DC1, activated pDCs, or activated mDCs.
FIGURE 1.
Vaccination was associated with increased glucocorticoid-induced tumor necrosis factor receptor (GITR) expression by regulatory T cells (TR) concurrent with a decrease in the percentage of TR. Peripheral blood mononuclear cells (PBMCs) were collected from imatinib-treated patients with chronic myeloid leukemia (CML) before they were vaccinated with human leukocyte antigen (HLA)-matched CML breakpoint heteroclitic peptides and at 3 months, 6 months, 9 months, and 12 months after vaccination. Patients who acquired a minimum 1-log reduction in BCR-ABL transcripts by Month 15 of follow-up were classified as responders (circles; n = 3 patients) and nonresponders (triangles; n = 7 patients). Staining and flow cytometric analysis for TR markers was performed in batches according to the manufacturer’s protocol (eBioscience; San Diego, Calif) with surface marker staining before permeabilization and staining with the forkhead box P3 (FoxP3) antibody (eBioscience). Responders had a significantly greater percentage of TR cells that expressed GITR at 6 months after vaccination (left plot) with a concurrent diminishing percentage of CD4-positive/CD25(high)FoxP3-positive cells in PBMCs (right plot) (asterisk: P < .05; Mann-Whitney U test). Plots show the mean ± standard error of the mean. The table below the plots represents individual responses by responders and nonresponders at each point of assessment.
FIGURE 2.
Vaccination had a minimal effect on leukocyte distribution. Leukocyte subsets were analyzed fresh by flow cytometry before vaccination with human leukocyte antigen (HLA)-matched chronic myeloid leukemia (CML) breakpoint heteroclitic pep-tides and at 3 months, 6 months, 9 months, and 12 months after vaccination. Patients who acquired a minimum of 1-log reduction in BCR-ABL transcripts by Month 15 of follow-up were classified as responders (circles; n = 3 patients) and nonresponders (triangles; n = 7 patients). A cross-sectional analysis indicated that CD3-positive/CD4-positive lymphocytes were significantly lower in nonresponders than in responders at 9 months (P = .033). Longitudinal analysis revealed a significant drop in the myeloid dendritic cell (mDC) count among responders (P = .009). No other significant differences or changes were observed. WBC indicates white blood cells; CD3, T cells; CD19, B cells; NK, natural killer cells; NKT, natural killer T cells; +, positive; CD8, suppressor/cytotoxic T cells; CD4, helper T cells; DC, dendritic cells; pDC, plasmacytoid dendritic cells.
Proliferation
There were increases in peptide-induced proliferation of CD4-positive T cells at 3 months after vaccination (P = .05; Wilcoxon signed-rank test for paired samples), and the trend was maintained at 12 months (Fig. 3). Nevertheless, there was no statistical difference in CD4-positive T cell proliferation in response to CML peptides between responders and nonresponders.
FIGURE 3.
Proliferation of peptide-stimulated CD4-positive T cells increased significantly 3 months after vaccination. CD4-positive T cells were isolated from peripheral blood mononuclear cells by positive selection using an AutoMACS cell separator (Miltenyi Biotec, Auburn, Calif). Then, 105 CD4-positive T cells were incubated with a mixture of vaccine peptides in quadruplicate wells for 4 days, and 1 µCi of 3H-thymidine was added to each well and incubated for an additional 24 hours. The amount of 3H-thymidine incorporation was measured on Day 4 using a scintillation counter. The proliferation of CD4-positive T cells, measured as the count per million (cpm), increased significantly at 3 months after vaccination (asterisk: P = .05; Wilcoxon signed-rank test for paired samples), and the trend was maintained at 12 months. Nevertheless, no statistically significant difference was observed between responders and nonresponders at baseline, at 3 months after vaccination, or at 12 months after vaccination.
DISCUSSION
Immune-mediated events may participate in the suppression of CML. A prominent example is graft-versus-leukemia, which is responsible for the eradication of CML after allogeneic stem cell transplantation.26 Immune modulation also may be responsible in part for the therapeutic effect of IFN.27 Furthermore, CTLs that were specific for the PR1 leukemia-associated antigen were identified in most patients with CML who responded to IFN or allogeneic stem cell transplantation but not in nonresponding patients or in patients who received chemotherapy.28 Thus, developing immunologic approaches to treat CML is attractive. One approach is to use vaccines to induce a tumor-specific immune response. Several of these have been investigated, including PR1,29 the bcr-abl junction peptide,13,15 and a heat-shock protein-based vaccine.30
In this study, our objective was to improve the molecular responses of patients with CML treated with imatinib using a junction peptide vaccine. Only 3 of the 10 patients who were treated achieved the primary endpoint of a 1-log reduction in BCR-ABL transcript levels, and all 3 responses were transient. These results are in contrast to the more favorable results from previous trials using a junction peptide vaccination (Table 3). Bocchia et al used junction peptides with a native sequence to treat 16 patients who had CML, including 10 patients who had received therapy with imatinib for a median of 16 months.15 In that study, 5 of 9 patients who were not in CCyR at the start of vaccinations achieved a CCyR, and 3 of them achieved a CMR. The 1 patient who was treated in CCyR (after 23 months of imatinib therapy) achieved a half-log reduction in BCR-ABL transcripts. In another study, Rojas et al14 reported on 19 patients who received a junction peptide cocktail using native sequence peptides. None of the 5 patients who entered that study without a major cytogenetic response (MCyR) to imatinib responded to the peptide vaccine, whereas 13 of 14 patients who had a MCyR at the start of vaccinations achieved a 1-log reduction in transcript levels.
Table 3.
Summary of Reported Chronic Myeloid Leukemia Vaccine Trials
| Reference | Eligible CML Patients |
HLA Types Allowed |
Peptides Sequences Used |
Type of Adjuvant Used |
No. of Patients |
No. of Patients Receiving Imatinib |
Median Time Receiving Imatinib, mo |
CCyR at Baseline |
Response |
|---|---|---|---|---|---|---|---|---|---|
| Pinilla-Ibarz 200012 | PR or CR with WBC <20,000; b3a2 transcript | All | Five peptides: HLA-A3 (KQSSKALQR), HLA-A11 (ATGFKQSSK), HLA-A3/11 (HSATGFKQSSK), HLA-B8 (GFKQSSKAL) and class II peptide (IVHSATGFKQSSKALQRPVASDFEP) | QS-21 | 12 | 0 | NR | NR | One CMR, 1 transient cytogenetic improvement |
| Cathcart 200413 | Measurable disease with PHR or CHR and WBC <20,000; b3a2 transcript | All | Six peptides: HLA-A2 (SSKALQRPV), HLA-A3 (KQSSKALQR), HLA-A11 (ATGFKQSSK), HLA-A3/11 (HSATGFKQSSK), HLA-B8 (GFKQSSKAL), and class II peptide (IVHSATGFKQSSKALQRPVASDFEP) | QS-21 | 14 | 2 | NR | Six patients (1 on matinib) | Six patients with CCyRs (3 with transient PCR improvement); 8 patients without CCyR (4 with cytogenetic improvement) |
| Bocchia 200515 | SD with >12 mo on matinib or 24 mo on FN; b3a2 transcript | HLA restricted | Six peptides: HLA-A2 (SSKALQRPV), HLA-A3 (KQSSKALQR), HLA-A11 (ATGFKQSSK), HLA-A3/11 (HSATGFKQSSK), HLA-B8 (GFKQSSKAL), and class II peptide IVHSATGFKQSSKALQRPVASDFEP) | QS-21 | 16 | 10 | 15.5 | One patient (on matinib) | Nine imatinib-treated patients without CCyR (5 achieved CCyR); 1 imatinib-treated patient in CCyR (0.5 log transcript reduction) |
| Rojas 200714 | SD with CHR and ≥6 mo of imatinib; b3a2 transcript | All | Patients with HLA-A3 and HLA-A11, KQSSKALQR and KQSSKALQR-PA-DRE; HLA-A3-negative and HLA-A11-negative patients, GFKQSSKAL and GFKQSSKAL-PADRE; all patients, GFKQSSKALQRPV-PADRE | PADRE | 19 | 19 | 17 (Imatinib responding patents only) | NR | Thirteen of 14 patients in MCyR (1 log reduction in PCR); 0/5 patients not in MCyR responded |
| Maslak 200819 | MCyR or CCyR on stable imatinib regimen; both b3a2 and b2a2 allowed | All | b3a2: Five peptides, including 2 hetero-clitic HLA-A2 binding peptides (KLLQRPVAV and YLKALQRPV) and 3 native sequences (HLA-A3, KQSSKALQR; HLA-B8, GFKQSSKAL; and class II peptide, IVHSATGFKQS-SKALQRPVASDFE); b2a2: 2 peptides, including 1 heteroclitic HLA-A2 binding peptide (YLINKEEAL) and 1 class II peptide (VHSIPLTINKEEALQRPVASDFE) | Montanide, GM-CSF | 13 | 13 | 27 | 10 | Two of 3 patients without CCyR achieved CCyR; inconsistent PCR results |
| Current study | CCyR with imatinib ≥12 mo; both b3a2 and b2a2 allowed | All | b3a2: Five peptides, including 2 heteroclitic HLA-A2-binding peptides (KLLQRPVAV and YLKALQRPV) and 3 native sequences (HLA-A3; KQSSKALQR; HLA-B8, GFKQSSKAL; and class II peptide, IVHSATGFKQS-SKALQRPVASDFE); b2a2: 2 peptides, including 1 heteroclitic HLA-A2-bind-ing peptide (YLINKEEAL) and 1 class II peptide (VHSIPLTINKEEALQRPVASDFE) | Montanide, GM-CSF | 10 | 10 | 62 | 10 | Three of 10 patients had a 1-log reduction in transcripts |
CML indicates chronic myeloid leukemia; HLA, human leukocyte antigen; CCyR, complete cytogenetic response; PR, partial response; CR, complete response; NR, not reported; CMR, complete molecular response; PHR, partial hematologic response; CHR, complete hematologic response; WBC, white blood cells; PCR, polymerase chain reaction analysis; SD, stable disease; IFN, interferon alpha; MCyR, major cytogenetic response; GM-CSF, granulocyte-macrophage colony-stimulating factor.
There are several differences between those 2 reports and our study, and these differences are summarized in Table 3. One difference is that both studies used only peptides with the native sequence; whereas, in the current study, we used heteroclitic peptides aimed at increasing the immunogenicity of the peptides. One other study used the same heteroclitic peptides that we used in our trial but with a different dose and schedule.19 In that study, 2 of 3 patients who had low levels of fluorescent in situ hybridization (FISH) positivity assessed before the start of vaccination had negative FISH results during the vaccinations. Molecular responses could not be assessed by RT-PCR in that study, but all patients had detectable transcript levels throughout the follow-up period. Immune responses were observed in 4 of 7 patients with the HLA-A02 phenotype and also in patients with the HLA-A3 or HLA-B8 phenotypes but not in patients with other phenotypes. Another important difference is that the previous studies only used peptides that corresponded to the B3A2 sequence, thus restricting eligibility to patients who had this breakpoint. In the study by Maslak et al19 and in our current study, the peptides that were used corresponded to both sequences. In both studies, in at least some instances, an immune and/or clinical response could be triggered with b2a2 peptides. In this regard, it is noteworthy that, in our study, all patients had the b2a2 sequence (2 patients also had the b3a2 sequence). The study by Maslak et al using the same peptides that we used appears to have induced more significant immune responses. This may have been caused in part because more patients expressed b3a2 in that study; however, differences in the methodology used to investigate immune response also may have been responsible for these differences. Other studies used different combinations of peptides, schedules of administration, and adjuvant. Currently, the contribution of these factors to the overall potential benefit of vaccination approaches cannot be determined.
In evaluating the responses achieved in these trials, it is important to consider that responses to imatinib improve over time. Thus, the contribution of the vaccine itself to any possible response is difficult to evaluate in these single-arm studies. In the study by Bocchia et al,15 for example, patients were included after a median of 15.5 months on imatinib; whereas, in the study by Rojas et al,14 evaluable information was provided for 11 of 19 patients, and they had been on imatinib for a median of 17 months. Similarly, in the study by Maslak et al using same peptides that we used in our study, the median time on imatinib was 27 months.19 According to the IRIS trial, the projected rate of CCyR improved from 69% at 12 months to 87% at 60 months. Similarly, the 5-year follow-up in the IRIS trial indicated that a 3-log reduction in BCR-ABL transcript levels was observed in 53% of patients at 1 year and in 80% of patients at 4 years (P < .001).31 In a recent analysis from a subset of patients from the IRIS trial, the incidence of CMR increased every year for the first 6 years of imatinib treatment, and 7% of patients achieved this response at 36 months compared with 45% of patients at 81 months.32 Therefore, the possibility that the responses observed in these trials could have occurred with continuation of imatinib alone cannot be ruled out. In our study, the median time on imatinib before the start of vaccination was 62 months, and the minimum was 34 months. Responses after this time tend to be more stable, thus making it more plausible that any improvement observed after vaccination may have been induced by the vaccine itself. Still, only a randomized trial can address the extent to which vaccination may improve the outcome of patients who have CML treated with imatinib.
In the current study, T-cell proliferation upon activation with pool peptides produced a sustained increase after 3 months of vaccination, suggesting a cross-reactive T-cell response to the synthetic peptides (Fig. 3). However, reduced percentages of FoxP3-expressing TR cells occurred only in patients who had achieved a 1-log reduction in Bcr-Abl transcript levels and may indicate disruption in immune tolerance leading to the elimination of the leukemic cells by CML-specific cytotoxic T cells. The higher percentage of GITR-expressing TR cells observed only in responders further supports our suggestion that the activation of CD4-positive/CD25-positive T cells through GITR may help to break immunologic tolerance.33 However, it is noteworthy that there was a time interval between the peak immune response (at approximately 6 months) and the time to best clinical response (approximately 15 months). The reason for this discordance is unclear.
In conclusion, heteroclitic peptides may transiently improve the molecular response in a subset of patients who have achieved a CCyR with imatinib. The approach presented here may extend the benefit to patients who have a B2A2 breakpoint, a group that has a not previously been targeted well. This approach may be combined with other methods to impact the immune-mediated control of CML.
Acknowledgments
Supported by National Institutes of Health grant PO1 CA23766.
Memorial Sloan-Kettering Cancer Center owns patents for inventions of David A. Scheinberg, MD, PhD, related to several of the peptides studied in this trial.
Footnotes
Conflict of Interest Disclosures
References
- 1.de Klein A, van Kessel AG, Grosveld G, et al. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1982;300:765–767. doi: 10.1038/300765a0. [DOI] [PubMed] [Google Scholar]
- 2.Rowley JD. A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining [letter] Nature. 1973;243:290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 3.Shtivelman E, Lifshitz B, Gale RP, Canaani E. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985;315:550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- 4.Shtivelman E, Lifshitz B, Gale RP, Roe BA, Canaani E. Alternative splicing of RNAs transcribed from the human abl gene and from the bcr-abl fused gene. Cell. 1986;47:277–284. doi: 10.1016/0092-8674(86)90450-2. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd P, Suffolk R, Halsey J, Allan N. Analysis of molecular breakpoint and m-RNA transcripts in a prospective randomized trial of interferon in chronic myeloid leukaemia: no correlation with clinical features, cytogenetic response, duration of chronic phase, or survival. Br J Haematol. 1995;89:546–554. doi: 10.1111/j.1365-2141.1995.tb08362.x. [DOI] [PubMed] [Google Scholar]
- 6.Hughes TP, Kaeda J, Branford S, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349:1423–1432. doi: 10.1056/NEJMoa030513. [DOI] [PubMed] [Google Scholar]
- 7.Marin D, Kaeda J, Szydlo R, et al. Monitoring patients in complete cytogenetic remission after treatment of CML in chronic phase with imatinib: patterns of residual leukaemia and prognostic factors for cytogenetic relapse. Leukemia. 2005;19:507–512. doi: 10.1038/sj.leu.2403664. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Peace DJ, Rovira DK, You SG, Cheever MA. T-cell immunity to the joining region of p210BCR-ABL protein. Proc Natl Acad Sci U S A. 1992;89:1468–1472. doi: 10.1073/pnas.89.4.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bocchia M, Wentworth PA, Southwood S, et al. Specific binding of leukemia oncogene fusion protein peptides to HLA class I molecules. Blood. 1995;85:2680–2684. [PubMed] [Google Scholar]
- 10.Bosch GJ, Joosten AM, Kessler JH, Melief CJ, Leeksma OC. Recognition of BCR-ABL positive leukemic blasts by human CD4+ T cells elicited by primary in vitro immunization with a BCR-ABL breakpoint peptide. Blood. 1996;88:3522–3527. [PubMed] [Google Scholar]
- 11.Yasukawa M, Ohminami H, Kojima K, et al. HLA class II-restricted antigen presentation of endogenous bcr-abl fusion protein by chronic myelogenous leukemia-derived dendritic cells to CD4(+) T lymphocytes. Blood. 2001;98:1498–1505. doi: 10.1182/blood.v98.5.1498. [DOI] [PubMed] [Google Scholar]
- 12.Pinilla-Ibarz J, Cathcart K, Korontsvit T, et al. Vaccination of patients with chronic myelogenous leukemia with bcr-abl oncogene breakpoint fusion peptides generates specific immune responses. Blood. 2000;95:1781–1787. [PubMed] [Google Scholar]
- 13.Cathcart K, Pinilla-Ibarz J, Korontsvit T, et al. A multivalent bcr-abl fusion peptide vaccination trial in patients with chronic myeloid leukemia. Blood. 2004;103:1037–1042. doi: 10.1182/blood-2003-03-0954. [DOI] [PubMed] [Google Scholar]
- 14.Rojas JM, Knight K, Wang L, Clark RE. Clinical evaluation of BCR-ABL peptide immunisation in chronic myeloid leukaemia: results of the EPIC study. Leukemia. 2007;21:2287–2295. doi: 10.1038/sj.leu.2404858. [DOI] [PubMed] [Google Scholar]
- 15.Bocchia M, Gentili S, Abruzzese E, et al. Effect of a p210 multipeptide vaccine associated with imatinib or interferon in patients with chronic myeloid leukaemia and persistent residual disease: a multicentre observational trial. Lancet. 2005;365:657–662. doi: 10.1016/S0140-6736(05)17945-8. [DOI] [PubMed] [Google Scholar]
- 16.Pinilla-Ibarz J, Korontsvit T, Zakhaleva V, Roberts W, Scheinberg DA. Synthetic peptide analogs derived from bcr/ abl fusion proteins and the induction of heteroclitic human T-cell responses. Haematologica. 2005;90:1324–1332. [PubMed] [Google Scholar]
- 17.Zugel U, Wang R, Shih G, Sette A, Alexander J, Grey HM. Termination of peripheral tolerance to a T cell epitope by heteroclitic antigen analogues. J Immunol. 1998;161:1705–1709. [PubMed] [Google Scholar]
- 18.Dyall R, Bowne WB, Weber LW, et al. Heteroclitic immunization induces tumor immunity. J Exp Med. 1998;188:1553–1561. doi: 10.1084/jem.188.9.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maslak PG, Dao T, Gomez M, et al. A pilot vaccination trial of synthetic analog peptides derived from the BCR-ABL breakpoints in CML patients with minimal disease. Leukemia. 2008;22:1613–1616. doi: 10.1038/leu.2008.7. [DOI] [PubMed] [Google Scholar]
- 20.Chianese-Bullock KA, Pressley J, Garbee C, et al. MAGE-A1-, MAGE-A10-, and gp100-derived peptides are immunogenic when combined with granulocyte-macrophage colony-stimulating factor and montanide ISA-51 adjuvant and administered as part of a multipeptide vaccine for melanoma. J Immunol. 2005;174:3080–3086. doi: 10.4049/jimmunol.174.5.3080. [DOI] [PubMed] [Google Scholar]
- 21.Hersey P, Menzies SW, Coventry B, et al. Phase I/II study of immunotherapy with T-cell peptide epitopes in patients with stage IV melanoma. Cancer Immunol Immunother. 2005;54:208–218. doi: 10.1007/s00262-004-0587-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slingluff CL, Jr, Petroni GR, Yamshchikov GV, et al. Clinical and immunologic results of a randomized phase II trial of vaccination using 4 melanoma peptides either administered in granulocyte-macrophage colony-stimulating factor in adjuvant or pulsed on dendritic cells. J Clin Oncol. 2003;21:4016–4026. doi: 10.1200/JCO.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Reuben JM, Lee BN, Li C, et al. Biologic and immunomodulatory events after CTLA-4 blockade with ticilimumab in patients with advanced malignant melanoma. Cancer. 2006;106:2437–2444. doi: 10.1002/cncr.21854. [DOI] [PubMed] [Google Scholar]
- 24.Lee BN, Follen M, Rodriquez G, et al. Deficiencies in myeloid antigen-presenting cells in women with cervical squamous intraepithelial lesions. Cancer. 2006;107:999–1007. doi: 10.1002/cncr.22092. [DOI] [PubMed] [Google Scholar]
- 25.Cortes J, Talpaz M, O’Brien S, et al. Molecular responses in patients with chronic myelogenous leukemia in chronic phase treated with imatinib mesylate. Clin Cancer Res. 2005;11:3425–3432. doi: 10.1158/1078-0432.CCR-04-2139. [DOI] [PubMed] [Google Scholar]
- 26.Barrett J. Allogeneic stem cell transplantation for chronic myeloid leukemia. Semin Hematol. 2003;40:59–71. doi: 10.1053/shem.2003.50003. [DOI] [PubMed] [Google Scholar]
- 27.Reuben JM, Lee BN, Johnson H, Fritsche H, Kantarjian HM, Talpaz M. Restoration of Th1 cytokine synthesis by T cells of patients with chronic myelogenous leukemia in cytogenetic and hematologic remission with interferon-alpha. Clin Cancer Res. 2000;6:1671–1677. [PubMed] [Google Scholar]
- 28.Molldrem J, Dermime S, Parker K, et al. Targeted T-cell therapy for human leukemia: cytotoxic T lymphocytes specific for a peptide derived from proteinase 3 preferentially lyse human myeloid leukemia cells. Blood. 1996;88:2450–2457. [PubMed] [Google Scholar]
- 29.Qazilbash MH, Wieder E, Rios R, et al. Vaccination with the PR1 leukemia-associated antigen can induce complete remission in patients with myeloid leukemia [abstract] Blood. 2004;104:77a. Abstract 259. [Google Scholar]
- 30.Li Z, Qiao Y, Liu B, et al. Combination of imatinib mesylate with autologous leukocyte-derived heat shock protein and chronic myelogenous leukemia. Clin Cancer Res. 2005;11:4460–4468. doi: 10.1158/1078-0432.CCR-05-0250. [DOI] [PubMed] [Google Scholar]
- 31.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 32.Branford S, Seymour JF, Grigg A, et al. BCR-ABL messenger RNA levels continue to decline in patients with chronic phase chronic myeloid leukemia treated with imatinib for more than 5 years and approximately half of all first-line treated patients have stable undetectable BCR-ABL using strict sensitivity criteria. Clin Cancer Res. 2007;13:7080–7085. doi: 10.1158/1078-0432.CCR-07-0844. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]