Abstract
Background and Purpose
Abnormal P-wave axis (aPWA) has been linked to incident atrial fibrillation (AF) and mortality; however, the relationship between aPWA and stroke has not been reported. We hypothesized that aPWA is associated with ischemic stroke independent of AF and other stroke risk factors and tested our hypothesis in the Atherosclerosis Risk In Communities study, a community-based prospective cohort study.
Methods
We included 15,102 participants (age 54.2 ± 5.7 years, 55.2% women, 26.5% blacks) who attended the baseline exam (1987–89) and without prevalent stroke. We defined aPWA as any value outside 0–75 degrees using 12-lead ECGs obtained during study visits. Each case of incident ischemic stroke was classified in accordance with criteria from the National Survey of Stroke by a computer algorithm and adjudicated by physician review. Multivariable Cox regression was used to estimate hazard ratios and 95% confidence intervals for the association of aPWA with stroke.
Results
During a mean follow-up of 20.2 years, there were 657 incident ischemic stroke cases. aPWA was independently associated with a 1.50-fold (95% CI, 1.22–1.85) increased risk of ischemic stroke in the multivariable model which included AF. When subtyped, aPWA was associated with a 2.04-fold (95% CI, 1.42–2.95) increased risk of cardioembolic stroke and a 1.32-fold (95% CI, 1.03–1.71) increased risk of thrombotic stroke.
Conclusions
aPWA is independently associated with ischemic stroke. This association appears to be stronger for cardioembolic strokes. Collectively, our findings suggest that alterations in atrial electrical activation may predispose to cardiac thromboembolism independent of AF.
Key Words/Subject Terms: atrial fibrillation, stroke, ECG/Stroke, Arrhythmia, Electrophysiology
Introduction
Atrial fibrillation (AF) is associated with a 5-fold increased risk of thromboembolic stroke.1 Thrombogenesis in the left atrium is a complex process relying on synergy between the three elements of Virchow’s triad.2 Pro-thrombotic remodeling of the atrial architecture is a critical component of this process and may precede development or diagnosis of AF. Hence, early detection of underlying atrial abnormality may potentially help improve risk stratification of AF-related stroke.
P-wave indices are markers derived from P-wave morphology on a 12-lead electrocardiogram (ECG) to characterize atrial conduction, which can be altered in the presence of structural remodeling. Abnormal P-wave indices, such as advanced inter-atrial block and abnormal p-wave terminal force in v1, have been associated with incident AF and ischemic stroke.3–5 Calculation of these indices, however, requires specialized analytic software and digitized ECGs, creating barriers for translation to the clinical domain. P-wave axis, on the other hand, is a routine measure reported on 12-lead ECGs that reflects the net vector component of atrial depolarization in the frontal plane. It has been associated with increased risk of AF;6 however, the relationship between aPWA and stroke has not been investigated. We hypothesized that aPWA is associated with ischemic stroke, independent of AF and other stroke risk factors. We tested this hypothesis in the Atherosclerosis Risk in Communities (ARIC) Study.
Methods
Study Population
The ARIC study is a prospective cohort study designed to identify and evaluate risk factors, etiology, and clinical manifestations of atherosclerotic coronary heart disease in the general population. Between 1987 and 1989, 15,792 men and women aged 45–64 years were recruited and enrolled from four United States communities (Washington County, MD; Forsyth County, NC; Jackson, MS; and suburban Minneapolis, MN).
There have been five study visits, the last in 2011–13. In between study visits, participants (or proxy) have been contacted annually by telephone to ascertain information on hospitalizations and deaths. Further, active community-wide surveillance of local hospitals has been performed to identify additional hospitalizations and cardiovascular events. Further details regarding outcome ascertainment procedures, study design, and population statistics have been previously described.7
For our analysis, we considered all 15,792 participants at the baseline visit and excluded those with missing ECG data (n=242), missing P-wave axis data (n=45), missing covariates (n=45), prevalent stroke (n=279), and those who were not white or black from all study sites, and nonwhite from Minneapolis and Washington County (due to small sample size; n=103) resulting in a final cohort of 15,078 participants.
Approval for the study was obtained from the institutional review board on human research at each participating institution and all participants provided informed consent.
Measurement of P-Wave Axis
ECGs obtained during the five ARIC study visits were recorded on MAC PC Personal Cardiographs (Marquette Electronics Inc. Milwaukee, WI) and processed at the EPICORE Center (University of Alberta, Edmonton, Alberta, Canada) and EPICARE Center (Wake Forest University, Winston-Salem, NC) during the early and late study phases, respectively. P-wave axis was determined by measuring the positive or negative P-wave deflections on all six limb leads and then calculating the net direction of electrical activity using the hexaxial reference system.8 Automated analysis of ECG data was conducted including selective averaging to obtain representative durations and amplitudes of ECG components to calculate the frontal P-wave axis. Abnormal P-wave axis was defined as any value outside 0–75 degrees.8
Definite Ischemic Stroke and Stroke Sub-Types
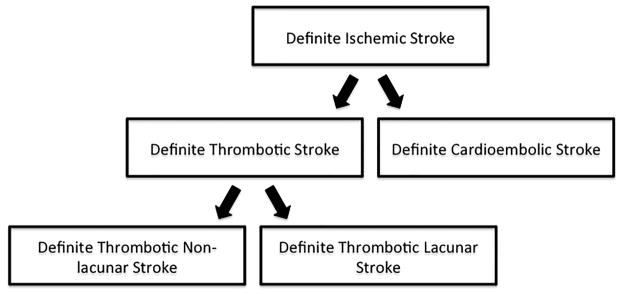
Annual participant phone interviews were conducted for the purposes of identifying hospitalizations and deaths. Potential cases of stroke were identified from review of hospital records and death certificates. Classification of stroke was then adjudicated by a panel of physicians with assistance of a computerized algorithm utilizing validated criteria from the National Survey of Stroke by the National Institute of Neurological Disorders.9 Strokes were classified as definite or probable thrombotic stroke, definite or probable cardioembolic stroke, definite or probable subarachnoid hemorrhage, definite or probable brain hemorrhage, and possible stroke of undetermined type. All definite thrombotic strokes were further sub-typed as definite thrombotic lacunar and definite thrombotic non-lacunar strokes. There were 26 definite thrombotic strokes that were unclassifiable. The primary endpoint in our study was definite ischemic stroke, which included all definite thrombotic strokes and all definite cardioembolic strokes. The secondary endpoints of our study included definite thrombotic stroke, definite cardioembolic stroke, definite thrombotic lacunar stroke, and definite thrombotic non-lacunar stroke (Figure 1). Further details on stroke identification and specific classification criteria in the ARIC study have been previously described.10,11
Figure 1.
Stroke classification scheme in the Atherosclerosis Risk in Communities Study. Definite ischemic strokes comprised of all definite cardioembolic strokes and all definite thrombotic strokes. Definite thrombotic strokes were then classified as lacunar and non-lacunar strokes.
Assessment of Covariates
The covariates included in our analysis were age, sex, race, study center, cigarette-smoking status, coronary heart disease, heart failure, diabetes, use of anti-hypertensive medications, systolic and diastolic blood pressure, use of anticoagulants, ECG-based left ventricular hypertrophy, AF, and body mass index. Baseline demographic data, medication use (use of anticoagulants or anti-hypertensive medications), and medical history were obtained by ARIC staff from participants during the study visit. Prevalent coronary heart disease was defined as a self-reported history of myocardial infarction, coronary artery bypass grafting, percutaneous coronary intervention, or ECG signs of coronary heart disease.12,13 Prevalent heart failure was defined as stage 3 “manifest heart failure” by the Gothenburg criteria or self-reported diagnosis of heart failure 14. Incident coronary heart disease and heart failure were identified by review of hospitalization records as previously described.15 Left ventricular hypertrophy was defined by the Cornell ECG criteria.16,17 Body mass index was defined as weight/height2 (kg/m2).12 Diabetes was defined as a fasting (minimum of 8 hours) glucose ≥126 mg/dL, non-fasting glucose ≥200 mg/dL, self-reported use of oral hypoglycemic agents or insulin, or self-reported physician diagnosis of DM.18 Smoking status was self-reported. Participants were classified as current smokers and non-current smokers. Education level was also self-reported and classified as less than high school, high school degree or vocational school, or more than high school. AF cases were ascertained by review of ECGs during study visits, hospital discharge records, and death certificates. Further details on specific ascertainment procedures have been previously described.19
Statistical Analysis
Person-years at risk were calculated from the date of baseline visit until the date of definite ischemic stroke, other death, loss to follow-up, or end of follow-up, whichever occurred first. For those with incident aPWA, time between baseline and aPWA diagnosis was considered as normal P-wave axis follow-up. The analysis was based on data obtained from 1987–2013.
The association between baseline P-wave axis and definite ischemic stroke was modeled with an unadjusted restricted cubic spline. We used Cox proportional hazard models with aPWA as a time-dependent exposure variable to calculate hazard ratios (HRs) and 95% confidence intervals (95% CI) of aPWA for definite ischemic stroke. Definite ischemic stroke was subtyped into definite cardioembolic stroke and definite thrombotic stroke. The latter was further subtyped into lacunar and non-lacunar stroke (Figure 1). For all stroke endpoints, we constructed 4 models. Model 0 was an unadjusted model. Model 1 was adjusted for age, sex, and race/study center. Model 2 was additionally adjusted for smoking status, body mass index, systolic and diastolic blood pressure, use of antihypertensive medication, diabetes, coronary heart disease, left ventricular hypertrophy, heart failure, use of anticoagulants. Model 3 was additionally adjusted for AF. To account for changes in covariates over time, all covariates in Models 1 and 2 were updated until the development of aPWA, censoring, or definite ischemic stroke, whichever occurred first. To determine whether our findings were mediated by development of AF, we updated AF until the time point just before the end of follow-up (Model 3). Finally, we conducted race and sex-stratified analysis for definite ischemic stroke using Model 3.
The proportional hazards assumption was assessed with scaled Schoenfeld residuals for both graphical and numerical tests, time interaction terms, and inspection of log negative log survival curves. Modeling assumptions were not violated in any model. Statistical analysis of ARIC data was performed using SAS version 9.3 (SAS Institute Inc., Cary, NC) and STATA 13.0 (StataCorp LP, College Station, TX). All P values reported are 2-sided, and statistical significance threshold was chosen as 0.05.
Results
We identified 657 cases of definite ischemic stroke over mean follow up time of 20.2 years. There were 1253 cases of prevalent aPWA and 1445 cases of incident aPWA. The baseline characteristics of participants are listed in Table 1. Participants with aPWA were more likely to be current smokers and on anti-coagulant medications.
Table 1.
Baseline Participant Characteristics by baseline P-wave axis status Atherosclerosis Risk in Communities Study, 1987–1989
| Characteristic* | Normal PWA (n=13,849) | aPWA (n=1,253) | P-Value |
|---|---|---|---|
| Age, mean (SD), years | 54.1 (5.7) | 55.1 (5.8) | <0.0001 |
| Female | 7629 (55%) | 694 (55%) | 0.83 |
| Black race | 3669 (26%) | 309 (25%) | 0.16 |
| Current smoker | 3488 (25%) | 445 (36%) | <0.0001 |
| Body mass index, mean (SD) kg/m2 | 27.9 (5.3) | 25.0 (5.3) | <0.0001 |
| Diabetes | 1641 (12%) | 115 (9%) | 0.005 |
| Hypertension medications | 4246 (31%) | 308 (25%) | <0.0001 |
| Systolic blood pressure, mmHg mean, (SD) | 121.3 (18.7) | 120.4 (19.8) | 0.12 |
| Diastolic blood pressure, mmHg mean, (SD) | 73.9 (11.2) | 72.2 (11.9) | <0.0001 |
| Heart failure | 623 (5%) | 66 (5%) | 0.21 |
| Coronary heart disease | 638 (5%) | 73 (6%) | 0.05 |
| Use of anticoagulants | 53 (0.4%) | 20 (2%) | <0.0001 |
| Left ventricular hypertrophy | 300 (2%) | 27 (2%) | 0.98 |
Data are presented as no. (%) unless as otherwise stated
Abbreviations: P-wave axis (PWA), abnormal P-wave axis (aPWA), standard deviation (SD)
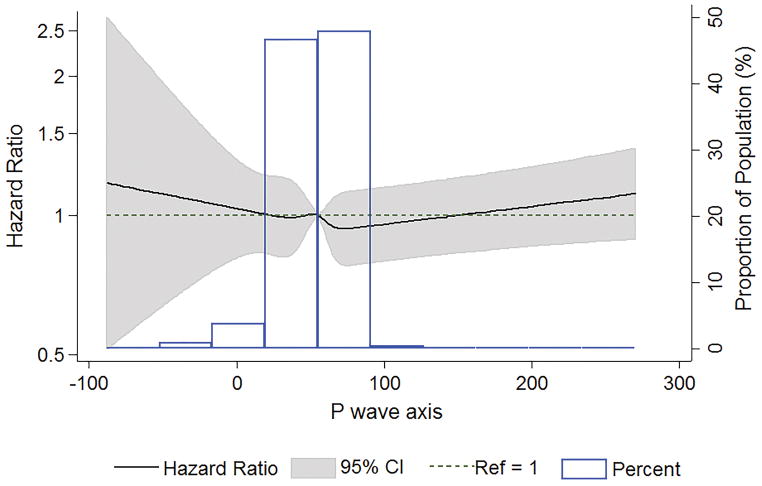
Figure 2 displays the association between baseline P-wave axis and definite ischemic stroke modeled as an unadjusted restricted cubic spline. The risk of ischemic stroke appeared to increase linearly at a P-wave axis of <32 degrees and >72 degrees. Table 2 lists the results of our Cox proportional hazards models for our primary endpoint, definite ischemic stroke. Compared with participants without aPWA, those with aPWA had a higher incidence of definite ischemic stroke. aPWA was associated with an increased risk of definite ischemic stroke after adjustment for sex, age, race/study center (Table 2, Model 1). This association remained significant after adjustment for stroke risk factors (Table 2, Model 2) and AF (Table 2, Model 3).
Figure 2.
Association between baseline P-wave axis and definite ischemic stroke modeled using an unadjusted restricted cubic spine. Data presented as hazard ratios (solid line) and 95% confidence intervals (shaded area).
Table 2.
Hazard Ratios of Abnormal P-wave Axis for Definite Ischemic Stroke, Atherosclerosis Risk in Communities Study, 1987–2013.
| Normal PWA (n=12,404) | aPWA (n=2698) | p-value | |
|---|---|---|---|
| Definite ischemic stroke (n=657) | 537 | 120 | |
| Person-years | 258,463 | 46,360 | |
| Incidence rate (95% CI)* | 2.08 (1.91–2.26) | 2.59 (2.16–3.08) | |
| HR (95% CI), Model 0† | 1 (REF) | 1.19 (0.97–1.45) | 0.09 |
| HR (95% CI), Model 1‡ | 1 (REF) | 1.44 (1.18–1.76) | 0.0004 |
| HR (95% CI), Model 2§ | 1 (REF) | 1.58 (1.29–1.94) | <0.0001 |
| HR (95% CI), Model 3|| | 1 (REF) | 1.50 (1.22–1.85) | 0.0001 |
per 1000 person-years
Unadjusted cox proportional hazard model
Model 0 + adjustment for age, sex, and race/study center variable updated until the development of aPWA, censoring, or definite ischemic stroke, whichever occurred first
Model 1 + adjustment for smoking status, body mass index, systolic and diastolic blood pressure, use of antihypertensive medication, diabetes, coronary heart disease, left ventricular hypertrophy, heart failure, use of anticoagulants updated until the development of aPWA, censoring, or definite ischemic stroke, whichever occurred first
Model 2 + atrial fibrillation updated to end of follow-up
Abbreviations: Confidence interval (CI), hazard ratio (HR), P-wave axis (PWA), abnormal P-wave axis (aPWA), reference (REF)
Table 3 lists the results of our Cox proportional hazards models for definite ischemic stroke subtypes—definite thrombotic stroke and definite cardioembolic stroke. The incidence rate of both subtypes was greater in participants with aPWA compared to those with normal P-wave axis. aPWA was associated with both subtypes after adjustment for age, sex, race/study center (Table 3, Model 1) as well as stroke risk factors (Table 3, Model 2) and AF (Table 3, Model 3). Of note, the effect size was larger for definite cardioembolic stroke relative to definite thrombotic stroke. In Model 3, aPWA was associated with a 2.04-fold (95% CI 1.42–2.95) increased risk of definite cardioembolic stroke compared to a 1.32-fold (95% CI 1.03–1.71) increased risk of definite thrombotic stroke.
Table 3.
Hazard Ratios of Abnormal P-wave Axis for Definite Ischemic Stroke Sub-Type, Atherosclerosis Risk in Communities Study, 1987–2013.
| Normal PWA (n=12,204) | aPWA (n=2698) | P-value | |
|---|---|---|---|
| Definite thrombotic stroke (n=481) | 405 | 76 | |
| Person-years | 258,463 | 46,360 | |
| Incidence rate (95% CI)* | 1.57 (1.42–1.73) | 1.64 (1.30–2.04) | |
| HR (95% CI), Model 0† | 1 (REF) | 1.01 (0.79–1.30) | 0.92 |
| HR (95% CI), Model 1‡ | 1 (REF) | 1.18 (0.92–1.52) | 0.19 |
| HR (95% CI), Model 2§ | 1 (REF) | 1.30 (1.01–1.67) | 0.04 |
| HR (95% CI), Model 3|| | 1 (REF) | 1.32 (1.03–1.71) | 0.03 |
| Definite cardioembolic stroke (n=176) | 132 | 44 | |
| Person-years | 258,463 | 46,360 | |
| Incidence rate (95% CI)* | 0.51 (0.43–0.60) | 0.95 (0.70–1.26) | |
| HR (95% CI), Model 0† | 1 (REF) | 1.69 (1.20–2.37) | 0.003 |
| HR (95% CI), Model 1‡ | 1 (REF) | 2.31 (1.63–3.28) | <0.0001 |
| HR (95% CI), Model 2§ | 1 (REF) | 2.55 (1.78–3.65) | <0.0001 |
| HR (95% CI), Model 3|| | 1 (REF) | 2.04 (1.42–2.95) | 0.0001 |
per 1000 person-years
Unadjusted cox proportional hazard model
Model 0 + adjustment for age, sex, and race/study center variable updated until the development of aPWA, censoring, or definite ischemic stroke, whichever occurred first
Model 1 + adjustment for smoking status, body mass index, systolic and diastolic blood pressure, use of antihypertensive medication, diabetes, coronary heart disease, left ventricular hypertrophy, heart failure, use of anticoagulants updated until the development of aPWA, censoring, or definite ischemic stroke, whichever occurred first
Model 2 + atrial fibrillation updated to end of follow-up
Abbreviations: Confidence interval (CI), hazard ratio (HR), P-wave axis (PWA), abnormal P-wave axis (aPWA), reference (REF)
Table 4 lists the results of our Cox proportional hazards for definite thrombotic stroke subtypes—definite thrombotic lacunar stroke and definite thrombotic non-lacunar stroke. Compared with participants with normal PWA, participants with aPWA had a higher incidence rate of definite thrombotic non-lacunar stroke, but not definite thrombotic lacunar stroke. aPWA was associated with definite thrombotic non-lacunar stroke after adjustment for age, sex, race/study center (Table 4, Model 1) as well as stroke risk factors (Table 4, Model 2) and AF (Table 4, Model 3). aPWA was not associated with definite thrombotic lacunar stroke in any of our models.
Table 4.
Hazard Ratios of Abnormal P-wave Axis for Definite Thrombotic Stroke Sub-Type, Atherosclerosis Risk in Communities Study, 1987–2013.
| Normal PWA (n=12,204) | aPWA (n=2698) | P-value | |
|---|---|---|---|
| Lacunar Stroke Cases (n=186) | 159 | 27 | |
| Person-years | 258,463 | 46,360 | |
| Incidence Rate (95% CI)* | 0.62 (0.53–0.72) | 0.58 (0.39–0.83) | |
| HR (95% CI), Model 0† | 1 (REF) | 0.91 (0.60–1.37) | 0.65 |
| HR (95% CI), Model 1‡ | 1 (REF) | 1.03 (0.68–1.56) | 0.89 |
| HR (95% CI), Model 2§ | 1 (REF) | 1.11 (0.72–1.69) | 0.64 |
| HR (95% CI), Model 3|| | 1 (REF) | 1.14 (0.75–1.75) | 0.53 |
| Non-Lacunar Stroke Cases (n=269) | 220 | 49 | |
| Person-years | 258,463 | 46,360 | |
| Incidence Rate (95% CI)* | 0.85 (0.74–0.97) | 1.06 (0.79–1.39) | |
| HR (95% CI), Model 0† | 1 (REF) | 1.20 (0.88–1.64) | 0.24 |
| HR (95% CI), Model 1‡ | 1 (REF) | 1.45 (1.06–1.99) | 0.02 |
| HR (95% CI), Model 2§ | 1 (REF) | 1.64 (1.19–2.26) | 0.003 |
| HR (95% CI), Model 3|| | 1 (REF) | 1.65 (1.20–2.29) | 0.002 |
per 1000 person-years
Unadjusted cox proportional hazard model
Model 0 + adjustment for age, sex, and race/study center variable updated until the development of aPWA, censoring, or definite ischemic stroke, whichever occurred first
Model 1 + adjustment for smoking status, body mass index, systolic and diastolic blood pressure, use of antihypertensive medication, diabetes, coronary heart disease, left ventricular hypertrophy, heart failure, use of anticoagulants updated until the development of aPWA, censoring, or definite ischemic stroke, whichever occurred first
Model 2 + atrial fibrillation updated to end of follow-up
Abbreviations: Confidence interval (CI), hazard ratio (HR), P-wave axis (PWA), abnormal P-wave axis (aPWA), reference (REF)
The results of our sex and race-stratified analyses are listed in Supplemental Table I. We did not find any sex or race-based interactions with the definite ischemic stroke endpoint (Supplemental Table I).
Discussion
In this large cohort of middle-aged, community-dwelling white and black individuals, we found that aPWA was significantly associated with increased risk of ischemic stroke independent of stroke risk factors including AF. This association appeared stronger for cardioembolic stroke compared with thrombotic stroke and thrombotic non-lacunar stroke compared with thrombotic lacunar stroke.
P-wave axis shift has been associated with left atrial enlargement on echocardiography and cardiac magnetic resonance imaging.20 There are limited data, however, on long-term clinical outcomes associated with aPWA. Recent analyses from the Cardiovascular Health Study and the Third National Health and Nutrition Examination Survey have indicated that aPWA is an independent risk factor for incident AF6 and cardiovascular and all-cause mortality.21 Our study is the first to demonstrate that aPWA is independently associated with ischemic stroke.
Analysis of P-wave morphology has been utilized to detect adverse atrial remodeling. Such atriopathy, which includes chamber enlargement and myocardial fibrosis, been recognized as a risk factor for AF22–29 as well as a critical component for thrombogenesis in the atrium.2,30 In fact, results from the Framingham Study indicate that increased left atrial size is an independent risk factor for stroke.31 Interestingly, our results indicate that the association between aPWA and ischemic stroke is not mediated by the development of AF. Rather, aPWA may reflect an intrinsically pro-thrombotic substrate.
The association between aPWA and stroke appeared strongest with definite cardioembolic stroke and definite thrombotic non-lacunar stroke. There was no association with definite thrombotic lacunar strokes. Theoretically, these deep brain infarcts are less likely to result from thromboembolism compared with non-lacunar infarcts.32–35 Thus, our findings support that aPWA may be associated with an embolic mechanism.
In the ARIC study, abnormal P-wave terminal force in V1 and advanced interatrial block have been associated with 1.3-fold36 and 1.70-fold3 increased risk of ischemic stroke independent of AF, respectively. Abnormal P-wave terminal force in V1 was also not associated with lacunar infarcts. We demonstrate that aPWA is associated with a comparable stroke risk. Our findings contribute to the emerging evidence that underscores the potential value of ECG-based markers for prediction of atrial thromboembolism irrespective of heart rhythm. Unlike other P-wave indices, P-wave axis is a routine ECG parameter that does not require digitization and specialized analytic software, thus lending itself for easy translation to clinical use.
Current stroke prediction models for patients with AF (CHA2DS2-VASc. etc) have modest discriminatory capacity.30,37,38 Markers that reflect atriopathy early in the disease course may improve stroke prediction in patients with AF. If our findings are replicated in other cohorts, further research to define the biological correlates of aPWA and to determine whether aPWA can improve stroke prediction should be conducted.
The principal strengths of our study include a large cohort with long follow-up duration, extensive measurement of covariates, and rigorous physician-adjudication of stroke. Some limitations should be noted. First, although we adjusted for potential confounders in our analyses, we cannot exclude residual confounding by imperfectly measured and unmeasured factors. Second, we were unable to account for subclinical AF or AF that was not detected on study visit ECGs, hospital discharge data, or death certificates. However, AF incidence in ARIC is consistent with other population-based studies and utilizing hospital discharge records for the purposes of AF detection has been previously validated in ARIC.39–42
Conclusion
In conclusion, our report—based on a large biracial population based-cohort study of middle-aged individuals—suggests that aPWA is associated with an increased risk of ischemic cardioembolic stroke independent of stroke risk factors including AF. If these findings are validated, further studies to investigate the clinical utility of aPWA in stroke prediction are warranted.
Supplementary Material
Acknowledgments
Funding Sources
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). WTO is supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number F32HL134290. Dr. Chen is supported by R01HL126637. Additional support was provided by grant 16EIA26410001.
The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
Disclosures
None
Contributor Information
Ankit Maheshwari, Cardiovascular Division, Department of Medicine, University of Minnesota, 420 Delaware Street SE, MMC 508, Minneapolis, MN 55455.
Faye L. Norby, Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, 420 Delaware St SE Mmc88, Minneapolis, MN 55455.
Elsayed Z. Soliman, Epidemiological Cardiology Research Center (EPICARE), Wake Forest School of Medicine, 1 Medical Center Blvd, Winston-Salem, NC 27103.
Ryan J. Koene, Cardiovascular Division, Department of Medicine, University of Minnesota, 420 Delaware Street SE, MMC 508, Minneapolis, MN 55455.
Mary R Rooney, Division of Epidemiology and Community Health, School of Public Health, University of Minnesota, 420 Delaware St SE Mmc88, Minneapolis, MN 55455.
Wesley T O’Neal, Department of Medicine, Division of Cardiology, Emory University School of Medicine, 101 Woodruff Circle, Woodruff Memorial Building, Atlanta, GA 30322.
Alvaro Alonso, Department of Epidemiology, Rollins School of Public Health, Emory University, 1518 Clifton Rd NE, Atlanta, GA.
Lin Y. Chen, Cardiovascular Division, Department of Medicine, University of Minnesota, 420 Delaware Street SE, MMC 508, Minneapolis, MN 55455.
References
- 1.Katsnelson M, Koch S, Rundek T. Stroke Prevention in Atrial Fibrillation. J Atr Fibrillation. 2007;3:53–64. doi: 10.4022/jafib.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watson T, Shantsila E, Lip GY. Mechanisms of thrombogenesis in atrial fibrillation: Virchow’s triad revisited. Lancet. 2009;373:155–166. doi: 10.1016/S0140-6736(09)60040-4. [DOI] [PubMed] [Google Scholar]
- 3.O’Neal WT, Kamel H, Zhang ZM, Chen LY, Alonso A, Soliman EZ. Advanced interatrial block and ischemic stroke: The Atherosclerosis Risk in Communities Study. Neurology. 2016;87:352–356. doi: 10.1212/WNL.0000000000002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Neal WT, Zhang ZM, Loehr LR, Chen LY, Alonso A, Soliman EZ. Electrocardiographic Advanced Interatrial Block and Atrial Fibrillation Risk in the General Population. Am J Cardiol. 2016;117:1755–1759. doi: 10.1016/j.amjcard.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC. Ethnic distribution of ecg predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the atherosclerosis risk in communities (ARIC) study. Stroke. 2009;40:1204–1211. doi: 10.1161/STROKEAHA.108.534735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rangel MO, O’Neal WT, Soliman EZ. Usefulness of the Electrocardiographic P-Wave Axis as a Predictor of Atrial Fibrillation. Am J Cardiol. 2015 doi: 10.1016/j.amjcard.2015.10.013. [DOI] [PubMed]
- 7.The ARIC Investigators. The Atherosclerosis Risk In Communities (ARIC) Study: Design and Objectives. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 8.Wagner G. Marriott’s Practical Electrocardiography. 11. Philadelphia, PA: Lippincott Williams and Wilkins; 2007. [Google Scholar]
- 9.Robins MWF. The National Survey of Stroke. Study design and methodology. Stroke. 1981;12:I7–11. [PubMed] [Google Scholar]
- 10.Rosamond WD, Folsom AR, Chambless LE, Wang C, Mcgovern PG, Howard G, et al. Stroke Incidence and Survival Among Middle-Aged Adults. Stroke. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 11.Atherosclerosis Risk in Communities Study. Manual 3: Surveillance component procedures, Version 6.2. Chapel Hill, NC: Atherosclerosis Risk In Communities Study; 2009. [Google Scholar]
- 12.Folsom AR, Yamagishi K, Hozawa A, Chambless LE. Absolute and attributable risks of heart failure incidence in relation to optimal risk factors. Circ Hear Fail. 2009;2:11–17. doi: 10.1161/CIRCHEARTFAILURE.108.794933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Heart, Lung, and Blood Institute. Atherosclerosis Risk in Communities (ARIC) study Operations Manual No. 5: Electrocardiograpy. Version 1.0. Chapel Hill, NC: ARIC Coordinationg Center, School of Public Health, University of North Carolina; 1987. [Google Scholar]
- 14.Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities Study) Am J Cardiol. 2008;101:1016–1022. doi: 10.1016/j.amjcard.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 15.Ndumele CE, Matsushita K, Lazo M, Bello N, Blumenthal RS, Gerstenblith G, et al. Obesity and Subtypes of Incident Cardiovascular Disease. J Am Heart Assoc. 2016;5:e003921. doi: 10.1161/JAHA.116.003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crow RS, Prineas RJ, Rautaharju P, Hannan P, Liebson PR. Relation between electrocardiography and echocardiography for left ventricular mass in mild systemic hypertension: results from Treatment of Mild Hypertension Study. Am J Cardiol. 1995;25:2377–2383. [PubMed] [Google Scholar]
- 17.Folsom AR, Yatsuya H, Psaty BM, Shahar E, Longstreth WT. Carotid Intima-Media Thickness, Electrocardiographic Left Ventricular Hypertrophy, and Incidence of Intracerebral Hemorrhage. Stroke. 2011;42:3075–3079. doi: 10.1161/STROKEAHA.111.623157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao D, Carnethon M, Evans GW, Cascio WE, Heiss G. Lower Heart Rate Variability Is Associated With the Development of Coronary Heart Disease in Individuals With Diabetes: The Atherosclerosis Risk in Communities (ARIC) Study. Diabetes. 2002;51:3524–3531. doi: 10.2337/diabetes.51.12.3524. [DOI] [PubMed] [Google Scholar]
- 19.Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsao CW, Josephson ME, Hauser TH, O’Halloran TD, Agarwal A, Manning WJ, et al. Accuracy of electrocardiographic criteria for atrial enlargement: validation with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2008;10:7. doi: 10.1186/1532-429X-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Shah AJ, Soliman EZ. Effect of electrocardiographic P-wave axis on mortality. Am J Cardiol. 2014;113:372–6. doi: 10.1016/j.amjcard.2013.08.050. [DOI] [PubMed] [Google Scholar]
- 22.Boldt A, Wetzel U, Lauschke J, Weigl J, Gummert J, Hindricks G, et al. Fibrosis in left atrial tissue of patients with atrial fibrillation with and without underlying mitral valve disease. Heart. 2004;90:400–405. doi: 10.1136/hrt.2003.015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Everett T, 4th, Olgin J. Atrial fibrosis and the mechanisms of atrial fibrillation. Hear Rhythm. 2006;4:S24–27. doi: 10.1016/j.hrthm.2006.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burstein B, Nattel S. Atrial fibrosis: mechanisms and clinical relevance in atrial fibrillation. J Am Coll Cardiol. 2008;51:802–809. doi: 10.1016/j.jacc.2007.09.064. [DOI] [PubMed] [Google Scholar]
- 25.Chimenti C, Russo M, Carpi A, Frustaci A. Histological substrate of human atrial fibrillation. Biomed Pharmacother. 2010;64:177–183. doi: 10.1016/j.biopha.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Kim S, Choisy S, Barman P, Zhang H, Hancox J, Jones S, et al. Atrial remodeling and the substrate for atrial fibrillation in rat hearts with elevated afterload. Circ Arrhythm Electrophysiol. 2011;4:761–769. doi: 10.1161/CIRCEP.111.964783. [DOI] [PubMed] [Google Scholar]
- 27.Tan A, Zimetbaum P. Atrial fibrillation and atrial fibrosis. Pharmacol. 2011;57:625–629. doi: 10.1097/FJC.0b013e3182073c78. [DOI] [PubMed] [Google Scholar]
- 28.Kottkamp H. Atrial fibrillation substrate: the “unknown species”– from lone atrial fibrillation to fibrotic atrial cardiomyopathy. Hear Rhythm. 2012;9:481–482. doi: 10.1016/j.hrthm.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 29.Koduri H, Ng J, Cokic I, Aistrup G, Gordon D, Wasserstrom J, et al. Contribution of fibrosis and the autonomic nervous system to atrial fibrillation electrograms in heart failure. Circ Arrhythm Electrophysiol. 2012;5:640–649. doi: 10.1161/CIRCEP.111.970095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldberger JJ, Arora R, Green D, Greenland P, Lee DC, Lloyd-Jones DM, et al. Evaluating the atrial myopathy underlying atrial fibrillation: Identifying the arrhythmogenic and thrombogenic substrate. Circulation. 2015;132:278–291. doi: 10.1161/CIRCULATIONAHA.115.016795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benjamin EJ, D’Agostino RB, Belanger AJ, Wolf PA, Levy D. Left Atrial Size and the Risk of Stroke and Death. Circulation. 1995;92:835–841. doi: 10.1161/01.cir.92.4.835. [DOI] [PubMed] [Google Scholar]
- 32.Inzitari D, Eliasziw M, Sharpe B, Fox A, Barnett H. Risk factors and outcome of patients with carotid artery stenosis presenting with lacunar stroke. North American Symptomatic Carotid Endarterectomy Trial Group. Neurology. 2000;54:660–666. doi: 10.1212/wnl.54.3.660. [DOI] [PubMed] [Google Scholar]
- 33.Besson G, Hommel M, Perret J. Historical aspects of the lacunar concept. Cerebrovasc Dis. 1991;1:306–310. [Google Scholar]
- 34.Helling T, Duke P, Beggs C, Crouse L. A prospective evaluation of 68 patients suffering blunt chest trauma for evidence of cardiac injury. J Trauma. 1989;29:961–6. doi: 10.1097/00005373-198907000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Futrell N. Lacunar Infarction: Embolism is the Key. Stroke. 2004;35:1778–1779. doi: 10.1161/01.STR.0000131930.41057.48. [DOI] [PubMed] [Google Scholar]
- 36.Kamel H, O’Neal WT, Okin PM, Loehr LR, Alonso A, Soliman EZ. Electrocardiographic left atrial abnormality and stroke subtype in the atherosclerosis risk in communities study. Ann Neurol. 2015;78:670–678. doi: 10.1002/ana.24482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Van Staa TP, Setakis E, Di Tanna GL, Lane DALG. A comparison of risk stratification schemes for stroke in 79,884 atrial fibrillation patients in general practice. J Thromb Haemost. 2011;9:39–48. doi: 10.1111/j.1538-7836.2010.04085.x. [DOI] [PubMed] [Google Scholar]
- 38.Lip G, Nieuwlaat R, Pisters R, Lane D, Crijns H. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 39.Miyasaka Y, Barnes ME, Gersh BJ, Cha SS, Bailey KR, Abhayaratna WP, et al. Secular trends in incidence of atrial fibrillation in Olmsted County, Minnesota, 1980 to 2000, and implications on the projections for future prevalence. Circulation. 2006;114:119–125. doi: 10.1161/CIRCULATIONAHA.105.595140. [DOI] [PubMed] [Google Scholar]
- 40.Benjamin E, Levy D, Vaziri S, D’Agostino R, Belanger A, Wolf P. Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 41.Psaty B, Manolio T, Kuller L, Kronmal R, Cushman M, Fried L, et al. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–61. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 42.Alonso A, Agarwal SK, Soliman E, Ambrose M, Chamberlain AM, Prineas RJ, et al. Incidence of atrial fibrillation in whites and African-Americans: the Atherosclerosis Risk in Communities (ARIC) Study. Am Hear J. 2009;158:111–117. doi: 10.1016/j.ahj.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.