Abstract
Objective
There is inconsistent evidence that zidovudine use during pregnancy increases overall, cardiac, and male genital malformations.
Design
We conducted a systematic review and meta-analysis of zidovudine use and malformations and, using Bayesian methods, combined it with data from a cohort study of mother-infant pairs in the nationwide Medicaid Analytic eXtract (MAX).
Methods
Using MAX data (2000–2010), we identified pregnant women with HIV treated with antiretroviral therapy (ART). Women with ≥1 zidovudine dispensing during the first trimester were compared to women receiving ART without zidovudine in the first trimester. Malformation outcomes were defined using diagnosis/procedure codes. To adjust for confounding, we performed 1:1 propensity score matching. Bayesian methods require specification of a prior, which we developed in the meta-analysis. Logistic regression models combined MAX data with the prior, estimating odds ratios (ORs) and 95% credible intervals.
Results
Fourteen articles contributed information on overall malformations, 7 on cardiac malformations, and 5 on male genital malformations. In MAX, matching led to a sample of 735 women each in the zidovudine and comparator groups. When comparing first trimester zidovudine use to other ART, the Bayesian procedure yielded OR estimates slightly above the null for overall (OR=1.11, 95% credible interval [0.80–1.55]) and cardiac (OR=1.30 [0.63–2.71]) malformations. There were no zidovudine-exposed cases of male genital malformations in MAX, but the meta-analysis yielded elevated OR estimates (OR=2.57 [1.26–5.24]).
Conclusions
For most malformations, first trimester zidovudine was not associated with increased risk. The potential increase in male genital malformations was small in absolute terms, and should be evaluated further.
INTRODUCTION
Use of antiretroviral drugs during pregnancy has dramatically reduced the risk of perinatal transmission of HIV to less than 1%.[1,2] However, there are lingering concerns about the safety of specific antiretroviral agents when used during pregnancy, and careful evaluation of the risks associated with specific drugs is needed to inform treatment decisions. Zidovudine is one antiretroviral agent that is frequently used to treat HIV during pregnancy, though it is no longer a component of the preferred first-line treatment, largely due to programmatic reasons unrelated to safety.[3]
Some epidemiological studies have found that zidovudine use, especially in the first trimester, is associated with modest elevations in the risk of overall malformations,[4] cardiac malformations,[5–7] and male genital malformations.[8,9] However, a number of other studies have not replicated the increased risk.[10–15] There are several potential explanations for these seemingly inconsistent results, including heterogeneity in study design, exposure definition, and outcome measurement. Further, because malformations are rare events, individual studies may lack the power to detect differences in risk, especially for specific malformation subgroups.
To provide more robust estimates of the association between zidovudine and overall, cardiac, and male genital malformations, we used Bayesian methods, which allow us to formally incorporate existing knowledge about an association into an analysis of new data. We conducted a systematic review and meta-analysis to develop a prior distribution for the risk of malformations associated with zidovudine, and incorporated data from the Medicaid Analytic eXtract (MAX) to provide a comprehensive assessment of the evidence available on this safety concern.
METHODS
Study population
This study used data from the Medicaid Analytic eXtract (MAX), a collection of enrollment information and healthcare claims for Medicaid beneficiaries nationwide in the United States. We had access to claims for inpatient and outpatient diagnoses and procedures, as well as outpatient pharmacy dispensing, from 2000–2010. An estimated 45% of all deliveries that occur in the United States are covered by Medicaid.[16]
We identified a cohort of pregnancies in MAX where distinct mothers and infants could be matched. The creation of this cohort has previously been described in detail,[17] and has been used in multiple studies of prescription drug safety during pregnancy[18–21]. Briefly, women between the ages of 12 and 55 years old with a code indicating a delivery were identified and linked to live-born infants based on shared family case numbers. We removed infants linked to more than one woman, as well as deliveries that were unreasonably close in time. The sample was restricted to women continuously enrolled in Medicaid, without supplementary private insurance or restricted benefits, for 3 months prior to the estimated last menstrual period (LMP) through 30 days after delivery, and infants were required to be continuously enrolled for 90 days after delivery or until death, whichever occurred sooner. We estimated LMP using an algorithm which correctly classifies pregnancy duration within 2 weeks in 99% of term and 75% of preterm deliveries.[22] For infants without diagnosis or procedure codes that indicated prematurity, date of LMP was 270 days prior to the delivery date. For preterm deliveries, date of LMP was 245 days prior to the delivery date. The first trimester was defined as the 90-day period after LMP, the second trimester as the period from 91 to 180 days after LMP, and the third trimester as the period from 181 days after LMP through delivery.
We included women who met any of our diagnostic criteria for HIV infection: (a) ≥2 claims for an HIV diagnosis; (b) ≥1 claim for HIV diagnosis and ≥1 HIV-related procedure; or (c) ≥1 claim for HIV diagnosis and ≥2 dispensings of antiretroviral drugs (see Supplemental Digital Content Table S1 for diagnostic and procedure codes). We further limited the sample to women who received some form of antiretroviral therapy (ART) during pregnancy, defined by at least one dispensing of an antiretroviral medication between LMP and delivery. We applied this restriction to create a comparative safety study design with an active comparator group, which produces results that are useful for clinical decision making and less susceptible to confounding by indication.[23]
Exposure and outcome definitions
A pregnancy was defined as having zidovudine exposure during the first trimester if at least one prescription for the drug was dispensed during the first trimester. Women in the exposed group were commonly co-prescribed other antiretroviral drugs in addition to zidovudine. The comparison group was comprised of pregnancies where the ART received did not include any dispensings of zidovudine during the first trimester.
Infant malformations were identified in the 90-day post-delivery period. An organ system was defined as having a malformation if there were at least two recorded diagnostic codes on separate dates for an anomaly in the organ system (or a diagnostic code and a surgical code), either from maternal or infant records, or one code and a recorded infant death within three months of delivery (see Supplemental Digital Content Table S2 for full list of codes). In this analysis, we focused on three outcomes: overall malformations from any organ system, cardiac malformations, and male genital malformations. A validation study found that cardiac malformations identified in MAX had a positive predictive value of 78%.[24]
Confounding and adjustment
We considered a variety of risk factors for malformations as potential confounders, including maternal demographic characteristics, markers of HIV disease severity, comorbid medical conditions (including the Obstetric Comorbidity Index[25]), obstetric characteristics, and prescription drugs. Confounders were defined in the 3-month baseline period prior to LMP and the first trimester.
To adjust for confounding, propensity scores were used to match each exposed pregnancy to an unexposed pregnancy. Propensity scores were calculated using a logistic regression model that estimated the probability of being dispensed zidovudine in the first trimester based on confounder values. All variables listed in Table 2 were included in the propensity score. We performed 1:1 fixed-ratio matching using a greedy algorithm,[26] based on the logit transformation of the propensity score. To minimize residual confounding, we used a caliper of 0.2 times the standard deviation of the logit transformation of the propensity score.[27]
Table 2.
Baseline characteristics for pregnant women living with HIV in Medicaid Analytic eXtract sample
| Full cohort | Matched cohort | |||||||
|---|---|---|---|---|---|---|---|---|
| Zidovudine in Trimester 1 (N = 824) | No zidovudine in Trimester 1 (N = 1,998) | Zidovudine in Trimester 1 (N = 735) | No zidovudine in Trimester 1 (N = 735) | |||||
| Categorical variables | n | % | n | % | n | % | n | % |
| Age | ||||||||
| 12–18 years | 29 | 3.5 | 122 | 6.1 | 28 | 3.8 | 26 | 3.5 |
| 19–25 years | 289 | 35.1 | 813 | 40.7 | 261 | 35.5 | 267 | 36.3 |
| 26–35 years | 390 | 47.3 | 841 | 42.1 | 343 | 46.7 | 357 | 48.6 |
| 36–55 years | 116 | 14.1 | 222 | 11.1 | 103 | 14.0 | 85 | 11.6 |
| Race/ethnicity | ||||||||
| White | 125 | 15.2 | 251 | 12.6 | 111 | 15.1 | 113 | 15.4 |
| Black/African American | 527 | 64.0 | 1,465 | 73.3 | 481 | 65.4 | 483 | 65.7 |
| Hispanic/Latino | 45 | 5.5 | 54 | 2.7 | 32 | 4.4 | 30 | 4.1 |
| Other/Unknown | 127 | 15.4 | 228 | 11.4 | 111 | 15.1 | 109 | 14.8 |
| Year of delivery | ||||||||
| 2000–2003 | 171 | 20.8 | 366 | 18.3 | 148 | 20.1 | 135 | 18.4 |
| 2004–2006 | 352 | 42.7 | 717 | 35.9 | 313 | 42.6 | 312 | 42.4 |
| 2007–2010 | 301 | 36.5 | 915 | 45.8 | 274 | 37.3 | 288 | 39.2 |
| Evidence of parity | 169 | 20.5 | 298 | 14.9 | 136 | 18.5 | 129 | 17.6 |
| Baseline antiretroviral dispensing | 481 | 58.4 | 460 | 23.0 | 394 | 53.6 | 383 | 52.1 |
| Diarrhea | 19 | 2.3 | 38 | 1.9 | 14 | 1.9 | 19 | 2.6 |
| Parasitic/fungal infection | 42 | 5.1 | 97 | 4.9 | 39 | 5.3 | 38 | 5.2 |
| Hepatitis C | 13 | 1.6 | 24 | 1.2 | 11 | 1.5 | 14 | 1.9 |
| Herpes simplex virus | 14 | 1.7 | 41 | 2.1 | 13 | 1.8 | 12 | 1.6 |
| Sexually transmitted infection | 54 | 6.6 | 163 | 8.2 | 51 | 6.9 | 51 | 6.9 |
| Overweight/obese | 21 | 2.5 | 38 | 1.9 | 17 | 2.3 | 17 | 2.3 |
| Hypertension | 42 | 5.1 | 98 | 4.9 | 35 | 4.8 | 41 | 5.6 |
| Diabetes | 32 | 3.9 | 50 | 2.5 | 27 | 3.7 | 30 | 4.1 |
| Dyslipidemia | 13 | 1.6 | 22 | 1.1 | 11 | 1.5 | 11 | 1.5 |
| Bipolar disorder | 25 | 3.0 | 37 | 1.9 | 18 | 2.4 | 24 | 3.3 |
| Anxiety disorder | 28 | 3.4 | 50 | 2.5 | 25 | 3.4 | 25 | 3.4 |
| Depression | 111 | 13.5 | 162 | 8.1 | 93 | 12.7 | 86 | 11.7 |
| Other psychiatric disorder | 42 | 5.1 | 69 | 3.5 | 36 | 4.9 | 32 | 4.4 |
| Alcohol abuse | 28 | 3.4 | 34 | 1.7 | 22 | 3.0 | 20 | 2.7 |
| Tobacco use | 29 | 3.5 | 66 | 3.3 | 25 | 3.4 | 28 | 3.8 |
| Illicit drug abuse | 65 | 7.9 | 97 | 4.9 | 52 | 7.1 | 49 | 6.7 |
| Antidepressant dispensing | 164 | 19.9 | 190 | 9.5 | 125 | 17.0 | 127 | 17.3 |
| Anticonvulsant dispensing | 39 | 4.7 | 59 | 3.0 | 30 | 4.1 | 30 | 4.1 |
| Stimulant dispensing | 11 | 1.3 | 14 | 0.7 | <111 | -- | <111 | -- |
| Antibiotic dispensing | 500 | 60.7 | 1,080 | 54.1 | 443 | 60.3 | 454 | 61.8 |
| Antihypertensive dispensing | 54 | 6.6 | 110 | 5.5 | 46 | 6.3 | 50 | 6.8 |
| Insulin dispensing | 20 | 2.4 | 25 | 1.3 | 15 | 2.0 | 15 | 2.0 |
| Antidiabetes medication dispensing | 18 | 2.2 | 21 | 1.1 | 15 | 2.0 | 13 | 1.8 |
| NSAID dispensing | 201 | 24.4 | 405 | 20.3 | 176 | 23.9 | 192 | 26.1 |
| Acetaminophen dispensing | 219 | 26.6 | 486 | 24.3 | 194 | 26.4 | 200 | 27.2 |
| Benzodiazapine dispensing | 34 | 4.1 | 50 | 2.5 | 28 | 3.8 | 32 | 4.4 |
| Opioid dispensing | 192 | 23.3 | 423 | 21.2 | 173 | 23.5 | 175 | 23.8 |
| Progestins dispensing | 14 | 1.7 | 28 | 1.4 | 12 | 1.6 | 12 | 1.6 |
| Corticosteroid dispensing | 208 | 25.2 | 341 | 17.1 | 169 | 23.0 | 174 | 23.7 |
| Fluconazole dispensing | 119 | 14.4 | 209 | 10.5 | 103 | 14.0 | 117 | 15.9 |
| ACE inhibitor dispensing | 16 | 1.9 | 31 | 1.6 | 13 | 1.8 | 15 | 2.0 |
| Continuous variables | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
|
| ||||||||
| Generic medications (excluding ARVs) | 7.34 | 6.64 | 5.09 | 5.00 | 6.85 | 6.08 | 7.09 | 5.71 |
| Distinct diagnoses | 11.84 | 7.68 | 9.48 | 7.19 | 11.44 | 7.42 | 12.04 | 7.94 |
| Outpatient visits | 8.36 | 7.75 | 6.48 | 7.64 | 8.16 | 7.45 | 8.60 | 9.66 |
| Emergency department visits | 1.12 | 4.73 | 1.05 | 1.75 | 1.13 | 4.98 | 1.28 | 2.01 |
| Inpatient hospitalizations | 0.13 | 0.43 | 0.13 | 0.55 | 0.13 | 0.44 | 0.12 | 0.39 |
| HIV-related procedures | 0.02 | 0.15 | 0.02 | 0.13 | 0.03 | 0.16 | 0.02 | 0.16 |
| Obstetric Comorbidity Index | 1.84 | 1.38 | 1.17 | 1.35 | 1.75 | 1.34 | 1.77 | 0.43 |
Cell sizes of 10 or less have been suppressed in accordance with Centers for Medicare and Medicaid Services cell size suppression policy.
Development of a Bayesian prior
Bayesian methods require specification of a prior probability distribution for each parameter included in the model. This prior can be conceptualized as a summary of beliefs about the true value of a variable before considering any new data. In this way, Bayesian analyses allowed us to incorporate existing evidence about zidovudine exposure and risk of congenital malformations into our analysis of the MAX data.
To develop our prior, we conducted a systematic review and meta-analysis for studies that examined the relationship between use of zidovudine in pregnancy and our three outcomes of interest: overall congenital malformation, cardiac malformation, and male genital malformation. We searched MEDLINE via PubMed, EMBASE, and Cochrane CENTRAL for abstracts with terms related to “zidovudine” and “pregnancy/congenital malformations.” The references cited in all included studies were reviewed to identify additional eligible articles. Search criteria are described in detail in Supplemental Digital Content Table S3.
Articles were included if they were written in English and reported sufficient information to calculate an odds ratio (OR) for zidovudine exposure during pregnancy and one of the outcomes of interest (overall, cardiac, and/or male genital malformation). We excluded conference abstracts, animal studies, basic science research, case reports, case series, and commentaries. When multiple reports were published from the same study, we only included the most recent publication to avoid duplication. In secondary analyses, we further restricted the meta-analysis to studies that defined exposure to zidovudine in the first trimester, had a comparison group that received ART, and controlled for confounding.
Two authors (KR, JWS) each screened the titles and abstracts of all identified articles according to the inclusion and exclusion criteria listed above. For articles passing the initial screen, the two authors independently performed full text review, finalized inclusion decisions, and extracted the relevant information using a standardized form. All discrepancies were resolved through discussion until reaching a consensus.
A random-effects meta-analysis was performed using the DerSimonian and Laird method[28] to summarize findings and construct a prior, and results were reported in forest plots. The I2 metric was computed to quantify between-study heterogeneity, and Egger’s test was performed to identify publication bias.[29,30] The meta-analysis was conducted using publically available user-written packages in Stata.[31,32]
Statistical analysis
The risk of each outcome was summarized in the full and matched MAX samples. Within the matched sample, we used a Bayesian approach to build a logistic model for the risk of malformation from exposure to zidovudine in pregnancy. Separate models were created for each malformation outcome. The prior distributions for the zidovudine-malformation relationships were set according to results of the meta-analysis, and a non-informative prior was specified for the model intercept term. Posterior estimates of the ORs and an accompanying 95% credible interval were developed using Markov Chain Monte Carlo methods. Because malformations are a rare outcome, the estimated OR closely approximates a risk ratio.[33,34] Bayesian analyses were performed in SAS, version 9.4 (SAS Institute, Cary, NC).
RESULTS
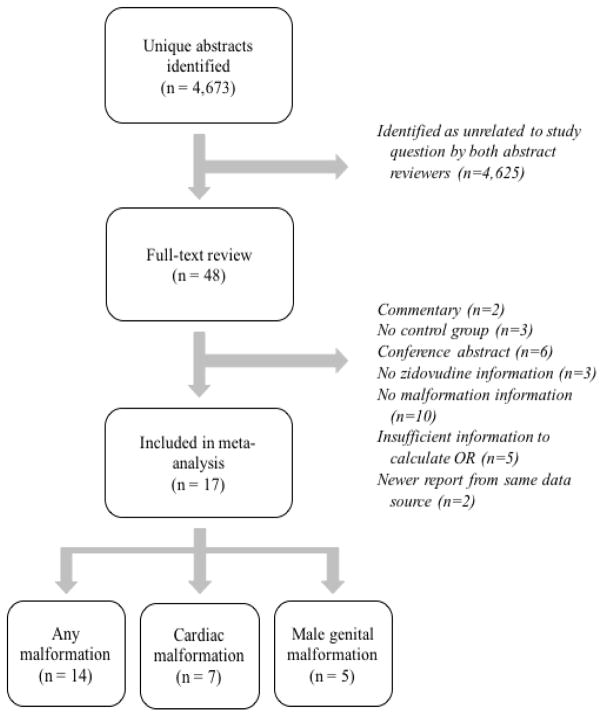
After removing duplicate references, the search strategy identified 4,673 unique citations, whose titles and abstracts were screened (Figure 1). After screening, 48 citations underwent a full-text review, and results from 17 articles were included in the meta-analysis.[4–15,35–39] For the outcome of overall malformation, 14 articles contributed information on over 27,239 infants with in utero zidovudine exposure and over 36,501 infants without zidovudine exposure. Seven studies contributed information on cardiac malformations (n=8,956 zidovudine exposed, n=15,100 unexposed), and 5 contributed information on male genital malformations (n=8,630 zidovudine exposed, n=4,643 unexposed). Study designs varied between articles: one study was a randomized controlled trial, while the remainder were observational cohorts; 14 defined exposure to zidovudine specifically in the first trimester; and 12 had control groups who received other forms of ART (Table 1). Nearly all studies were conducted in the United States or Europe.
Figure 1.
Flowchart of article inclusion in meta-analysis
Table 1.
Description of studies identified in systematic review and included in meta-analyses
| Citation | Cohort | Geographic setting | Years | Timing of zidovudine exposure | Comparison group | Malformation definitions | Number exposed | Number unexposed | Malformation outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Sperling et al 1998 | ACTG 076 | France, USA | 1991–1994 | T2/T3 | Placebo in T2/T31 | Not reported | 214 | 210 | Overall, Cardiac, Male genital |
| Newschaffer et al 2000 | New York Medicaid | New York, USA | 1993–1996 | T1 | No zidovudine in pregnancy1 | ICD-9 coding2 | Not reported | Not reported | Overall, Cardiac |
| Watts et al 2007 | WITS | USA | 1990–2004 | T1 | No zidovudine in T1 | MACDP guidelines & APR criteria | 621 | 1,289 | Overall, Male genital |
| Townsend et al 2009 | NSHPC | Ireland, UK | 1990–2007 | Anytime in pregnancy | No zidovudine in pregnancy | Reported by treating physician | 6,711 | 792 | Male genital |
| Brogly et al 2010 | PACTG 219/219C | USA | 1993–2006 | T1 | No zidovudine in T11 | MACDP guidelines & APR criteria2 | 605 | 1,428 | Overall, Cardiac |
| Joao et al 2010 | NISDI Perinatal Study | Argentina, Brazil | 2002–2007 | Anytime in pregnancy | No zidovudine in pregnancy | MACDP guidelines & APR criteria | 954 | 41 | Overall |
| Watts et al 2011 | PACTG 316 | Brazil, Bahamas, Europe, USA | 1997–2000 | T1 | No zidovudine in T1 | MACDP guidelines & APR criteria2 | 517 | 897 | Overall, Cardiac |
| Tariq et all 2011 | NSHPC and ECS | Europe | 2000–2009 | T1 | No zidovudine in T1 | ICD-10 coding | 1,077 | 1,477 | Overall |
| Knapp et al 2012 | IMPAACT P1025 | USA | 2002–2007 | T1 | No zidovudine in pregnancy | MACDP guidelines2 | 356 | 187 | Overall |
| Floridia et al 2013 | Italian NPSATP | Italy | 2001–2011 | T1 | No ART in T1 | MACDP guidelines & APR criteria | 358 | 561 | Overall, Cardiac, Male genital |
| Prieto et al 2014 | The Madrid Cohort | Madrid, Spain | 2000–2009 | T1 | No zidovudine in pregnancy1 | EUROCAT criteria | 287 | 189 | Overall |
| Sibiude et al 2014 | EPF ANRS CO1/CO11 | France | 1994–2010 | T1 | No zidovudine in pregnancy | ICD-10 coding according to EUROCAT criteria | 3,267 | 2152 | Overall |
| Phiri et al 2014 | Tennessee Medicaid | Tennessee, USA | 1994–2009 | T1 | No zidovudine in T11 | ICD-9 coding & vital record data according to MACDP guidelines2 | 156 | 650 | Overall |
| Sibiude et al 2015 | EPF ANRS CO1/CO11 | France | 1994–2010 | T1 | No zidovudine in T1 | Previously identified cardiac defects reviewed by pediatric cardiologist2 | 3,262 | 9626 | Cardiac |
| Williams et al 2015 | PHACS SMARTT | USA | 1995–2012 | T1 | No zidovudine in T1 | MACDP guidelines & APR criteria2 | 726 | 1,791 | Overall, Male genital |
| Vannappagari et al 2016 | APR | USA, 65 additional countries | 1989–2013 | T1 | No zidovudine in pregnancy | MACDP guidelines & APR criteria | 4,000 | 2,378 | Cardiac |
| APR Steering Committee 2016 | APR | USA, 65 additional countries | 1989–2016 | T1 | No zidovudine in T1 | MACDP guidelines & APR criteria | 4,128 | 12,833 | Overall |
Abbreviations: APR, Antiretroviral Pregnancy Registry; ACTG, AIDS Clinical Trials Group; WITS, Women and Infants Transmission Study; NSHPC, National Study of HIV in Pregnancy and Childhood; PACTG, Pediatric AIDS Clinical Trials Group; NISDI, NICHD International Site Development Initiative; NSHPC, National Study of HIV in Pregnancy and Childhood; ECS, European Collaborative Study; IMPAACT, International Maternal, Pediatric, Adolescent Clinical Trials Network; NPSATP, National Programme on Surveillance on Antiretroviral Treatment in Pregnancy; EPF, Enquete Perinatale Francaise; PHACS, Pediatric HIV/AIDS Cohort Study; SMARTT, Surveillance Monitoring for ART Toxicities; ART, antiretroviral therapy; T1, first trimester; T2, second trimester; T3, third trimester; EUROCAT, European Surveillance of Congenital Anomalies; MACDP, Metropolitan Atlanta Congenital Defects Program
Comparison group included women who were not treated during pregnancy
Case determination included blinded clinician review
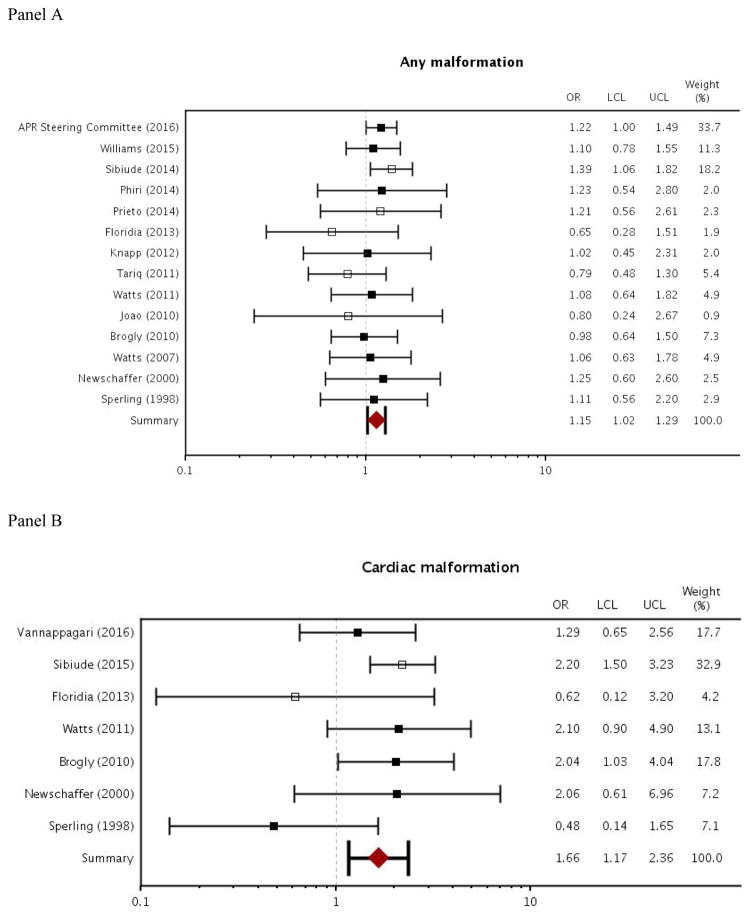
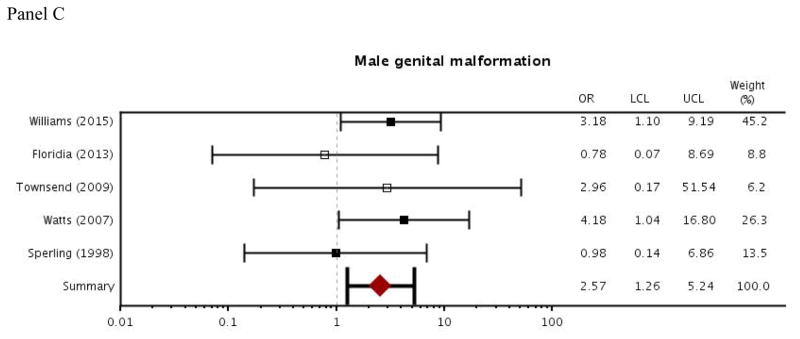
For zidovudine exposure during pregnancy, results from the meta-analysis indicated slightly increased odds of overall malformation and cardiac malformation (overall malformation: OR=1.15, 95% CI [1.02–1.29]; cardiac malformation: OR=1.66 [1.17–2.36]; Figure 2). Odds of a male genital malformation more than doubled with zidovudine exposure during pregnancy (OR=2.57 [1.26–5.24]; Figure 2). Between-study heterogeneity was low to moderate for each of the malformation outcomes. I2, which represents the percentage of variance in meta-analysis that is attributable to between-study heterogeneity, ranged from 0 to 28 (overall malformation: I2=0 [0–55]; cardiac malformation: I2=28 [0–69]; male genital malformation: I2= 0 [0–79]). There was also some evidence of publication bias according to Egger’s test for small study effects, where a small p-value indicates asymmetry in the funnel plot (p=0.04 for overall malformation; p=0.08 for cardiac malformation; p=0.26 for male genital malformation).
Figure 2.
Forest plot of meta-analysis results: odds ratios for zidovudine use in pregnancy and outcomes of overall malformation, cardiac malformation, and male genital malformation
Abbreviations: APR, Antiretroviral Pregnancy Registry; OR, odds ratio; LCL, lower 95% confidence limit; UCL, upper 95% confidence limit
Filled boxes indicate studies that recruited participants from the US and may have some overlap with Medicaid data. Empty boxes indicate studies that did not recruit participants from the US.
In the MAX cohort, 824 women were dispensed zidovudine in the first trimester and 1,998 were dispensed ART that did not include zidovudine in the first trimester. Before matching, there were some small differences in baseline characteristics between the exposure groups; women with first trimester zidovudine exposures were slightly older, less likely to be black, had deliveries earlier in the study period, had more psychiatric diagnoses and antidepressant use, and were more likely to be dispensed an antiretroviral medication in the 3 months prior to pregnancy (Table 2). The 1:1 matching procedure resulted in a sample of 735 women each in the zidovudine and comparator groups. In the matched sample, these differences between baseline characteristics decreased (Table 2). Women with first trimester zidovudine exposure who were unmatched, and thus dropped from the analysis, were slightly more likely to receive antiretroviral drugs in the baseline period (59% in unmatched group versus 54% in matched group) and were dispensed more prescriptions overall (mean=7.34 in unmatched group versus mean=6.85 in the matched group).
Prior to matching, women with a first trimester dispensing of zidovudine had a 4.6% risk of overall malformations, compared to 4.0% in the comparison group (Table 3). After implementing the matching procedure, these risks shifted to 4.6% and 4.9%, respectively. In both the full sample and the matched sample, women with first trimester zidovudine exposure and those without had similar risk of cardiac malformations (1.5% versus 1.5% in the full sample; 1.5% versus 1.6% in the matched sample). Among women with first trimester zidovudine exposure, there were no male genital malformations in the full sample.
Table 3.
First trimester zidovudine exposure and risk of malformations: MAX and posterior Bayesian analysis results
| Risk in full sample: zidovudine in Trimester 1 (N=823) | Risk in full sample: no zidovudine in Trimester 1 (N=1,998) | Risk in matched sample: zidovudine in Trimester 1 (N=735) | Risk in matched sample: no zidovudine in Trimester 1 (N=735) | Posterior estimates: Bayesian analysis results | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | OR | 95% credible interval | ||
|
|
|||||||||||
| Overall malformation | 38 | 4.6 | 79 | 4.0 | 34 | 4.6 | 36 | 4.9 | 1.11 | 0.80 | 1.55 |
| Cardiac malformation | 12 | 1.5 | 29 | 1.5 | 11 | 1.5 | 12 | 1.6 | 1.30 | 0.63 | 2.71 |
| Male genital malformation | 0 | 0.0 | <111 | -- | 0 | 0.0 | <111 | -- | N/A | -- | -- |
Abbreviations: MAX, Medicaid Analytic eXtract; OR, odds ratio
Cell sizes of 10 or less have been suppressed in accordance with Centers for Medicare and Medicaid Services cell size suppression policy.
Among infants with first trimester exposure to zidovudine, cardiac malformations were the most common type of malformation. The remainder of malformations were heterogeneous, with no more than five linked to any single organ system. Within the category of cardiac malformations, there was also considerable diversity, including diagnoses of patent ductus arteriosus, right ventricular outflow tract obstruction, left ventricular outflow tract obstruction, secundum atrial septal defects, single ventricle defects, and conotruncal defects.
When comparing ART with first trimester zidovudine to ART without first trimester zidovudine, the Bayesian posterior OR estimates were slightly above the null for overall malformation (OR=1.11; 95% credible interval: 0.80–1.55) and cardiac malformation (OR=1.30; 95% credible interval: 0.63–2.71). Because there were no exposed cases of male genital malformations in the MAX cohort, the Bayesian model did not converge for that outcome.
For the outcome of overall malformation, restricting the meta-analysis to studies with similar designs to what was implemented in the MAX cohort (i.e., classified zidovudine exposure during the first trimester, required that women in the comparison group received ART, and controlled for confounding) resulted in very similar posterior OR estimates, though the credible interval became wider (OR=1.08; 95% credible interval: 0.70–1.69). We were unable to conduct similar sensitivity analyses for the cardiac and male genital malformation outcomes because there were a prohibitively small number of studies that met the more restrictive criteria.
DISCUSSION
The use of antiretroviral agents, including zidovudine, has dramatically lowered the risk of perinatal HIV transmission. In a nationwide cohort of Medicaid-enrolled pregnant women with HIV from the years 2000 to 2010, we found that first trimester exposure to zidovudine was relatively common, comprising 30% of deliveries for ART-treated women with HIV. Our systematic review and meta-analysis captured existing information in the literature, which used Bayesian methods to incorporate novel information from Medicaid. Compared to women with ART regimens that did not include zidovudine in the first trimester, those with first trimester zidovudine exposure had a modest increase in the odds of overall malformation and cardiac malformation, though the 95% credible intervals in the Bayesian analysis included the null value of 1. No exposed cases of male genital malformation were observed in the MAX cohort. However, our meta-analysis of previous studies reporting estimates for this outcome indicate that there may be a substantial increase in risk of male genital malformations for infants with in utero zidovudine exposure, though estimates were imprecise due to the limited sample size and the rare nature of the outcome.
The severity and clinical impact of the specific malformations observed is unclear. In the MAX data, the cardiac malformations identified among infants with first trimester zidovudine exposure were largely heterogeneous. Studies included in the meta-analysis identified an excess of ventricular septal defects among infants with in utero zidovudine exposure, and these defects are often managed non-surgically[40]. A 2015 study in France found that most identified cardiac malformations were minor and less than 10% required a surgical intervention.[7] The male genital malformations identified in previous studies were predominantly hypospadias, which generally has a good prognosis.[41]
Because of the relatively smaller size of MAX compared to the number of women included across all studies in the meta-analysis, posterior credible intervals from the Bayesian analysis were wider than confidence intervals from the meta-analysis alone. In sensitivity analyses, we undertook an alternative analytical approach, treating the MAX data as another entry in the meta-analysis. This led to odds ratio estimates of 1.13 [1.01, 1.27] for overall malformations and 1.53 [1.08, 2.17] for cardiac malformations, which are similar to the findings of the meta-analysis before including the MAX data.
When interpreting the results of the meta-analysis, it is important to be aware of substantial diversity in the designs of the included studies. In addition to random error, heterogeneity in results may be due to a number of important differences, including timing of exposure measurement (e.g., zidovudine use in first trimester versus any time in pregnancy), comparator group (e.g., no antiretroviral therapy in pregnancy versus no zidovudine exposure in the first trimester), outcome definitions (e.g., MACDP versus EUROCAT), internal study validity (e.g., amount of confounding control), or geographic differences (e.g. Europe versus United States).
Findings from this study must also be interpreted within the context of existing knowledge about the use of zidovudine and other antiretroviral drugs during pregnancy. In addition to potential teratogenicity, many considerations influence treatment decisions for pregnant women with HIV, including treatment availability, tolerability of side effects, interactions with other medications, drug resistance, and other maternal and infant safety concerns.
An important infant safety concern for zidovudine use in pregnancy is its possible link to mitochondrial dysfunction, a debilitating disorder caused by disturbances in the mitochondrial oxidative phosphorylation system. Animal models have suggested zidovudine causes transplacental mitochondrial toxicity,[42] and studies of mitochondrial function biomarkers have found inverse relationships with in utero exposure to zidovudine [43] and nucleoside drugs [44]. Epidemiological evidence of zidovudine use during pregnancy leading to mitochondrial dysfunction has been mixed, with some studies supporting the link,[45,46] but others finding no relationship.[47–50] We did not examine mitochondrial dysfunction in this study but, alongside malformations, it is a key safety consideration when contemplating the use of zidovudine during pregnancy.
Our study has several limitations. First, it is possible that some children were enrolled in multiple studies, including MAX, which would artificially increase the sample size and decrease the variance. However, we do not expect bias in our estimates because the prospective nature of nearly all included studies makes it unlikely that repeated observations are differential with respect to outcome. Second, classification of exposure to zidovudine during the first trimester was based on an algorithm to estimate LMP. This may result in some non-differential misclassification of the exposure, which would bias estimates towards the null. Third, we were only able to follow infants for 3 months after delivery, limiting outcome sensitivity, and were not able to review medical records for cases of suspected malformations, limiting outcome specificity. However, a previous validation study showed a good positive predictive value for claims-based definitions of cardiac malformations in MAX.[24] We expect any outcome misclassification to be non-differential, and therefore biased towards the null. Fourth, the MAX dataset and nearly all studies included in the meta-analysis were observational cohorts, and there is potential for residual confounding. However, this should be limited by use of propensity score matching and an active comparator group. Fifth, because MAX and some studies in the meta-analysis are restricted to only include live births, there is a potential for selection bias if malformations due to first trimester zidovudine were so severe that pregnancies ended in miscarriage or stillbirth. Finally, the data from MAX and most studies included in the meta-analysis were conducted in high-income countries in North America and Europe. However, most women receiving ART during pregnancy are from low-income countries, and it is unclear how our results may generalize to these settings.
Our study also has multiple strengths. Because exposure was measured through pharmacy dispensing records, our measurements will not be impacted by inconsistencies in recall or memory. In addition, the active comparator design also makes our results clinically interpretable and minimizes the potential for bias due to confounding. Finally, our posterior estimates summarize all currently available information, and are especially useful in this context because of the rare nature of organ-specific malformations.
In conclusion, these findings provide reassurance that for most types of congenital malformations, first trimester exposure to zidovudine results in minimal differences in risk compared to other treatment strategies. The potential increase in male genital malformations appears small in absolute magnitude, but should continue to be monitored. It will be important to conduct similar analyses to monitor adverse events associated with other antiretroviral agents used during pregnancy, including other nucleoside agents and newer agents from other classes with limited safety data. Due to the Bayesian approach used, estimates from this study reflect the most comprehensive evidence available on zidovudine and malformations that can be used as a resource for women with HIV, their healthcare providers, and policy makers to assess options for treatment of HIV during pregnancy.
Supplementary Material
Acknowledgments
We would like to thank Carol Ann Mita for her help in creating the systematic search criteria. We would also like to thank Helen Mogun for her assistance in preparing the analytic datasets.
Funding: KR was supported by grant number T32 AI007433 from the National Institute of Allergy and Infectious Diseases (NIAID) and a training grant from the Pharmacoepidemiology Concentration at the Harvard T.H. Chan School of Public Health. KFH was supported by a career development grant K01MH099141 from the National Institute of Mental Health. BTB was supported by career development grant 4K08HD075831-04 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. SHD was supported by grant R01MH100216 from the National Institute of Mental Health.
Footnotes
Author contributions: KR, GRS, PLW, and SHD were the authors who conceived and designed the study. KR and JWS conducted the systematic review. KR had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. KR was responsible for conducting statistical analyses, and led the drafting of the manuscript. KFH, BTB, and SHD provided leadership and oversight of the creation of the MAX pregnancy cohort. All authors provided input on the study design, interpretation of analyses, and revisions to manuscript.
CONFLICTS OF INTEREST
KH is co-investigator of a grant to the Brigham and Women’s Hospital from Eli Lilly and from Pfizer, unrelated to the topic of this manuscript. BTB is co-investigator of a grant to the Brigham and Women’s Hospital or Massachusetts General Hospital from Eli Lilly, GSK, Pacira, Baxalta, and Pfizer. SHD received salary support from the North American AED Pregnancy Registry; and consulted for UCB, Teva, and Boehringer-Ingelheim; her institution received training grants from Pfizer, Takeda, Bayer, and Asisa. She is co-investigator grants to the Harvard T.H. Chan School of Public Health from Eli Lilly, GSK and Pfizer, unrelated to the topic of this manuscript.
References
- 1.Fowler MG, Qin M, Fiscus SA, Currier JS, Flynn PM, Chipato T, et al. Benefits and Risks of Antiretroviral Therapy for Perinatal HIV Prevention. N Engl J Med. 2016;375:1726–1737. doi: 10.1056/NEJMoa1511691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper ER, Charurat M, Mofenson L, Hanson IC, Pitt J, Diaz C, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defici Syndr. 2002;29:484–94. doi: 10.1097/00126334-200204150-00009. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. World Health Organization; 2016. http://www.who.int/hiv/pub/arv/arv-2016/en/ [PubMed] [Google Scholar]
- 4.Sibiude J, Mandelbrot L, Blanche S, Le Chenadec J, Boullag-Bonnet N, Faye A, et al. Association between prenatal exposure to antiretroviral therapy and birth defects: an analysis of the French perinatal cohort study (ANRS CO1/CO11) PLoS Med. 2014;11:e1001635. doi: 10.1371/journal.pmed.1001635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brogly SB, Abzug MJ, Watts DH, Cunningham CK, Williams PL, Oleske J, et al. Birth defects among children born to human immunodeficiency virus-infected women: pediatric AIDS clinical trials protocols 219 and 219C. Pediatr Infect Dis J. 2010;29:721–7. doi: 10.1097/INF.0b013e3181e74a2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watts DH, Huang S, Culnane M, Kaiser KA, Scheuerle A, Mofenson L, et al. Birth defects among a cohort of infants born to HIV-infected women on antiretroviral medication. J Perinat Med. 2011;39:163–70. doi: 10.1515/JPM.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sibiude J, Le Chenadec J, Bonnet D, Tubiana R, Faye A, Dollfus C, et al. In utero exposure to zidovudine and heart anomalies in the ANRS French perinatal cohort and the nested PRIMEVA randomized trial. Clin Infect Dis. 2015;61:270–80. doi: 10.1093/cid/civ260. [DOI] [PubMed] [Google Scholar]
- 8.Williams PL, Crain MJ, Yildirim C, Hazra R, Van Dyke RB, Rich K, et al. Congenital anomalies and in utero antiretroviral exposure in human immunodeficiency virus-exposed uninfected infants. JAMA Pediatr. 2015;169:48–55. doi: 10.1001/jamapediatrics.2014.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watts DH, Li D, Handelsman E, Tilson H, Paul M, Foca M, et al. Assessment of birth defects according to maternal therapy among infants in the Women and Infants Transmission Study. J Acquir Immune Defic Syndr. 2007;44:299–305. doi: 10.1097/QAI.0b013e31802e2229. [DOI] [PubMed] [Google Scholar]
- 10.Phiri K, Hernandez-Diaz S, Dugan KB, Williams PL, Dudley JA, Jules A, et al. First Trimester Exposure to Antiretroviral Therapy and Risk of Birth Defects. Pediatr Infect Dis J. 2014;33:741–746. doi: 10.1097/INF.0000000000000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Floridia M, Mastroiacovo P, Tamburrini E, Tibaldi C, Todros T, Crepaldi A, et al. Birth defects in a national cohort of pregnant women with HIV infection in Italy, 2001–2011. BJOG. 2013;120:1466–75. doi: 10.1111/1471-0528.12285. [DOI] [PubMed] [Google Scholar]
- 12.Vannappagari V, Albano JD, Koram N, Tilson H, Scheuerle AE, Napier MD. Prenatal exposure to zidovudine and risk for ventricular septal defects and congenital heart defects: data from the Antiretroviral Pregnancy Registry. Eur J Obs Gynecol Reprod Biol. 2016;197:6–10. doi: 10.1016/j.ejogrb.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Sperling RS, Shapiro DE, McSherry GD, Britto P, Cunningham BE, Culnane M, et al. Safety of the maternal-infant zidovudine regimen utilized in the Pediatric AIDS Clinical Trial Group 076 Study. AIDS. 1998;12:1805–13. doi: 10.1097/00002030-199814000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Knapp KM, Brogly SB, Muenz DG, Spiegel HML, Conway DH, Scott GB, et al. Prevalence of congenital anomalies in infants with in utero exposure to antiretrovirals. Pediatr Infect Dis J. 2012;31:164–70. doi: 10.1097/INF.0b013e318235c7aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tariq S, Townsend CL, Cortina-Borja M, Duong T, Elford J, Thorne C, et al. Use of zidovudine-sparing HAART in pregnant HIV-infected women in Europe: 2000–2009. J Acquir Immune Defic Syndr. 2011;57:326–33. doi: 10.1097/QAI.0b013e31821d34d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Governors Association. 2014 Maternal and Child Health Update: States are Using Medicaid and CHIP to Improve Health Outcomes for Mothers and Children. Washington, D.C: 2015. http://www.nga.org/files/live/sites/NGA/files/pdf/MCHUPDATE2014.PDF. [Google Scholar]
- 17.Palmsten K, Huybrechts KF, Mogun H, Kowal MK, Williams PL, Michels KB, et al. Harnessing the Medicaid Analytic eXtract (MAX) to Evaluate Medications in Pregnancy: Design Considerations. PLoS One. 2013;8:e67405. doi: 10.1371/journal.pone.0067405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huybrechts KF, Palmsten K, Avorn J, Cohen LS, Holmes LB, Franklin JM, et al. Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med. 2014;370:2397–407. doi: 10.1056/NEJMoa1312828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desai RJ, Huybrechts KF, Hernandez-Diaz S, Mogun H, Patorno E, Kaltenbach K, et al. Exposure to prescription opioid analgesics in utero and risk of neonatal abstinence syndrome: population based cohort study. BMJ. 2015;350:h2102. doi: 10.1136/bmj.h2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bateman BT, Patorno E, Desai RJ, Seely EW, Mogun H, Maeda A, et al. Late Pregnancy Beta Blocker Exposure and Risks of Neonatal Hypoglycemia and Bradycardia. Pediatrics. 2016;138:e20160731–e20160731. doi: 10.1542/peds.2016-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huybrechts KF, Hernández-Díaz S, Patorno E, Desai RJ, Mogun H, Dejene SZ, et al. Antipsychotic Use in Pregnancy and the Risk for Congenital Malformations. JAMA Psychiatry. 2016;73:938. doi: 10.1001/jamapsychiatry.2016.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Margulis AV, Palmsten K, Andrade SE, Charlton RA, Hardy JR, Cooper WO, et al. Beginning and duration of pregnancy in automated health care databases: review of estimation methods and validation results. Pharmacoepidemiol Drug Saf. 2015;24:335–342. doi: 10.1002/pds.3743. [DOI] [PubMed] [Google Scholar]
- 23.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58:323–337. doi: 10.1016/j.jclinepi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Palmsten K, Huybrechts KF, Kowal MK, Mogun H, Hernández-Díaz S. Validity of maternal and infant outcomes within nationwide Medicaid data. Pharmacoepidemiol Drug Saf. 2014;23:646–655. doi: 10.1002/pds.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bateman BT, Mhyre JM, Hernandez-Diaz S, Huybrechts KF, Fischer MA, Creanga AA, et al. Development of a Comorbidity Index for Use in Obstetric Patients. Obs Gynecol. 2013;122:957–965. doi: 10.1097/AOG.0b013e3182a603bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergstralh E, Kosanke J. Gmatch SAS macro. 1995. [Google Scholar]
- 27.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–161. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 29.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.StataCorp. Stata Statistical Software: Release 13. 2013. [Google Scholar]
- 32.Sterne JAC, Harris RJ, Harbord RM, Steichen TJ. Meta-Analysis in Stata: An Updated Collection from the Stata Journal, Second Edition. College Station, TX: StataCorp LP; 2016. [Google Scholar]
- 33.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 34.Hernán MA, Hernández-Díaz S, Werler MM, Mitchell AA. Causal knowledge as a prerequisite for confounding evaluation: an application to birth defects epidemiology. Am J Epidemiol. 2002;155:176–84. doi: 10.1093/aje/155.2.176. [DOI] [PubMed] [Google Scholar]
- 35.Newschaffer CJ, Cocroft J, Anderson CE, Hauck WW, Turner BJ. Prenatal zidovudine use and congenital anomalies in a Medicaid population. J Acquir Immune Defic Syndr. 2000;24:249–56. doi: 10.1097/00126334-200007010-00009. [DOI] [PubMed] [Google Scholar]
- 36.Townsend CL, Willey BA, Cortina-Borja M, Peckham CS, Tookey PA. Antiretroviral therapy and congenital abnormalities in infants born to HIV-infected women in the UK and Ireland, 1990–2007. AIDS. 2009;23:519–24. doi: 10.1097/QAD.0b013e328326ca8e. [DOI] [PubMed] [Google Scholar]
- 37.Joao EC, Calvet GA, Krauss MR, Freimanis Hance L, Ortiz J, Ivalo SA, et al. Maternal antiretroviral use during pregnancy and infant congenital anomalies: the NISDI perinatal study. J Acquir Immune Defic Syndr. 2010;53:176–85. doi: 10.1097/QAI.0b013e3181c5c81f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prieto LM, González-Tomé MI, Muñoz E, Fernández-Ibieta M, Soto B, Álvarez A, et al. Birth defects in a cohort of infants born to HIV-infected women in Spain, 2000–2009. BMC Infect Dis. 2014;14:700. doi: 10.1186/s12879-014-0700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antiretroviral Pregnancy Registry Steering Committee. Antiretroviral Pregnancy Registry International Interim Report for 1 January 1989 through 31 January 2016. Wilmington, NC: 2016. [Google Scholar]
- 40.Penny DJ, Vick GW. Ventricular septal defect. Lancent. 2011;377:1103–1112. doi: 10.1016/S0140-6736(10)61339-6. [DOI] [PubMed] [Google Scholar]
- 41.Baskins L. Hypospadias. In: Stringer O, Mouriquand P, editors. Pediatric Surgery and Urology: Long Term Outcomes. Cambridge: University Press; 2006. pp. 611–620. [Google Scholar]
- 42.Olivero OA, Anderson LM, Diwan BA, Haines DC, Harbaugh SW, Moskal TJ, et al. Transplacental effects of 3′-azido-2′,3′-dideoxythymidine (AZT): tumorigenicity in mice and genotoxicity in mice and monkeys. J Natl Cancer Inst. 1997;89:1602–8. doi: 10.1093/jnci/89.21.1602. [DOI] [PubMed] [Google Scholar]
- 43.Poirier MC, Divi RL, Al-Harthi L, Olivero OA, Nguyen V, Walker B, et al. Long-term mitochondrial toxicity in HIV-uninfected infants born to HIV-infected mothers. J Acquir Immune Defic Syndr. 2003;33:175–83. doi: 10.1097/00126334-200306010-00010. [DOI] [PubMed] [Google Scholar]
- 44.Noguera A, Fortuny C, Muñoz-Almagro C, Sanchez E, Vilaseca MA, Artuch R, et al. Hyperlactatemia in human immunodeficiency virus-uninfected infants who are exposed to antiretrovirals. Pediatrics. 2004;114:e598–603. doi: 10.1542/peds.2004-0955. [DOI] [PubMed] [Google Scholar]
- 45.Barret B, Tardieu M, Rustin P, Lacroix C, Chabrol B, Desguerre I, et al. Persistent mitochondrial dysfunction in HIV-1-exposed but uninfected infants: clinical screening in a large prospective cohort. AIDS. 2003;17:1769–85. doi: 10.1097/00002030-200308150-00006. [DOI] [PubMed] [Google Scholar]
- 46.Brogly SB, Ylitalo N, Mofenson LM, Oleske J, Van Dyke R, Crain MJ, et al. In utero nucleoside reverse transcriptase inhibitor exposure and signs of possible mitochondrial dysfunction in HIV-uninfected children. AIDS. 2007;21:929–38. doi: 10.1097/QAD.0b013e3280d5a786. [DOI] [PubMed] [Google Scholar]
- 47.Bulterys M, Nesheim S, Abrams EJ, Palumbo P, Farley J, Lampe M, et al. Lack of evidence of mitochondrial dysfunction in the offspring of HIV-infected women. Retrospective review of perinatal exposure to antiretroviral drugs in the Perinatal AIDS Collaborative Transmission Study. Ann N Y Acad Sci. 2000;918:212–21. doi: 10.1111/j.1749-6632.2000.tb05491.x. [DOI] [PubMed] [Google Scholar]
- 48.Dominguez K, Bertolli J, Fowler M, Peters V, Ortiz I, Melville S, et al. Lack of definitive severe mitochondrial signs and symptoms among deceased HIV-uninfected and HIV-indeterminate children ≤ 5 years of age, Pediatric Spectrum of HIV Disease project (PSD), USA. Ann N Y Acad Sci. 2000;918:236–46. doi: 10.1111/j.1749-6632.2000.tb05493.x. [DOI] [PubMed] [Google Scholar]
- 49.Lindegren ML, Rhodes P, Gordon L, Fleming P Perinatal Safety Review Working Group, State and Local Health Department HIV/AIDS Surveillance Programs. Drug safety during pregnancy and in infants. Lack of mortality related to mitochondrial dysfunction among perinatally HIV-exposed children in pediatric HIV surveillance. Ann N Y Acad Sci. 2000;918:222–35. [PubMed] [Google Scholar]
- 50.Nucleoside exposure in the children of HIV-infected women receiving antiretroviral drugs: absence of clear evidence for mitochondrial disease in children who died before 5 years of age in five United States cohorts. J Acquir Immune Defic Syndr. 2000;25:261–8. doi: 10.1097/00126334-200011010-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.