Abstract
Cooling a 1:1 (v/v) solution of acetonitrile and water at −16° C is known to result in two clear phases. We will refer to this event as “cold-induced aqueous acetonitrile phase separation (CIPS)”. On a molar basis, acetonitrile is 71.7% and 13.6% in the upper and lower phases, respectively, in our study. The phase separation proceeds as a descending cloud of microdroplets. At the convenient temperature (typical freezer) employed here the lower phase is rather resistant to solidification, although it emerges from the freezer as a solid if various insoluble matter is present at the outset. In a preliminary way, we replaced the initial (salting-out) step of a representative QuEChERS procedure with CIPS, applying this modified procedure (“CIPS-QuEChERS”) to a homogenate of salmon (and partly to beef). Three phases resulted, where only the upper, acetonitrile-rich phase is a liquid (that is completely clear). The middle phase comprises ice and precipitated lipids, while the lower phase is the residual matrix of undissolved salmon or meat. Treating the upper phase from salmon, after isolation, with anhydrous MgSO4 and C18-Si (typical QuEChERS dispersive solid phase extraction sorbents), and injecting into a GC–MS in a nontargeted mode, gives two-fold more preliminary hits for chemicals, and also number of spiked pesticides recovered, relative to that from a comparable QuEChERS method. In part, this is because of much higher background signals in the latter case. Further study of CIPS-QuEChERS is encouraged, including taking advantage of other QuERChERS conditions.
Keywords: Extraction, Fish, Pesticides, QuEChERS, Acetonitrile
1. Introduction
It is well known that acetonitrile can be phase separated from a water/acetonitrile solution by the addition of kosmotropic salts such as MgSO4 or NaCl. This technique is useful for extracting organic substances from aqueous samples for chemical analysis. Such salt-based phase separation of aqueous acetonitrile is the key and initial step in the QuEChERS (quick, easy, cheap, effective, rugged, safe) methods, which are widely employed for sample preparation, as has been reviewed [1–5]. Kits for this technique are available from several companies. The analytes of interest are mainly pesticides, industrial pollutants, natural toxins, and drugs. QuEChERS methods have been applied to foods (fruits, vegetables, beef, fish), environmental samples (water, soil, sludge), clinical specimens (blood, plasma, urine), and alcoholic beverages (wine, beer, milk). In the original method [6] a homogenized fruit or vegetable sample is extracted with acetonitrile; copious anhydrous MgSO4 and NaCl are added to give a phase separation; centrifugation is done to yield an acetonitrile-rich supernatant; dispersive solid phase extraction (d-SPE) is performed with anhydrous MgSO4 (to remove residual water) plus a primary/secondary amine (for additional removal of matrix interferences); and the supernatant, after centrifugation, is injected into a GC-MS to detect pesticides.
Many variations and improvements of the original method have taken place through the years such as use of buffers [7]; substitution of NH4HCO2 instead of MgSO4/NaCl for initial phase separation [8]; addition or substitution of other d-SPE (such as chitin [9], CaCl2 [10], zirconium oxide [8,11], C18/zirconium oxide [8–11], or carbons [8,12,13]); testing or use of other extraction solvents such as ethyl acetate [14]; evaporative concentration into toluene [15,16]; shortening the method to a single step [17]; use of in-vial filtration [8,18]; use of low pressure GC–MS [18,19]; automated mini-column SPE [20]; and detection by LC–MS methods [1].
Cold-induced aqueous acetonitrile phase separation (referred to as “CIPS” here) was previously used by Gu and coworkers for the removal of the majority of the solvent after reverse phase LC [21,22]. They subsequently used it for the phase partitioning of antibiotics, peptides and amino acids [23]. Here we report a preliminary, modified QuEChERS procedure on salmon (and partly on beef) with such phase separation as the initial step, instead of a conventional salting-out step. Fish have been subjected to QuEChERS previously [8,11,15,16,18,24].
2. Experimental section
2.1. Chemicals and materials
EPA 505/525 Update Pesticides Mix B (47728-U, 500 μg/mL each, in acetone) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Note that one of the compounds, namely hexachlorocylopentadiene, is a pesticide precursor rather than a pesticide. The working solution of pesticides was made by dilution of above stock solution to 2 μg/mL with acetonitrile, which was kept at 4 ° C and used for one month. Paper towels (Kimwipes KIMTECH Science Brand) were from Kimberly-Clark (Roswell, GA, USA). Sodium chloride (S640-500), magnesium sulfate anhydrous (M65-500), acetonitrile (A996-4), aluminum foil (heavy duty), Fisherbrand Total Immersion thermometer (Cat. No. 14-986B), and borosilicate glass tubes (12 × 75 mm, cat.no. 14-961-26) were from Fisher Scientific (Fair Lawn, NJ, USA). The cap for this type of glass tube was purchased from Wheaton (Millville, NJ, USA). The aluminum foil was cleaned before use by wiping with methanol then acetonitrile using a Kimwipe. Parafilm was from Demis (Neenah, WI, USA). Discovery C18 (C18-Si) and Discovery SAX were from Supelco (North Harrison, PA, USA). Glass culture tubes (25 × 150 mm) with caps were purchased from Kimble & Chase (45066A-25150, Mexico). Each tube was first capped with aluminum foil and then the supplied cap was installed. Nonfrozen salmon and beef were purchased from the local grocery store just before each experiment.
2.2. Solidification-Inducing reagents
The reagents used to give a solid lower phase when acetonitrile water (1:1, volume/volume) was kept at −16° C overnight were as follows: Sephadex G25-300 (Sigma); Anion Exchanger DE53 (Whatman, Pittsburgh, PA, USA); Rexyn 201 (CI-SO, Fisher Scientific); Activated Carbon, Darco G60 (Sigma Aldrich); Celite 520 (Sigma Aldrich, St. Louis, MO 63178, USA); Florisil (100–200 mesh/Sigma Aldrich); Ficoll 400 (Pharmacia); WPC18 Prepscale Bulk Packing (40 μm, Baker, Sanford, ME, USA); and Bio-Gel P-2 (50–100 mesh, Bio-Rad, Hercules, CA, USA).
2.3. Equipment
Homogenization was performed with a Handy Homogenizer (ESGE, M133/1281-0, Switzerland). The centrifuge was a Damon IEC (CRU-5000, USA). The Mini Vortexer (Model No. 945404) was from Fisher Scientific (Houston, TX, USA). The GC–MS was a 5973 Network MSD connected to a 6890 GC system from Agilent (Wilmington, Delaware, USA). The system was equipped with a 7683 autosampler and a DB-5MS capillary column (30.0 m × 250 μm × 0.25 μm, Agilent). A conventional freezer (Frigidaire freezer/refrigerator) was employed, where our sample rack was always placed in the middle of the freezer compartment in the same place, having a constant temperature of−16 °C (measured with a thermometer).
2.4. Sample preparation
Salmon (50 g for testing of 4 aliquots to explore different conditions for subsequent steps) was homogenized in 10mL of dd water (to avoid a thick paste that was awkward to process) with a Handy Homogenizer in a 1000mL glass beaker, then each 12g aliquot (equal to 10 g original salmon) of the homogenate was transferred into a glass culture tube (25 × 150 mm). For spiking, 1 mL of the working solution of pesticides plus 10mL of acetonitrile was added. The tube was sealed (see above), shaken by hand end-to-end intensely for 30 s, and spun at 1750g for 3 min. After storage −16°C for 12h, 1 mL of the top layer was transferred to a borosilicate glass tube (12 × 75 mm). After addition of 50 mg of C18-Si and 150mg anhydrous MgSO4, the tube was capped and sealed with parafilm. After shaking on Mini Vortexer for 2 min, the tube was spun at 1750 g for 2 min, and 2 μL was injected into the GC–MS.
2.5. GC–MS analysis
The GC–MS injector temperature was held at 255 °C with a helium flow rate of 1.0mL/min. The column was held at 60° C for 1 min after injection in the splitless mode, then programmed at 15° C/min to 180° C, followed by ramping to 280 °C at 12° C/min, which was held for 2 min. Ionization was by electron impact at 70 eV with the AUX temperature held at 260°C. The data were analyzed by searching the database of NIST MS Search 2.0 (2014).
3. Results and discussion
3.1. Cold-induced aqueous acetonitrile phase separation (CIPS)
If a 1:1 solution of acetonitrile/water (v/v) is placed in a −80 °C freezer, the entire solution turns to a homogenous translucent solid (an ordinary result of little interest here). However, if the solution instead is kept at −16°C overnight (12h), two liquid phases form. NMR analysis reveals that, on a molar basis, the upper phase is 71.7% acetonitrile, and the lower phase similarly is 13.6%. In an earlier study of a 65:35, v/v, solution of acetonitrile:aqueous 0.1% trifluoroacetic acid, storage at −17 ° C for several hours or overnight gave a top phase of 88% acetonitrile and a bottom phase of 35% acetonitrile based on refractive index measurements [21].
The lower phase is rather resistant to solidification. Seeding it with ice (and certain solids, see below); scratching the glass tube in contact with the lower liquid phase with a file; and tapping the tube vigorously did not immediately solidify this phase. Storing the sample for longer than 12 h at −16°C can eventually solidify the lower layer, but the time for this varies. The shortest time for solidifying the lower layer at −16°C has been 3 days without additional measures. However, placing the phase-separated sample in a −80° C freezer readily solidifies the lower (but not the upper) phase. We were, however, able to readily obtain a solid lower phase by adding any of a series of insoluble, arbitrary particulate materials (that are listed in the Experimental section), to the initial sample of aqueous acetonitrile prior to keeping it at −16°C. Scratching a test tube heavily, but not lightly, with a file prior to adding aqueous acetonitrile, and then storing at −16°C overnight, also gave a solid lower phase. In prior work involving dilute protein solutions and a higher ratio of acetonitrile to water at the outset, no freezing of the lower phase took place [21,22].
When the 1:1 aqueous acetonitrile solution is kept at −16°C, first it turns uniformly cloudy throughout. This is followed by the formation of a boundary layer having a flat top. This flat boundary layer slowly descends, leading to the product of two clear layers.
3.2. Volume aspects, and absence of phasing with other organic solvents
Aqueous solutions of acetone, methanol, and 2-propanol fail to undergo a similar phase separation at −16°C, as do the semi-miscible solvents ethyl acetate and butanol beyond their initial, partial phasing at room temperature. As is well known, mixing water and acetonitrile immediately shrinks the overall volume: when we mix 1 mL each of acetonitrile and water, the immediate volume is 1.92 mL at room temperature. The volumes of the upper and lower phases after phasing at −16 °C for 12 h are approximately 0.7 and 1.2 mL, respectively (based on measuring the meniscus of each).
3.3. Extraction of salmon by CIPS-QuEChERS
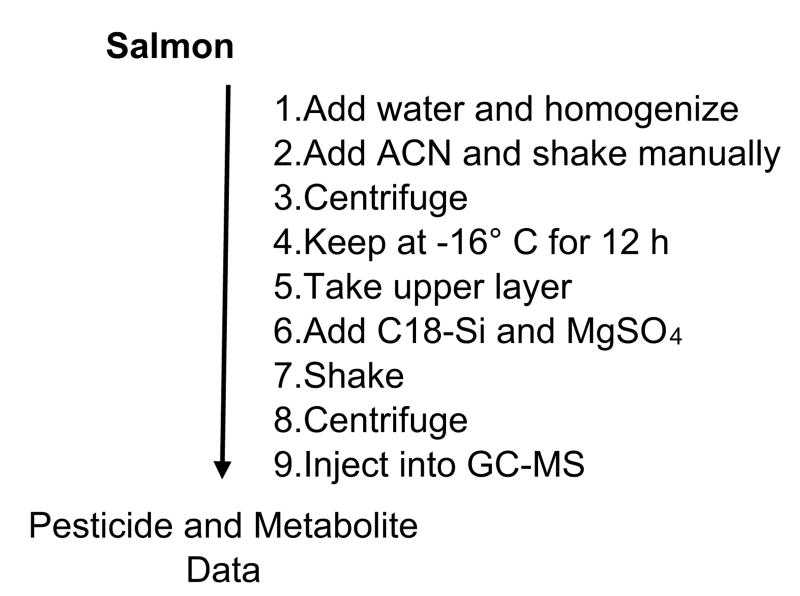
We subjected salmon to a preliminary “CIPS-QuEChERS procedure”, consisting of the sequence of steps shown in Fig. 1. After step 1, where the sample was a tissue homogenate, it was spiked with a standard mixture of eight pesticides plus one pesticide precursor. At the end of step 3, where the sample was a centrifuged homogenate at room temperature, two phases were present (liquid phase of aqueous acetonitrile on top, and solid phase of salmon homogenate below) as shown in Fig. 2a. After step 4 (−16° C, 12h) of Fig. 1, three phases were present, as seen in Fig. 2b: an upper, completely transparent, acetonitrile-rich phase (which was 77.0% acetonitrile on a molar basis); a middle, whitish, phase of ice; and a bottom, frozen solid phase which already had formed at room temperature as just noted. We assume that the middle phase is whitish primarily from a combination of being ice and having precipitated lipid. Others have precipitated lipid from acetonitrile or other solvents by cooling in a QuEChERS (conventional QuEChERS) or related procedure [25–31]. As has been reviewed, removal of lipids from organic solvents by cooling was also practiced prior to the introduction of QuEChERS methods [32]. When we subjected beef to steps 1–4 of Fig. 1 (Fig. S1), similar results were obtained. The subsequent steps of Fig. 1 were not applied to the beef sample.
Fig. 1.
Scheme for sample preparation by cold-induced aqueous acetonitrile phase separation (CIPS)-QuEChERS.
Fig. 2.

Pictures of a salmon sample: A, after step 2 in Fig. 1; and B, after step 4 in Fig. 1.
We recovered a part of the upper layer (step 5 of Fig. 1), and subjected it to d-SPE cleanup with C18-Si and anhydrous MgSO4 (steps 6 and 7), using amounts of the reagents previously employed by others in a comparable QuEChERS method applied to catfish preparations [11] After centrifugation (step 8), an aliquot was injected into a GC–MS, giving the chromatogram shown in Fig. 3a. Corresponding data for the indicated peaks is summarized in Table 1.
Fig. 3.
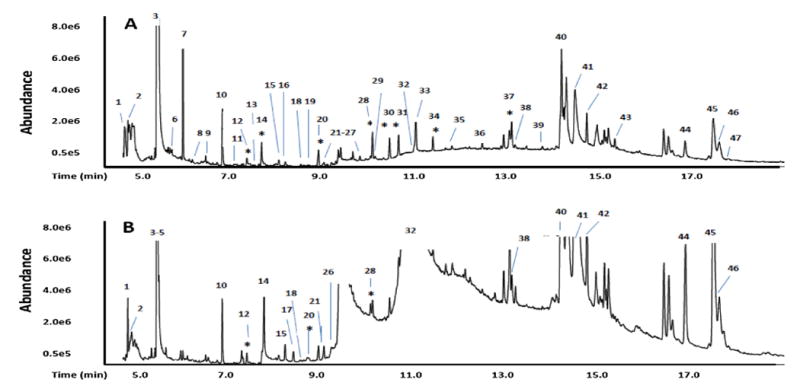
GC–MS chromatograms of salmon extracts formed by the scheme shown in Fig. 1 (A), and by a similar scheme in which steps 2–3 of this figure were conducted with MgSO4 and NaCl with omission of step 4(B). Preliminary hits for the numbered peaks are provided in Table 1.The peaks for the added pesticide standards are marked with an asterisk. Criteria for a hit: relatively symmetrical peak where at least two spectra, taken from the two sides of the peak, give the same hit with consistent probability values above 25%. Unlabeled peaks, even when relatively high, which gave different hits on the two sides, were not assigned.
Table 1. Hits for chemicals and recovery of internal standards for salmon by GC–MS after sample clean-up via CIPS/QuEChERS (CQ) or QuEChERS (Q)a.
| Chemical or Internal Standard | RT (min) | Prob. (%) on NIST MS 2014 | ||
|---|---|---|---|---|
|
| ||||
| CQ | Q | |||
| 1 | Nonanoic acid | 4.63 | 43 | 26 |
| 2 | Cyclotetrasiloxane, octamethyl- | 4.76 | 62 | 83 |
| 3 | Niacinamide | 5.41 | 74 | 73 |
| 4 | 2,4,7,9-Tetramethyl-5-decyn-4,7-diol | 5.44 | 30 | |
| 5 | Cyclopentasiloxane, decamethyl- | 5.56 | 31 | |
| 6 | 4,6-di-tert-Butyl-m-cresol | 5.73 | 26 | |
| 7 | Butylated Hydroxytoluene | 5.96 | 70 | |
| 8 | 1,2,4-Trioxolane, 3,3,5-triphenyl- | 6.11 | 49 | |
| 9 | 2-Methyl-5,5-diphenyl-4-(methylthio)imidazole | 6.55 | 28 | |
| 10 | Benzophenone | 6.83 | 83 | 89 |
| 11 | 2H-1-Benzopyran, 2,2-diphenyl | 7.21 | 68 | |
| 12 | Benzene, hexachloro- (24± 7)b | 7.37 | 35 | 34 |
| 13 | 1,7-diisopropylnaphthalene | 7.54 | 49 | |
| 14 | Atrazine(79±4) | 7.69 | 98 | 97 |
| 15 | Tetradecanoic acid | 7.74 | 47 | 80 |
| 16 | 9H-Fluorene, 9-methylene- | 8.07 | 27 | |
| 17 | 2-Pentadecanone, 6,10,14-trimethyl- | 8.39 | 69 | |
| 18 | 9,12,15-Octadecatrienoic acid, oxymethylethyl ester | 8.51 | 41 | 55 |
| 19 | 1,2-Benzenedicarboxylic acid, butyl octyl ester | 8.55 | 26 | |
| 20 | Heptachlor (59 ± 5) | 8.94 | 97 | 96 |
| 21 | 2H-Pyran-2-one, tetrahydro-6-nonyl- | 9.06 | 29 | 50 |
| 22 | 5,10-Diethoxy-2,3,7,8-tetrahydro-1H,6H-dipyrrolopyrazine | 9.22 | 41 | |
| 23 | Octadecanoic acid | 9.26 | 33 | |
| 24 | Cyclopropanetetradecanoic acid, 2-octyl-, methyl ester | 9.28 | 25 | |
| 25 | 1,2-Benzenedicarboxylic acid, butyl octyl ester | 9.31 | 26 | |
| 26 | n-Hexadecanoic acid | 9.37 | 65 | 76 |
| 27 | 1,3,6,10-Cyclotetradecatetraene,-trimethyl-14-(1-methylethyl)- | 9.42 | 33 | |
| 28 | Heptachlor epoxide (80 ± 5) | 10.1 | 85 | 56 |
| 29 | 1-Acetyl-2,2,4-trimethyl-4-phenyl-1,2,3,4-tetrahydroquinoline | 10.4 | 29 | |
| 30 | cis-Chlordane (63 ± 7) | 10.5 | 36 | |
| 31 | trans-Chlordane(61±4) | 10.7 | 41 | |
| 32 | Oleic Acid | 10.8 | 36 | 30 |
| 33 | Butyl citrate | 11.1 | 85 | |
| 34 | Endrin(99±11) | 11.4 | 70 | |
| 35 | Estra-1,3,5(10)-trien-17β-ol | 11.7 | 39 | |
| 36 | Nonanedioic acid, dibutyl ester | 12.5 | 29 | |
| 37 | Methoxychlor (93 ± 7) | 13.1 | 31 | |
| 38 | 4-Hexyl-1-(7-methoxycarbonylheptyl)bicyclodeca-2,5,7-triene | 13.2 | 24 | 28 |
| 39 | o,p'-Methoxychlor | 13.8 | 32 | |
| 40 | 9,10-Secocholesta-triene-1,3-diol, 25-[(trimethylsilyl)oxy]- | 14.3 | 31 | 25 |
| 41 | 9-Octadecenoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | 14.6 | 42 | 35 |
| 42 | 1,3-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester | 14.8 | 29 | 41 |
| 43 | Acetic acid, 17-acetoxy-phenanthren-10-ylmethyl ester | 15.4 | 27 | |
| 44 | γ-Tocopherol | 16.9 | 39 | 45 |
| 45 | Cholesterol | 17.5 | 28 | 32 |
| 46 | Vitamin E | 17.7 | 30 | 26 |
| 47 | 1-Monolinoleoylglycerol trimethylsilyl ether | 17.9 | 26 | |
| Total | 42 | 23 | ||
Internal standards are bolded.
Percent recoveries of spiked standards (n = 3) as mean values ±SD.
Overall, 8 of the 9 spiked chemicals were recovered in yields (from spiking) ranging from 24 to 99% (hexachlorocyclopentadiene was not recovered). Aside from the spiked pesticides, the preliminary hits (tentative identifications) in Table 1 by CIPS QuEChERS comprise two siloxanes (apparently from the GC column: they show up when pure solvent is injected); two trimethylsilyl compounds (apparently formed in the GC–MS); and a mixture of environmental contaminants plus metabolites. Criteria for a hit: at least two spectra, taken from the two sides of a peak, give the same hit with both probability values above 25%. Unlabeled peaks, even if relatively high, were not assigned if they gave different hits on their two sides. In Fig. 2S the hit peaks of Fig. 3a are enlarged.
3.4. Reproducibility of GC–MS chromatograms
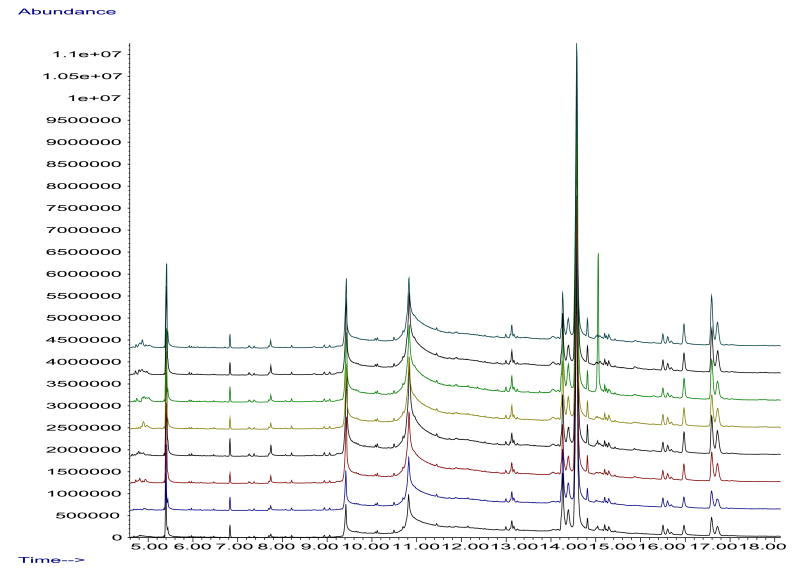
In Fig. 4 are shown GC–MS chromatograms formed by making eight injections of a salmon extract (with a blank injection of acetonitrile between each salmon injection), where the extract was obtained according to the scheme shown in Fig. 1. Good reproducibility is seen. However, continued injections (not shown) begin to yield degraded chromatograms, a typical tradeoff of minimizing sample preparation in order to increase the coverage provided by non-targeted chemical analysis. Currently, we heat the GC–MS after every 16 such injections to restore it, but potentially a more stable performance by GC–MS can be achieved in the future by increasing sample cleanup and/or improving the GC–MS conditions [18,21]. This is important because presently we are only able to conduct 4 such cycles of 16 injections each before the GC–MS needs to be cleaned.
Fig. 4.
GC–MS chromatograms from eight injections (bottom to top) of a salmon sample according to Fig. 1, with a blank injection of acetonitrile between each salmon injection.
3.5. CIPS-QuEChERS with SAX
Often a primary-secondary amine is employed as a secondary d-SPE in a QuEChERS procedure to further reduce background peaks in the subsequent analysis by GC–MS or LC–MS [18]. We explored, a preliminary way, a strong anion exchanger (SAX) for this purpose (especially intended for removal of fatty acids and some phospholipids) in our CIPS-QuEChERS method by conducting step 6 of Fig. 1 with MgSO4 alone, and in the presence of 25 and 50 mg of SAX. The background was the same in each case (Fig. 3S). We speculate that the descending microdroplets that yield the middle phase in the cold phase separation step might have already removed polar contaminants that SAX could extract, although this is uncertain since GC–MS is limited in its ability to reveal polar contaminants.
3.6. Comparative extraction of salmon by MgSO4/NaCl-QuEChERS
To compare CIPS-QuEChERS with a similar QuEChERS method based on initial phase separation with salt [11] (that we will now designate as “salt-QuEChERS”), we conducted the phase separation of steps 2–3 of Fig. 1 instead with MgSO4 (4g) and NaCl (1 g), and omitted step 4. This led to the chromatographic data shown in Fig. 3b, which is summarized in Table 1. As seen, compared to the salt-QuEChERS method, our technique results in significantly lower background signals. Apparently, largely as a result of this high background, we only recovered 4 of 9 spiked chemicals with the salt-QuEChERS method, but detected 8 of 9 with our procedure. Even worse, the sample prepared using salt-QuEChERs severely contaminated our GC–MS system after only one injection, and extensive cleaning was required to bring it back to a normal condition. Compared to the salt-QuEChERS procedure, which results in 23 detected compounds (largely as preliminary hits: tentative identifications), CIPS-QuEChERS similarly gives 43 detected compounds. Once again, apparently this is largely due to the higher background signals in the salt-QuEChERS procedure. Some of the hits by CIPS-QuEChERS that are missed by salt-QuEChERS show up before the elution of the high background signals in the latter technique. Three of the hits (4, 5, and 17) arise only by salt-QuEChERS.
These data do not establish that CIPS-QuEChERS is better than salt-QuEChERS. There are many QuEChERS methods, including recent ones that achieve very high performance including high throughput for the targeted analysis of pesticides and environmental contaminants in meat and fish samples [18,24]. The data shown in Fig. 2 and Table 1 instead only encourages further study of the conditions and usefulness of CIPS-QuEChERS methods.
3.7. Benefits and potential benefits of CIPS-QuERChERS
CIPS is convenient and, in this study, where it was used to modify a conventional QuERChERS procedure, it was found to improve the performance of a subsequent GC–MS analysis. The CIPS-QuERChERS method utilizes less reagents (which have a cost and may add impurities) than a conventional QuEChERS procedure, where initial phase separation is achieved by adding a large quantity of one or more salts. Sometimes CIPS in this role may be advantageous where further analysis of a precipitated phase is of interest, since now there is no burden of high salt there.
4. Conclusions
Cold-induced aqueous acetonitrile phase separation (CIPS), a known technique, which is now seen to proceed through a micro-droplet cloud that can yield a lower solid phase, is interesting as a new way to begin a QuEChERS procedure. It is convenient, conserves reagents, has improved the performance of a subsequent GC–MS step, and provides three phases at the outset, none of which is burdened with salt for subsequent analysis. However, work remains to fully define the usefulness of CIPS-QuEChERS relative to QuEChERS, since there are many interacting variables that can play a role: type of sample, other CIPS conditions, choice of QuEChERS steps and reagents, type of detection, targeted vs non-targeted analysis, cost, throughput, and range of analytes that are detected.
Supplementary Material
Acknowledgments
This work was supported by NIEHS Grant P42 ES017198 and NIFA Grant 2015-67030-23755. The authors thank Clemens Anklin at Bruker Biospin for sample analysis by NMR, and Yelena Sapozhnikova at the US Department of Agriculture for valuable discussions.
Footnotes
Appendix A. Supplementary data: Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.chroma.2017.05.045
References
- 1.Lehotay SJ. QuEChERS sample preparation approach for mass spectrometric analysis of pesticide residues in food. Methods Mol Biol. 2011;774:65–91. doi: 10.1007/978-1-61779-136-9_4. [DOI] [PubMed] [Google Scholar]
- 2.Lehotay SJ, Son KA, Kwon H, Koesukwiwat U, Fu W, Mastovska K, et al. Comparison of QuEChERS sample preparation methods for the analysis of pesticide residues in fruits and vegetables. J Chromatogr A. 2010;1217:2548–2560. doi: 10.1016/j.chroma.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 3.Rejczak T, Tuzimski T. A review of recent developments and trends in the QuEChERS sample preparation approach. Open Chem. 2015;13:980–1010. [Google Scholar]
- 4.Bruzzoniti MC, Checchini L, Carlo RMD, Orlandini S, Rivoira L, Bubba MD. QuEChERS sample preparation for the determination of pesticides and other organic residues in environmental matrices: a critical review. Anal Bioanal Chem. 2014;406:4089–4116. doi: 10.1007/s00216-014-7798-4. [DOI] [PubMed] [Google Scholar]
- 5.Rejczak T, Tuzimski T. A review of recent developments and trends in the QuEChERS sample preparation approach. Open Chem. 2015;13:980–1010. [Google Scholar]
- 6.Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck F. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and dispersive solid-phase extraction for the determination of pesticide residues in produce. J AOAC Int. 2011;86(2):412–431. [PubMed] [Google Scholar]
- 7.Lehotay SJ, Mastovska K, Lightfield AR. Use of buffering and other means to improve results of problematic pesticides in a fast and easy method for residue analysis of fruits and vegetables. J AOAC Int. 2005;88(2):615–629. [PubMed] [Google Scholar]
- 8.Han L, Sapozhnikova Y, Lehotay SJ. Streamlined sample cleanup using combined dispersive solid-phase extraction and in-vial filtration for analysis of pesticides and environmental pollutants in shrimp. Anal Chim Acta. 2014;827:40–46. doi: 10.1016/j.aca.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Cerqueira MBR, Caldas SS, Primel EG. New sorbent in the dispersive solid phase extraction step of quick easy, cheap, effective, rugged, and safe for the extraction of organic contaminants in drinking water treatment sludge. J Chromatogr A. 2014;1336:10–22. doi: 10.1016/j.chroma.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Lozano A, Rajski L, Belmonte-Valles N, Uclés A, Uclés S, Mezcua M, Fernández-Alba AR. Pesticide analysis in teas and chamomile by liquid chromatography and gas chromatography tandem mass spectrometry using a modified QuEChERS method: validation and pilot survey in real samples. J Chromatogr A. 2012;1268:109–122. doi: 10.1016/j.chroma.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Sapozhnikova Y, Lehotay SJ. Multi-class multi-residue analysis of pesticides, polychlorinated biphenyls, polycylic aromatic hydrocarbons, polybrominated diphenyl ethers and novel flame retardants in fish using fast, low-pressure gas chromatography-tandem mass spectrometry. Anal Chim Acta. 2013;758:80–90. doi: 10.1016/j.aca.2012.10.034. [DOI] [PubMed] [Google Scholar]
- 12.Luo YB, Li X, Jiang XY, Cai BD, Zhu FP, Zhang HF, Chen ZG, Pang YQ, Feng YQ. Magnetic graphene as modified quick easy, cheap, effective, rugged and safe adsorbent for the determination of organochlorine pesticide residues in tobacco. J Chromatogr A. 2015;1406:1–9. doi: 10.1016/j.chroma.2015.05.066. [DOI] [PubMed] [Google Scholar]
- 13.Han Y, Song L, Zou N, Chen R, Qin Y, Pan C. Multi-residue determination of 171 pesticides in cowpea using a modified QuEChERS method with multi-walled carbon nanotubes as reversed-dispersive solid-phase extraction materials. J Chromatogr B. 2016;1031:99–108. doi: 10.1016/j.jchromb.2016.07.043. [DOI] [PubMed] [Google Scholar]
- 14.Kalachova KJ, Pulkrabova T, Cajka L, Drabova, Hajslova J. Implementation of comprehensive two-dimensional gas chromatography?time-of-flight mass spectrometry for the simultaneous determination of halogenated contaminants and polycyclic aromatic hydrocarbons in fish. Anal Bio Anal Chem. 2012;403:2813–2824. doi: 10.1007/s00216-012-6095-3. [DOI] [PubMed] [Google Scholar]
- 15.Lehotay SJ. Determination of pesticide residues in foods by acetonitrile extraction and partitioning with magnesium sulfate: collaborative study. J AOAC Int. 2007;48:485–520. [PubMed] [Google Scholar]
- 16.Krol WJ, Eitzer BD, Arsenault T, Mattina MJI, White JC. Significant improvements in pesticide residue analysis in fodd using the QuEChERS method. LCGC North Am. 2014;32:115–125. [Google Scholar]
- 17.Zheng J, Ding SJ, Zheng QW, Yu BF, Yuan YQ. Magneticone-step Quick, Easy, Cheap Effective Rugged and Safe Method for the Fast Determination of Pesticide Residues in Freshly Squeezed Juice. 2015;1398:1–10. doi: 10.1016/j.chroma.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 18.Han L, Sapozhnikova Y, Lehotay SJ. Method validation for 243 pesticides and environmental contaminants in meats and poultry by tandem mass spectrometry coupled to low-pressure gas chromatography and ultrahigh-performance liquid chromatography. J Food Control. 2016;66:270–282. [Google Scholar]
- 19.Sapozhnikova Y, Lehotay SJ. Review of decent developments and applications in low-pressure (vacuum outlet) gas chromatography. Anal Chim Acta. 2015;899:13–22. doi: 10.1016/j.aca.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Lehotay SJ, Han L, Sapozhnikova Y. Automated mini-Column solid-Phase extraction cleanup for high-Throughput analysis of chemical contaminants in foods by low-Pressure gas Chromatography—Tandem mass spectrometry. Chromatographia. 2016;79:1113–1130. doi: 10.1007/s10337-016-3116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu T, Gu Y, Zheng Y, Wiehl PE, Kopchick JJ. Phase separation of acetonitrile-water mixture in protein purification. Sep Technol. 1994;4:258–260. [Google Scholar]
- 22.Pence DN, Gu T. Liquid-liquid equilibrium of the acetonitrile-water system for protein purification. Sep Technol. 1996;6:261–264. [Google Scholar]
- 23.Gu T, Zhang L. Partition coefficients of some antibiotics, peptides and amino acids in liquid-Liquid partitioning of the acetonitrile-Water system at subzero temperatures. Chem Eng Comm. 2007;194:828–834. [Google Scholar]
- 24.Sapozhnikova Y. Rapid sample preparation and fast GC–MS–MS for the analysis of pesticides and environmental contaminants in fish. LCGC North Am. 2014;32(11):878–886. [Google Scholar]
- 25.Hong J, Kima HY, Kim DG, Seo J, Kimb KJ. Rapid determination of chlorinated pesticides in fish by freezing-lipid filtration, solid-phase extraction and gas chromatography-mass spectrometry. J Chromatogr A. 2004;1038:27–35. doi: 10.1016/j.chroma.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 26.Chen S, Yu X, He X, Xie D, Fan Y, Peng J. Simplified pesticide multiresidue analysis in fish by low-temperature cleanup and solid-phase extraction coupled with gas chromatography/mass spectrometry. Food Chem. 2009;113:1297–1300. [Google Scholar]
- 27.Seo J, Kim HY, Ching BC, Hong J. Simultaneous determination of anabolic steroids and synthetic hormones in meat by freezing-lipid filtration, solid-phase extraction and gas chromatography-mass spectrometry. J Chromatogr A. 2005;1067:303–309. doi: 10.1016/j.chroma.2004.12.063. [DOI] [PubMed] [Google Scholar]
- 28.Ahn YG, Shin JH, Kim HY, Khim J, Lee MK, Hong J. Application of solid-phase extraction coupled with freezing-lipid filtration clean-up for the determination of endocrine-disrupting phenols in fish. Anal Chim Acta. 2007;603:67–75. doi: 10.1016/j.aca.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 29.Norli HR, Christiansen A, Deribe E. Applications of QuEChERS method for extraction of selected persistent organic pollutants in fish tissue and analysis by gas chromatography mass spectrometry. J Chromatogr A. 2011;1218:7234–7241. doi: 10.1016/j.chroma.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 30.Naksen W, Prapamontol T, Mangklabruks A, Chantara S, Thavornyuikarn P, Robson MG, Ryan PB, Barr DB, Panuwet P. A single method for detecting 11 organophosphate pesticides in human plama and breastmilk using GC-FPD. J Chromatogr B. 2016;1025:92–104. doi: 10.1016/j.jchromb.2016.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rawn DFK, Judge J, Roscoe V. Application of the QuEChERS method for analysis of pyrethins and pyrethroids in fish tissue. Anal Bioanal Chem. 2010;397:2525–2531. doi: 10.1007/s00216-010-3786-5. [DOI] [PubMed] [Google Scholar]
- 32.Fletouris DJ. In: Clean-up and Fractionation Methods, Food Toxicants Analysis: Techniques, Strategies and Developments. Pico Y, editor. Elsevier B.V; 2007. pp. 299–348. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.