Abstract
Background
Studies conducted in male rats report that social contact can either facilitate or inhibit drug intake depending on the behavior of social partners. The purpose of the present study was to: (1) examine the effects of social contact on cocaine intake in female rats, (2) examine the behavioral mechanisms by which social contact influences cocaine intake, and (3) examine whether the estrous cycle moderates the effects of social contact on cocaine intake.
Methods
Female rats were assigned to either isolated or pair-housed conditions in which a social partner either had access to cocaine (cocaine partner) or did not have access to cocaine (abstinent partner). Pair-housed rats were tested in custom-built operant conditioning chambers that allowed both rats to be tested simultaneously in the same chamber.
Results
Rats housed with a cocaine partner self-administered more cocaine than isolated rats and rats housed with an abstinent partner. A behavioral economic analysis indicated that these differences were driven by a greater intensity of cocaine demand (i.e., greater intake at lower unit prices) in rats housed with a cocaine partner. Multivariate modeling revealed that the estrous cycle did not moderate the effects of social contact on cocaine intake.
Conclusions
These findings indicate that: (1) social contact influences cocaine self-administration in females in a manner similar to that reported in males, (2) these effects are due to differences in the effects of social contact on the intensity of cocaine demand, and (3) these effects are consistent across all phases of the estrous cycle.
Keywords: behavioral economics, demand curve, estrous, substance use, social environment, social learning
1. Introduction
Peers have a strong influence on an individual’s likelihood of using drugs (Bahr et al., 2005; Simons-Morton and Chen, 2006). According to social learning theory, an individual learns to take drugs in small, informal groups (Bahr et al., 1998; Petraitis et al., 1995). In these settings, individuals are taught through imitation and reinforcement to hold attitudes that are favorable or unfavorable to drug use (Reed and Rountree, 1997). Indeed, individuals may experiment with drugs or alcohol to gain peer approval, but they may also stop using drugs or adopt anti-drug norms because of peer pressure (Teunissen et al., 2012). Adolescent males and females are particularly vulnerable to social influence, but it is thought that adolescent girls are more likely to succumb to peer influence because they tend to have more interpersonal relationships than boys (Downs, 1985).
Preclinical studies have shown that the social environment is a contributing factor to drug use (see reviews by Bardo et al., 2013; Neisewander et al., 2012; Strickland and Smith, 2015; Zernig et al., 2013). For instance, our laboratory has used modified operant conditioning chambers to examine intravenous drug self-administration in multiple rats at the same time and in the same chamber. Using these chambers, we found that cocaine self-administration was facilitated in male rats paired with a cocaine-using partner and inhibited in male rats paired with an abstaining partner (Smith, 2012). In addition, an experienced cocaine-using partner facilitated the acquisition of cocaine self-administration whereas an abstaining partner inhibited the acquisition of cocaine self-administration and reduced the escalation of cocaine intake over time (Robinson et al., 2016; Smith et al., 2014). Taken together, these data emphasize the critical role of the social environment in drug self-administration in males. These findings have not yet been extended to females, which is relevant given the increasing prevalence of substance use disorders in females (Brady et al., 2009; Greenfield et al., 2010) and the fact that females might be more vulnerable to social factors that influence drug use (Downs, 1985; Frajzyngier et al., 2007).
Recent investigators have argued for the expanded use of quantitative analyses of behavior to isolate potential mechanisms that may be responsible for drug effects (Pitts, 2014). Drug self-administration is particularly suited to such quantitative analysis and this may be accomplished through the use of procedures borrowed from economics. For instance, using an econometric analysis, the influence of both the intensity of drug demand (i.e., the consumption of the drug when it is free) and the elasticity of drug demand (i.e., how rapidly consumption decreases when the price increases) may be examined. We previously reported that social housing influences cocaine self-administration in male rats by altering the intensity of cocaine demand but not the elasticity of cocaine demand (Peitz et al., 2013), but these findings have not been extended to females. Males and females often differ in their patterns of drug intake (Lynch et al., 2002), suggesting that the behavioral mechanisms influencing cocaine intake may not be the same for both sexes.
Clinical studies indicate that females are more vulnerable to substance use disorders than males and that this may be attributed to gonadal hormones. Indeed, females initiate cocaine use sooner, take less time to become addicted to cocaine, opioids, and alcohol after initial use, and are at a greater risk for relapse following abstinence compared to males (Becker and Hu, 2008; Lex, 1991). In females, drug-seeking behavior differs across the menstrual cycle in primates (human and non-human) and estrous cycle in rodents (Lynch et al., 2000; Newman et al., 2006; Sofuoglu et al., 1999). For example, women reported increased subjective ratings of euphoria and craving following administration of amphetamine and smoked cocaine during the follicular phase of the menstrual cycle compared to the luteal phase (Evans and Foltin, 2006; Evans et al., 2002; Justice and deWit, 1999; White et al., 2002). Similarly, studies in rodents found that relative to non-estrus females, female rats in estrus reached higher cocaine breakpoints (Hecht et al., 1999; Roberts et al., 1989) and displayed greater responding during extinction and cocaine-primed reinstatement (Feltenstein and See, 2007). These studies provide evidence that fluctuating ovarian hormones influence the subjective effects and self-administration of cocaine and other stimulants, but it is not known how these hormones influence the effects of social contact on drug intake.
The primary aim of the present study was to determine if social contact influences cocaine self-administration in female rats. Female rats were assigned to either isolated or pair-housed conditions in which a social partner either had access to cocaine or did not have access to cocaine. We predicted that cocaine intake would be greatest in rats that had a partner with access to cocaine and least in rats that had a partner without access to cocaine. To determine the mechanisms by which social contact influences cocaine intake we performed an econometric analysis on the dose-response data to see if changes in drug intake were due to changes in the intensity or elasticity of cocaine demand. We also tracked the estrous cycle to determine if estrous moderates the effects of social contact on drug self-administration.
2. Materials and Methods
2.1. Subjects
Female, Long-Evans rats were obtained at weaning (~21 days) and randomly assigned to isolated or socially housed conditions upon arrival and placed in polycarbonate “shoebox” cages (interior dimensions: 50 × 28 × 20 cm) for six weeks. After six weeks, rats were transferred to custom-built, operant conditioning chambers that served as home cages for the remainder of the study (see description below). Isolated subjects (n = 13) were housed individually without a social partner. Socially housed rats were subdivided randomly into two groups: in one group (n = 10), subjects were housed with a social partner with access to cocaine (cocaine partner); in the other group, subjects (n = 10) were housed with a social partner that did not have access to cocaine (abstinent partner). Food and water were freely available in the home cages except during the brief period of lever press training (see below). Throughout the study, subjects were maintained on a 12-hr light/dark cycle (lights on: 0500) in a temperature- and humidity-controlled colony room. All subjects were maintained in accordance with the guidelines of the Animal Care and Use Committee of Davidson College.
2.2. Apparatus
Rats were trained to lever press using food reinforcement in commercially available operant conditioning chambers (Med Associates Inc., St. Albans, VT). Each chamber was equipped with a single houselight, two response levers, and a food hopper. Experimental events were programmed and data were collected with software and interfacing from Med Associates, Inc. (St. Alban, VT, USA).
All drug self-administration sessions took place in operant conditioning chambers custom made by Faircloth Machine Shop (Winston-Salem, NC, USA). These chambers were IACUC-approved for the long-term housing of rats and served as home cages throughout the period of behavioral testing. Chambers for isolated rats consisted of one 30 × 30 × 30 cm chamber constructed with stainless steel and aluminum. Chambers for pair-housed rats were constructed from two chambers, each with one sidewall removed, and connected with a wire screen. The wire screen permitted rats full visual, auditory, olfactory, and limited tactile contact, but prevented one rat from accessing the response lever and infusion lines of its partner. Each chamber was equipped with one retractable response lever and an infusion pump mounted outside the chamber. Drug infusions were delivered through a Tygon tube protected by a stainless steel spring and attached to a counterbalanced swivel at the top of the chamber. Response levers, syringe pumps, interfacing, and computer software were obtained from Med Associates, Inc. Foam insulation panels (2.5 cm thickness) were placed between chambers to attenuate extraneous sounds and prevent a direct line of sight to other rats in the colony (for further description and images, see Lacy et al., 2014b; Smith, 2012)
2.3. Lever-Press Training
Five weeks after arrival and one week prior to catheter implantation, rats were lightly food restricted to no less than 90% of their free-feeding body weight and trained to press a response lever on a fixed ratio (FR1) schedule of food reinforcement. On this schedule, each response produced a 45 mg food pellet delivered to a food hopper located between the two response levers. Sessions terminated automatically once 40 reinforcers were delivered or 2 hr elapsed, whichever occurred first. Training continued in this manner until a rat earned the maximum number of reinforcers over four days.
2.4. Estrous cycle monitoring
Concurrent with the beginning of lever press training, daily collection of vaginal cells (via lavage) began in female subjects. Samples were collected and analyzed using light microscopy (×100) less than 1 hr before each self-administration session. The cells were categorized into one of four estrous phases: metaestrus, diestrus, proestrus, and estrus (Goldman et al., 2007; Hubscher et al., 2005; Marcondes et al., 2002).
2.5. Catheter implantation
Rats were anesthetized with a combination of ketamine (100 mg/kg, ip) and xylazine (15 mg/kg, ip) and a catheter was implanted in the right jugular vein and exited on the dorsal surface between the scapulae (Lacy et al., 2014a; Smith et al., 2008). Ketoprofen (3.0 mg/kg, sc) was given immediately after surgery as a post-operative analgesic and again 24 hr later. Beginning on the day of surgery, a solution of heparinized saline and ticarcillin (20 mg/kg, iv) was infused through the catheter daily to prevent infection and maintain patency. After seven days, ticarcillin was discontinued and only heparinized saline was used to maintain catheter patency. Wounds were treated with a topical antibiotic ointment for two days after surgery. All animals were allowed to recover for at least three days before beginning self-administration training.
2.6. Self-a2dministration training
Following recovery from surgery, self-administration sessions were conducted daily for rats in the isolated condition and pair-housed rats assigned to self-administer cocaine. Each training and testing session began promptly at the start of the dark cycle (1700) with insertion of the retractable lever into the chamber and a noncontingent infusion of the dose of cocaine available during that session. Pair-housed rats assigned to no-access conditions had an inactive response lever during their partner’s self-administration sessions. Self-administration training sessions were conducted daily for five days. During these sessions, responding was reinforced on an FR1 schedule of reinforcement with 0.5 mg/kg/infusion cocaine. Coincident with each infusion, the response lever retracted for 20 s to signal a post-infusion timeout in which cocaine was not available. After 20 s, the lever extended back into the chamber and cocaine was once again available. All sessions terminated automatically after 2 hr. Each infusion delivered cocaine over a 2.0- to 2.4-s infusion duration (based on body weight).
2.7. Self-administration testing
After seven days of training, self-administration testing commenced and a cocaine dose-effect curve was generated over the next five consecutive sessions. During each of these daily test sessions, a single dose of cocaine was available following each lever press on the FR1 schedule throughout the session. The dose of cocaine was changed prior to the beginning of each session; otherwise, all conditions were identical to those present during training. Four doses of cocaine (0.03, 0.1, 0.3 and 1.0 mg/kg/infusion) and saline were tested across sessions in a pseudo-random order with the stipulation that no more than two ascending or descending doses could be tested in a row.
2.8. Data analysis
For the dose-response analysis, the number of infusions was examined via mixed-factor ANOVA, with group serving as the between-subjects factor and dose serving as the repeated measure. An area under the curve (AUC) analysis was then conducted to isolate the effects of group using the trapezoidal rule. Linear and quadratic patterns of active lever pressing (cocaine rats), inactive lever pressing (abstinent rats), and cocaine intake were analyzed where appropriate using polynomial contrasts.
Economic measures of demand intensity and demand elasticity were determined using the exponential demand equation described by Hursh and Silberburg (2008):
Where Q = consumption; Q0 = intensity of demand (consumption at unconstrained price); k = constant denoting consumption range in log10 units (k = 3.0 for observed data set); C = unit price (responses/mg cocaine); and α = elasticity of demand. Greater values of Q0 indicate greater consumption at unconstrained price (i.e., a hypothetical zero price). Greater values of α indicate greater elasticity (i.e., a greater sensitivity to increase in unit price). These values were compared across groups via one-way ANVOA.
Effect sizes were calculated as partial eta-squared (ηp2) and were considered large if ηp2 ≥ .14 according to standard statistical definitions (Cohen, 1988). All post-hoc tests were conducted using Tukey’s honestly significant difference (HSD) test for multiple comparisons. All statistical tests were two-tailed and the alpha level was set at .05.
The effects of estrous were examined using multilevel, multivariate modeling techniques. We chose to use multilevel modeling because of the unbalanced design of the estrous data (i.e., a rat was tested only once at a given dose, and thus only one phase of estrous is represented for that rat at that dose) resulting in subjects without complete data across all doses and all estrous phases. Multilevel analyses are preferred in these types of samples because they allow for inclusion of subjects with “missing” data as opposed to ANOVA methods that require complete data from all cases. Such methods improve power by allowing for improved estimation with a larger sample while also protecting against type I error rates. In this study, a longitudinal mixed model analysis was performed using SAS proc.mixed (Littell et al., 2006) to account for the nesting of the data. Restricted maximum likelihood (REML) estimation was used along with a random intercept. Akaike’s information criterion (AIC) was used to compare the fit of various error covariance structures: compound symmetry, autoregressive (1), and unstructured. The unstructured model formulation provided the best fit. The final model included dose and estrous cycle as level 1 terms, group as a level 2 term, and the dose x group cross-level interaction.
3. Results
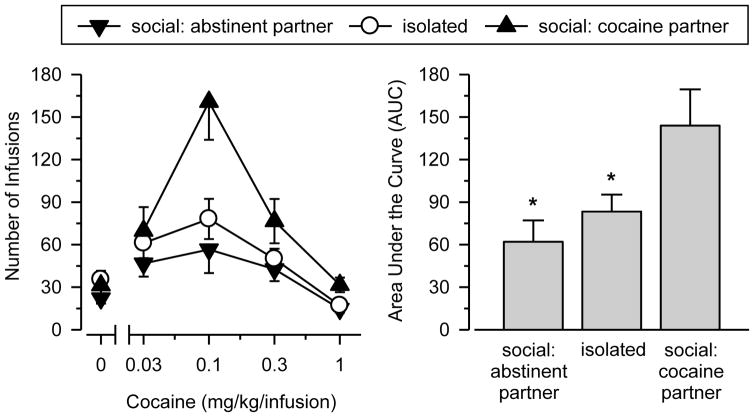
All rats acquired cocaine self-administration on the first day of testing and showed regular patterns of responding (characterized by a “load-up” phase followed by stable post-infusion pauses for the remainder of the session) by the fifth day of testing. Figure 1 shows that cocaine self-administration was characterized by an inverted U-shaped dose-effect curve, with peak responding occurring at 0.1 mg/kg/infusion in all groups [main-effect of dose: F (3, 90) = 38.780, p < .001]. Cocaine self-administered also differed significantly across groups [main effects of group: F (2, 30) = 4.686, p = .017]. Differences across groups varied as a function of dose [dose x group interaction: F (6, 90) = 6.402, p < .001], with the greatest differences observed at 0.1 (p = .002) and 1.0 (p = .002) mg/kg/infusion. No significant differences were observed across groups in the saline substitution test. An AUC analysis of the dose-response data revealed a statistically significant effect of group [F (2, 30) = 5.434, p = .010] and a large effect size (ηp2 = .266). In the AUC analysis, socially housed rats with a cocaine partner yielded significantly greater AUC values than either isolated rats (p = .048) or socially housed rats with an abstinent partner (p = .010).
Figure 1. Social Contact Influences Cocaine Self-Administration.
Left Panel: Responding maintained by cocaine on an FR1 schedule of reinforcement. Data are shown for socially housed rats with an abstinent partner [social: abstinent partner; n = 10], isolated rats [isolated; n = 13], and socially housed rats with a cocaine partner [social: cocaine partner = 10]. Vertical axis depicts number of infusions during 2-hr session. Horizontal axis depicts dose of cocaine in mg/kg/infusion. Points above 0.0 depict the effects of saline. Vertical lines indicate SEM; where not indicated, the SEM fell within the data point. Right Panel: Area under the curve (AUC) estimates for cocaine in rats responding on an FR1 schedule of reinforcement. Vertical lines indicate SEM. Asterisks (*) indicate significant difference from socially housed rats with a cocaine partner (p < .05).
Using the dose-response data, we performed an economic demand analysis to isolate the effects of demand intensity and demand elasticity for cocaine. In this analysis, consumption was plotted as a function of unit price and an exponential demand curve was fit to the data (Figure 2). Nonlinear mixed effects modeling indicated that the intensity of cocaine demand (Q0) differed significantly across groups [F (2, 30) = 7.499, p = .002] with a large effect size (ηp2 = .333). A post-hoc analysis further indicated that socially housed rats with a cocaine partner exhibited greater intensity of cocaine demand than either isolated rats (p = .008) or socially housed rats with an abstinent partner (p = .008). Differences in the elasticity of cocaine demand (α) were also observed across groups; these differences were characterized by a large effect size (ηp2 = .144), but they failed to reach statistical significance (p = .097).
Figure 2. Social Contact Influences Intensity of Cocaine Demand.
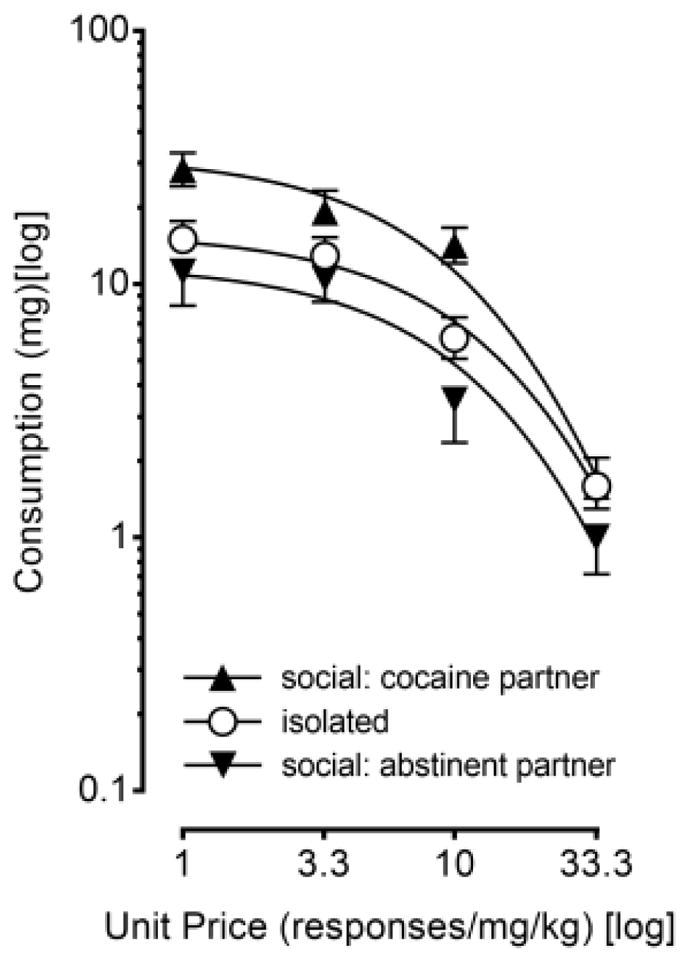
Demand curves computed from the dose-response data of socially housed rats with an abstinent partner, isolated rats, and socially housed rats with a cocaine partner. Vertical axis depicts consumption (measured as intake in mg/kg in log units). Horizontal axis depicts unit price (depicted as responses/mg/kg in log units). An exponential demand equation was fit to the data and plotted as mean values. Vertical lines surrounding data points represent the SEM; where not indicated, the SEM fell within the data point.
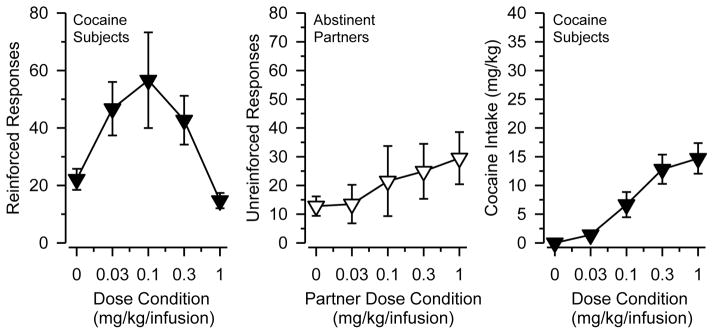
Figure 3 (center) depicts non-reinforced responses in abstinent rats in comparison to the cocaine-reinforced responses (left) and total cocaine intake (right) of their cocaine self-administering partners. The non-reinforced responses of abstinent rats did not vary significantly as a function of their partners’ dose condition; however, a polynomial contrast revealed a significant linear trend in these data [F (1, 9) = 6.440, p = .032]. A similar linear trend was observed for their social partners’ total cocaine intake [F (1, 9) = 35.024, p < .001]. In contrast, the number of cocaine-reinforced responses of their social partner followed a quadratic trend [F (1, 9) = 10.699, p = .010]. Thus, non-reinforced responding in abstinent rats varied more as a function of their partner’s presumed level of intoxication (i.e., cocaine intake) than as a function of their partner’s level of cocaine-reinforced lever pressing.
Figure 3. Non-reinforced Responding of Abstinent Rats Mirrors Cocaine Intake of Social Partners.
Left Panel: Cocaine-reinforced responses of socially housed rats with an abstinent partner (data are redrawn from Figure 1). Center Panel: Unreinforced responses of abstinent partners without access to cocaine. Right Panel: Cocaine intake (mg/kg) of socially housed rats with an abstinent partner. Horizontal axes reflect cocaine dose condition (mg/kg/infusion) of the partner with access to cocaine. Vertical lines indicate SEM; where not indicated, the SEM fell within the data point. Polynomial contrasts revealed that the non-reinforced responding of abstinent partners followed a linear (but not quadratic) trend. Cocaine-reinforced responding of their social partners followed a quadratic (but not linear) trend, but cocaine-intake of their social partners followed a linear (but not quadratic) trend.
A mixed linear effects model was used to evaluate the effects of the estrous cycle on responding. When data were collapsed across dose and group, responding was greatest during estrous, but the main effect was not statistically significant (p = .053). Importantly, the estrous cycle did not interact significantly with any other factor of the model (i.e., group and dose), suggesting that estrous does not moderate the group and dose effects described above.
4. Discussion
The goals of the present study were to determine the effects of social contact on cocaine self-administration in female rats, to determine the behavioral mechanisms through which social contact influences cocaine self-administration, and to determine if these effects change across the estrous cycle. Rats were assigned to either isolated or pair-housed conditions in which a social partner either had access to cocaine or did not have access to cocaine. Our results indicate that rats that were socially housed with a cocaine partner self-administered significantly more cocaine than isolated rats and rats housed with an abstinent partner. An econometric analysis of the data suggested the increase in cocaine intake in rats with a cocaine partner was due to a greater intensity of cocaine demand in this group. These effects were consistent across all four phases of the estrous cycle, suggesting that estrous does not influence the effects of social contact on cocaine self-administration.
We examined cocaine intake during self-administration sessions in which female rats were isolated or paired with a cocaine or abstinent partner. Rats that were paired with a cocaine partner self-administered more cocaine than both isolated rats and rats paired with an abstinent partner. These results are consistent with previous studies with male rats that found cocaine self-administration was greater in male rats housed with a cocaine partner than male rats housed with an abstinent partner (Smith, 2012; Smith et al., 2014). In addition, female rats housed with a cocaine partner self-administered more cocaine than isolated rats, indicating a robust facilitation effect of the social environment in females. These results differ somewhat from findings with male rats that found rats housed with an abstinent partner self-administered less cocaine than isolated rats, suggesting a more robust inhibitory effect of the social environment in males (Peitz et al., 2013; Robinson et al., 2016). The facilitation of cocaine intake in females is especially relevant given that females have more interpersonal relationships than males (Downs, 1985) and are more vulnerable to substance use disorders than males (for review see, Anker and Carroll, 2010; Roth et al., 2004). It should be noted that repeated vaginal lavage (as was done in the present study) attenuates cocaine-induced locomotor activity and abolishes the effects of the estrous cycle on cocaine-induced locomotor activity (Walker et al., 2002). Thus, caution should be taken when comparing these female rats to male rats from previous studies on measures of cocaine-mediated effects.
An econometric analysis was performed to determine how social contact influences the reinforcing strength of cocaine in female rats. This analysis isolated two independent contributors of reinforcing strength: intensity of drug demand and elasticity of drug demand. Demand intensity refers to the consumption of the drug when it is free and demand elasticity refers to how rapidly consumption decreases when the price increases. Our results indicate that socially housed rats with a cocaine partner exhibited greater intensity of cocaine demand than either isolated rats or socially housed rats with an abstinent partner. In other words, socially housed rats with a cocaine partner had higher levels of cocaine intake when the cost of the drug approached zero. Demand elasticity showed a similar pattern of effects, but those effects failed to reach statistical significance despite a large effect size. A previous study with male rats also found that social contact influenced the intensity of cocaine demand (Peitz et al., 2013). In that study, male rats housed with an abstinent partner exhibited lower demand intensity than isolated rats or rats housed with a cocaine partner, and those effects were observed in the absence of significant differences in demand elasticity. Taken collectively, these data provide further evidence that social contact may have more of an inhibitory effect in male rats (at least when housed with an abstinent partner) and more of a facilitation effect in female rats (at least when housed with a drug-using partner). Regardless, the present study indicates that demand intensity and demand elasticity can impact drug consumption independently, and that the intensity of cocaine demand is particularly sensitive to the social environment.
A critical aim of this study was to determine how the estrous cycle moderates the effects of social contact on cocaine self-administration. Using a multilevel multivariate analysis, responding was greatest during estrus, but the main effect of cycle failed to reach statistical significance (p = .053). Previous studies have generally reported that cocaine seeking varies across the estrous cycle, with greater levels of responding observed during estrus (Feltenstein and See, 2007; Hecht et al., 1999; Lacy et al., 2016; Lynch et al., 2000; Roberts et al., 1989). Importantly, the estrous cycle did not moderate the effects of social contact on cocaine self-administration, meaning that the effects of social contact described above were consistent across all four phases of estrous. We previously reported that social contact did not influence the effects of the estrous cycle on heroin self-administration (Lacy et al., 2016), suggesting that these findings are consistent across different drug classes. Collectively, these results suggest that ovarian hormones do not moderate the effects of social contact on measures of drug-seeking behavior.
In addition to the behavioral mechanisms revealed by the econometric analysis, a number of social learning mechanisms may also be influencing cocaine intake in pair-housed rats. Indeed, factors such as imitation, conditioned reinforcement, social reinforcement, social facilitation, stimulus enhancement, and reinforcement enhancement may all be playing a role in the facilitation of cocaine self-administration observed in this study (for descriptions and examples of these various mechanisms, see Strickland and Smith, 2014). We previously reported that patterns of cocaine-maintained responding become progressively more similar over time in pair-housed male rats in which both partners have access to cocaine, and these effects are observed in the absence of changes in cocaine intake (Lacy et al., 2014b). Specifically, patterns of responding on a fixed-interval schedule became more similar within (but not across) social dyads across five consecutive days, suggesting that pair-housed rats learn to imitate the behavior of their social partners. Although this finding does not rule out other social learning mechanisms, it is consistent with epidemiological studies describing drug use within peer groups (Bot et al., 2005; Salvy et al., 2014).
A previous study conducted in male rats revealed that non-reinforced responding of abstinent rats mimicked the cocaine-reinforced responding of their cocaine partners (Smith, 2012). In that study, the number of inactive lever presses emitted by abstinent partners mirrored the active lever responding of their cocaine partners (which followed a biphasic pattern), but did not track the cocaine intake of their cocaine partners (which followed a linear pattern). In the present study, the number of inactive lever presses emitted by abstinent females tracked the cocaine intake of their cocaine partners (which increased linearly) but not the number of active lever presses (which followed a biphasic pattern). These data suggest that social learning mechanisms related to modeling and imitation may differ between males and females; however, it must be noted that the mechanisms controlling non-reinforced responding are likely to be different from those controlling cocaine-reinforced responding.
From a translational perspective, these findings suggest that a female’s social environment may influence the likelihood that she will use (and potentially abuse) cocaine. Specifically, extensive social contact with another cocaine-using female may increase the use of cocaine and similar drugs. Furthermore, females may be especially susceptible to these effects when cocaine is readily available and the cost is low. Consequently, future interventions aimed at substance use among females should focus on strategies to reduce drug use when drugs are easily accessible and available at low costs.
Highlights.
The effects of social contact on cocaine intake were examined in female rats
A partner with access to cocaine increased cocaine self-administration
A partner with access to cocaine increased the intensity of cocaine demand
Estrous cycle did not influence the effects of social contact on cocaine intake
Acknowledgments
The authors thank Charlotte Magee and Alexander Casimir for expert technical assistance and the National Institute on Drug Abuse for supplying the study drug.
Footnotes
Contributors
M.A. Smith developed the project. A.M. Robinson, G.E. Fronk, and H. Zhang collected all data for the project. S. Tonidandel assisted in data analysis and interpretation. A.M. Robinson drafted the manuscript and M.A. Smith edited the manuscript. All authors approved the manuscript for submission.
Conflict of Interest
No conflict declared.
Role of Funding Sources
This study was funded by NIH Grant DA031725 (MAS). The NIH had no role in the design of the study; in the collection, analysis, and interpretation of the data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anker JJ, Carroll ME. Females are more vulnerable to drug abuse than males: Evidence from preclinical studies and the role of ovarian hormones. In: Neill JC, Kulkarni J, editors. Biological Basis of Sex Differences in Psychopharmacology. Springer Berlin Heidelberg; New York: 2010. pp. 73–96. [DOI] [PubMed] [Google Scholar]
- Bahr SJ, Hoffmann JP, Yang X. Parental and peer influences on the risk of adolescent drug use. J Prim Prev. 2005;26:529–551. doi: 10.1007/s10935-005-0014-8. [DOI] [PubMed] [Google Scholar]
- Bahr SJ, Maughan SL, Marcos AC, Li B. Family, religion, and the risk of adolescent drug use. J Marriage Fam. 1998;60:979–992. [Google Scholar]
- Bardo MT, Neisewander JL, Kelly TH. Individual differences and social influences on the neurobehavioral pharmacology of abused drugs. Pharmacol Rev. 2013;65:255–290. doi: 10.1124/pr.111.005124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Hu M. Sex differences in drug abuse. Front Neuroendocrinol. 2008;29:36–47. doi: 10.1016/j.yfrne.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Back SE, Greenfield SF, editors. Women And Addiction: A Comprehensive Handbook. 1. Guilford Press; New York: 2009. [Google Scholar]
- Bot SM, Engels RC, Knibbe RA, Meeus WH. Friend’s drinking behaviour and adolescent alcohol consumption: The moderating role of friendship characteristics. Addict Behav. 2005;30:929–947. doi: 10.1016/j.addbeh.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Downs WR. Using panel data to examine sex differences in causal relationships among adolescent alcohol use, norms, and peer alcohol use. J Youth Adolesc. 1985;14:469–486. doi: 10.1007/BF02139521. [DOI] [PubMed] [Google Scholar]
- Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659–674. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, See RE. Plasma progesterone levels and cocaine-seeking in freely cycling female rats across the estrous cycle. Drug Alcohol Depend. 2007;89:183–189. doi: 10.1016/j.drugalcdep.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frajzyngier V, Neaigus A, Gyarmathy VA, Miller M, Friedman SR. Gender differences in injection risk behaviors at the first injection episode. Drug Alcohol Depend. 2007;89:145–152. doi: 10.1016/j.drugalcdep.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: Characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Back SE, Lawson K, Brady KT. Substance abuse in women. Psychiatr Clin North Am. 2010;33:339–355. doi: 10.1016/j.psc.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht GS, Spear NE, Spear LP. Changes in progressive ratio responding for intravenous cocaine throughout the reproductive process in female rats. Dev Psychobiol. 1999;35:136–145. [PubMed] [Google Scholar]
- Hubscher CH, Brooks DL, Johnson JR. A quantitative method for assessing stages of the rat estrous cycle. Biotechnic Histochem. 2005;80:79–87. doi: 10.1080/10520290500138422. [DOI] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A. Economic demand and essential value. Psychol Rev. 2008;115:186–98. doi: 10.1037/0033-295X.115.1.186. [DOI] [PubMed] [Google Scholar]
- Justice AJ, de Wit H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- Lacy RT, Strickland JC, Brophy MK, Witte MA, Smith MA. Exercise decreases speedball self-administration. Life Sci. 2014a;114:86–92. doi: 10.1016/j.lfs.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy RT, Strickland JC, Feinstein MA, Robinson AM, Smith MA. The effects of sex, estrous cycle, and social contact on cocaine and heroin self-administration in rats. Psychopharmacology. 2016;233:3201–3210. doi: 10.1007/s00213-016-4368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy RT, Strickland JC, Smith MA. Cocaine self-administration in social dyads using custom-built operant conditioning chambers. J Neurosci Methods. 2014b;236:11–18. doi: 10.1016/j.jneumeth.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lex BW. Some gender differences in alcohol and polysubstance users. Health Psychol. 1991;10:121–132. doi: 10.1037//0278-6133.10.2.121. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for Mixed Models. SAS Institute; Cary, NC: 2006. [Google Scholar]
- Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology. 2000;152:132–139. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: Preclinical and clinical studies. Psychopharmacology. 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Bio. 2002;62:609–614. doi: 10.1590/s1519-69842002000400008. [DOI] [PubMed] [Google Scholar]
- Neisewander JL, Peartree NA, Pentkowski NS. Emotional valence and context of social influences on drug abuse-related behavior in animal models of social stress and prosocial interaction. Psychopharmacology. 2012;224:33–56. doi: 10.1007/s00213-012-2853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JL, Thorne JJ, Batulis DK, Carroll ME. Effects of menstrual cycle phase on the reinforcing effects of phencyclidine (PCP) in rhesus monkeys. Pharmacol Biochem Behav. 2006;85:584–591. doi: 10.1016/j.pbb.2006.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peitz GW, Strickland JC, Pitts EG, Foley M, Tonidandel S, Smith MA. Peer influences on drug self-administration: An econometric analysis in socially housed rats. Behav Pharmaco. 2013;24:114–123. doi: 10.1097/FBP.0b013e32835f1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petraitis J, Flay BR, Miller TQ. Reviewing theories of adolescent substance use: Organizing pieces in the puzzle. Psychol Bull. 1995;117:67–86. doi: 10.1037/0033-2909.117.1.67. [DOI] [PubMed] [Google Scholar]
- Pitts RC. Reconsidering the concept of behavioral mechanisms of drug action. J Exp Anal Behav. 2014;101:422–441. doi: 10.1002/jeab.80. [DOI] [PubMed] [Google Scholar]
- Reed MD, Rountree PW. Peer pressure and adolescent substance use. J Quant Criminol. 1997;13:143–180. [Google Scholar]
- Roberts DCS, Bennett SAL, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology. 1989;98:408–411. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- Robinson AM, Lacy RT, Strickland JC, Magee CP, Smith MA. The effects of social contact on cocaine intake under extended-access conditions in male rats. Exp Clin Psychopharmacol. 2016;24:285. doi: 10.1037/pha0000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth ME, Cosgrove KP, Carroll ME. Sex differences in the vulnerability to drug abuse: A review of preclinical studies. Neurosci Biobehav Rev. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Salvy SJ, Pedersen ER, Miles JN, Tucker JS, D’Amico EJ. Proximal and distal social influence on alcohol consumption and marijuana use among middle school adolescents. Drug Alcohol Depend. 2014;144:93–101. doi: 10.1016/j.drugalcdep.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons-Morton B, Chen RS. Over time relationships between early adolescent and peer substance use. Addict Behav. 2006;31:1211–1223. doi: 10.1016/j.addbeh.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmaco. 1999;7:274–283. doi: 10.1037//1064-1297.7.3.274. [DOI] [PubMed] [Google Scholar]
- Smith MA, Schmidt KT, Iordanou JC, Mustroph ML. Aerobic exercise decreases the positive-reinforcing effects of cocaine. Drug Alcohol Dep. 2008;98:129–135. doi: 10.1016/j.drugalcdep.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA. Peer influences on drug self-administration: Social facilitation and social inhibition of cocaine intake in male rats. Psychopharmacology. 2012;224:81–90. doi: 10.1007/s00213-012-2737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Lacy RT, Strickland JC. The effects of social learning on the acquisition of cocaine self-administration. Drug Alcohol Dep. 2014;141:1–8. doi: 10.1016/j.drugalcdep.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland JC, Smith MA. The effects of social contact on drug use: Behavioral mechanisms controlling drug intake. Exp Clin Psychopharmacol. 2014;22:23–34. doi: 10.1037/a0034669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland JC, Smith MA. Animal models of social contact and drug self-administration. Pharmacol Biochem Behav. 2015;136:47–54. doi: 10.1016/j.pbb.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunissen HA, Spijkerman R, Prinstein MJ, Cohen GL, Engels RC, Scholte RH. Adolescents’ conformity to their peers’ pro- alcohol and anti- alcohol norms: The power of popularity. Alcohol Clin Exp Res. 2012;36:1257–1267. doi: 10.1111/j.1530-0277.2011.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Nelson CJ, Smith D, Kuhn CM. Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats. Pharmacol Biochem Behav. 2002;73:743–752. doi: 10.1016/s0091-3057(02)00883-3. [DOI] [PubMed] [Google Scholar]
- White TL, Justice AJ, de Wit H. Differential subjective effects of D-amphetamine by gender, hormone levels and menstrual cycle phase. Pharmacol Biochem Behav. 2002;73:729–741. doi: 10.1016/s0091-3057(02)00818-3. [DOI] [PubMed] [Google Scholar]
- Zernig G, Kummer KK, Prast JM. Dyadic social interaction as an alternative reward to cocaine. Front Psychiatry. 2013;4:100. doi: 10.3389/fpsyt.2013.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]