Introduction
Many health interventions are used by far fewer patients and at less frequent intervals than recommended by clinical guidelines. Preventive care, screenings, vaccinations, and treatments may be highly cost-effective when used as directed [1]. However, when interventions are not aligned with patients’ needs, low uptake and poor adherence can squander limited resources. To improve uptake, adherence, and efficiency, interventions must take into account the preferences of the intended target populations.
Health interventions often require more than a patient’s consent and out-of-pocket payment: they also require the patient’s time, energy, and attention, and they require acceptance of risks and side effects. For example, a vaccination or a session with a physical therapist may be recommended and free, but the patient can choose to forgo these services. Behavioral economics asks a simple question as a model of rational behavior: Do the expected benefits of an intervention exceed the perceived costs? If the answer to this question is “No” for an individual patient at a given point in time, uptake declines, adherence fails, and resources are wasted—regardless of guidelines or scientific evidence on cost-effectiveness. In this paper on health preference research (HPR), we review motivations for patient preference studies, discuss common assumptions about their relevance, and outline the next steps toward improving uptake, adherence, and efficiency of high-value health interventions.
Why Conduct a Patient Preference Study?
Occasionally, the balance of benefits and costs of an intervention can be adjusted in simple ways, like by reducing the amount paid out-of-pocket. However, in addition to out-of-pocket costs, patients incur costs in terms of time, inconvenience, invasiveness, and embarrassment, and each patient has different constraints: some lack money, a few lack time, and others lack attention. Health preference tools systematically elicit patients’ preferences and constraints so that policy makers and providers can adapt the healthcare experience to patients’ needs.
Traditionally, the reasons underlying the suboptimal use of health interventions have been identified retrospectively (e.g., through program monitoring, satisfaction surveys, and statistical analysis of claims). Preference evidence, by contrast, may concurrently or even prospectively identify misalignments between intervention attributes and the needs of target populations. Once misalignments are known, systematic adaptations of attributes may improve uptake and efficiency and avoid incremental trial-and-error implementations that are often costly, time consuming, non-systematic, and limited in scope.
Methods for Understanding Patient Preferences
HPR conceptualizes interventions as combinations of positive and negative attributes [2,3]. HPR methods identify patients’ preferences for these attributes as well as any misalignments between preferences and intervention attributes. To advance the use of HPR for designing preference-informed health interventions, future research should evaluate the validity of four assumptions.
Assumption 1
HPR can be used to elicit patient preferences on intervention attributes, and the resulting preference estimates are valid and reliable.
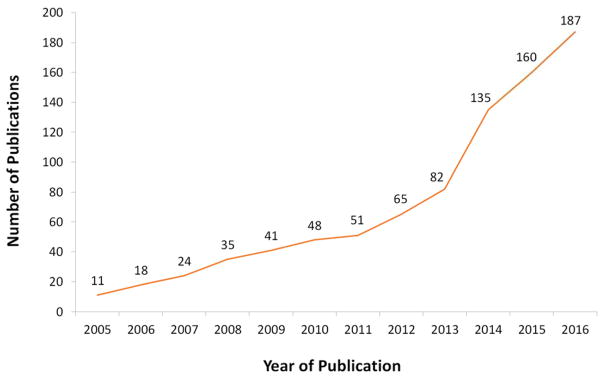
There has been a rapid increase in the number of HPR studies in recent years (Figure 1). The range of populations, topics, and geographic settings studied has largely resolved concerns about the feasibility of HPR methods, including discrete choice experiments (DCEs), within different economic or cultural contexts.
Figure 1.
Publications on Discrete Choice Experiments (DCEs) in PubMed
Despite the growing popularity, questions remain. In HPR, internal validity is a matter of cognitive burden. Real-world choices may involve almost unlimited factors, but preference surveys focus on a handful of attributes chosen by researchers. Because the validity of DCEs depends on the accuracy of the underlying decision model, the field must systematically compare the potential bias garnered by omitting relevant intervention attributes with the effects of an overly complex, but more realistic survey (for example, when asking participants to consider too many attributes or complex attributes such as risks). External validity is also a concern. How applicable are preference estimates from one population, time, intervention, or setting to another?
Assumption 2
Patient preference evidence can be used to improve the design and implementation of health interventions.
Until recently, HPR studies have only estimated the average preferences of populations. With an increasing focus on preference heterogeneity, HPR holds great potential to inform patient-centered care by matching interventions to preference profiles in specific target populations. Adoption of statistical techniques like latent class models and Bayesian analyses can increase the utility of HPR by generating estimates for subgroups or even individual patients [4]. Preference-elicitation tools for individual patients can inform the design of customized health interventions, and help to increase the efficiency of care by focusing providers’ limited time on discussing what matters most to each patient. By focusing on patient preferences and trade-offs using an experimental approach, systematically designed stated preference surveys may reduce social desirability and other biases that are often seen with more direct questioning [5]. (For example: patients may be reluctant to indicate that they would have preferred less interaction with a counselor; or they might mark all in a series of intervention characteristics as ‘very important.’) Finally, HPR can complement implementation science when used as a diagnostic tool to identify barriers to and facilitators of treatment engagement as well as to help policymakers and clinicians customize interventions and communication strategies in new settings [6,7].
Assumption 3
Designing health interventions to better meet the needs of patients not only improves patient satisfaction, but improves patient behaviors such as uptake and adherence.
So far, we have assumed that the availability of preference-informed interventions will impact patients’ behaviors about uptake and adherence. However, two intermediate conditions may not be met. First, a patient may have imperfect information about an intervention and its alternatives, including all of their attributes (e.g., availability, risks, costs, and benefits). When eliciting preferences using DCEs, participants are asked to make choices on the basis of specific information that is described in the survey instrument. This level of control may be difficult to achieve in practice. Because of this, future research should investigate the extent to which factors such as misinformation or uncertainty can explain the differences between stated preferences and actual behavior. Second, some patients may not want full decision-making control in all circumstances, and caregivers, providers, or payers may limit their control.
Assumption 4
Improvements in patient satisfaction, uptake, and adherence due to HPR lead to better health and, ultimately, to more efficient healthcare systems.
Preference-informed health interventions may increase patient satisfaction, uptake, and adherence; however, not all increases provide equal value and/or are desirable from a payer perspective. For example, it is plausible that some negative attributes of health interventions were designed to deter patients from over-utilization (e.g., waiting periods), and it is plausible that some patients prefer less intensive or less cost-effective care. Ultimately HPR will be judged by the effects of preference-informed interventions on health (e.g., survival and health-related quality of life) and on the amount of resources that are required to achieve these improvements.
Next Steps in Patient Preference Studies
By identifying which attributes matter most, HPR promises to identify which attribute combinations are most valued by patients and may, therefore, facilitate the design of preference-informed health interventions. The four assumptions above outline a far-reaching research agenda for the field of HPR. One additional consideration that remains is the hypothetical nature and framing of DCEs. While preference studies on health interventions intend to resemble real-world choices, responses to a survey may differ from patient choices because: (1) actual choices have actual implications for time investments, expenditures, pain, risks, or health outcomes; (2) these choices may take into account the preferences and recommendations of others; and (3) the patients who face these choices may differ from those who complete the surveys. Several recent studies showed good concordance between patients’ stated and revealed preferences [8–10]. However, additional studies will be needed to evaluate in which situations preference evidence can accurately predict uptake and adherence of various types of health interventions. Despite these limitations and the need to further test common assumptions as detailed above, there is great potential for HPR to serve as a broader health system resource for guiding patient-centered care and customizing interventions. A robust, patient-centered approach to the design of health interventions holds significant potential for improving uptake, adherence, and efficiency.
Acknowledgments
The authors gratefully acknowledge the administrative support for the preparation of this manuscript from the International Academy of Health Preference Research (IAHPR). Drs. Mühlbacher and Brown serve on the Board of the IAHPR Foundation. Dr. Mühlbacher is also a founding co-chair of the Health Preference Research special interest group at the International Society of Quality of Life Research and co-chair of the Stated Preference Research in the European Union Working Group of the International Society for Pharmacoeconomics and Outcomes Research.
Funding: No funding was received for the preparation of this article.
Footnotes
Compliance with Ethical Standards
Conflict of interest: Jan Ostermann, Derek S. Brown, Esther W. de Bekker-Grob, Axel C. Mühlbacher, and Shelby D. Reed have no conflicts of interest directly relevant to the content of this article.
Contributor Information
Jan Ostermann, Department of Health Services Policy & Management, Arnold School of Public Health, University of South Carolina, 915 Greene Street, Discovery Bldg., Ste. 358, Columbia, SC 29208, USA, Center for Health Policy and Inequalities Research, Duke Global Health Institute, Duke University, Durham, NC USA.
Derek S. Brown, Washington University in St. Louis, Brown School, Campus Box 1196, One Brookings Drive, St. Louis, Missouri 63130, USA.
Esther W. de Bekker-Grob, Section of Health Technology Assessment & Erasmus Choice Modelling Centre, Institute of Health Policy and Management, Erasmus University Rotterdam, Burgemeester Oudlaan 50, 3062 PA Rotterdam, The Netherlands, Section of Medical Decision Making & Erasmus Choice Modelling Centre, Department of Public Health, Erasmus MC – University Medical Centre, Dr. Molewaterplein 50, 3000 CA Rotterdam, The Netherlands.
Axel C. Mühlbacher, Professur Gesundheitsökonomie und Medizinmanagement, Hochschule Neubrandenburg, Postfach 11 01 21, 17041 Neubrandenburg, Germany, Center for Health Policy and Inequalities Research, Duke Global Health Institute, Duke University, Durham, NC USA
Shelby D. Reed, Center for Clinical and Genetic Economics, Duke Clinical Research Institute, Duke University, 2400 Pratt Street, Durham, NC 27705.
References
- 1.Tengs TO, et al. Five-hundred life-saving interventions and their cost-effectiveness. Risk Anal. 1995;15(3):369–90. doi: 10.1111/j.1539-6924.1995.tb00330.x. [DOI] [PubMed] [Google Scholar]
- 2.Craig BM, et al. Health Preference Research: An Overview. The Patient. 2017 doi: 10.1007/s40271-017-0253-9. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 3.Mühlbacher A, Johnson FR. Choice Experiments to Quantify Preferences for Health and Healthcare: State of the Practice. Appl Health Econ Health Policy. 2016 doi: 10.1007/s40258-016-0232-7. [DOI] [PubMed] [Google Scholar]
- 4.Whitty JA, Fraenkel L, Saigal CS, Groothuis-Oudshoorn C, Regier DA, Marshall DA. Assessment of Individual Patient Preferences to Inform Clinical Practice. The Patient. 2017 doi: 10.1007/s40271-017-0254-8. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallander L. 25 years of factorial surveys in sociology: A review. Social Science Research. 2009;38(3):505–520. [Google Scholar]
- 6.van Helvoort-Postulart D, van der Weijden T, Dellaert B, de Kok M, von Meyenfeldt MF, Dirksen CD. Investigating the complementary value of discrete choice experiments for the evaluation of barriers and facilitators in implementation research: a questionnaire survey. Imp Sci. 2009;4:10. doi: 10.1186/1748-5908-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terris-Prestholt F, Quaife M, Vickerman P. Parameterising User Uptake In Economic Evaluations: The Role Of Discrete Choice Experiments. Health Economics. 2016;25(Suppl 1):116–123. doi: 10.1002/hec.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hainmueller J, Hangartner D, Yamamoto T. Validating vignette and conjoint survey experiments against real-world behavior. PNAS. 2015;112(8):2395–2400. doi: 10.1073/pnas.1416587112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lambooij MS, Harmsen IA, Veldwijk J, de Melker H, Mollema L, van Weert Y, de Wit GA. Consistency between stated and revealed preferences: a discrete choice experiment and a behavioural experiment on vaccination behaviour compared. BMC Medical Research Methodology. 2015;15:19. doi: 10.1186/s12874-015-0010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salampessy BH, Veldwijk J, Schuit AJ, van den Brekel-Dijkstra K, Neslo EJ, de Wit GA, Lambooij MS. The Predictive Value of Discrete Choice Experiments in Public Health: An Exploratory Application. The Patient. 2015;8(6):521–529. doi: 10.1007/s40271-015-0115-2. [DOI] [PMC free article] [PubMed] [Google Scholar]