Abstract
Acute kidney injury (AKI) is a common complication in the critically ill. Current standard of care mainly relies on identification of patients at risk, haemodynamic optimization, avoidance of nephrotoxicity and the use of renal replacement therapy (RRT) in established AKI. The detection of early biomarkers of renal tissue damage is a recent development that allows amending the late and insensitive diagnosis with current AKI criteria. Increasing evidence suggests that the consequences of an episode of AKI extend long beyond the acute hospitalization. Citrate has been established as the anticoagulant of choice for continuous RRT. Conflicting results have been published on the optimal timing of RRT and on the renoprotective effect of remote ischaemic preconditioning. Recent research has contradicted that acute tubular necrosis is the common pathology in AKI, that septic AKI is due to global kidney hypoperfusion, that aggressive fluid therapy benefits the kidney, that vasopressor therapy harms the kidney and that high doses of RRT improve outcome. Remaining uncertainties include the impact of aetiology and clinical context on pathophysiology, therapy and prognosis, the clinical benefit of biomarker-driven interventions, the optimal mode of RRT to improve short- and long-term patient and kidney outcomes, the contribution of AKI to failure of other organs and the optimal approach for assessing and promoting renal recovery. Based on the established gaps in current knowledge the trials that must have priority in the coming 10 years are proposed together with the definition of appropriate clinical endpoints.
Keywords: Acute kidney injury, Biomarkers, Fluid therapy, Renal replacement therapy, Research agenda, Trial endpoints
Introduction
The field of acute kidney injury (AKI) has been hampered by the long-standing lack of a standardized system for identifying and classifying this syndrome. The concept of AKI dates from the consensus RIFLE definition of 2004, which rationalized a plethora of historic definitions of clinical acute renal failure (ARF) and pathologic acute tubular necrosis (ATN), into a standardized definition based on fold increase in serum creatinine (SCr) or progressively severe oliguria [1]. Armed with this standard definition a vast amount of literature rapidly emerged describing the risks and associations of acute renal dysfunction, accompanied by the newly coined term AKI, better “encompassing” a full clinical spectrum of renal dysfunction following focal and systemic insults. Through multiple iterations a unified consensus definition, the Kidney Disease Improving Global Outcomes (KDIGO) classification, has finally been introduced (Fig. 1) [2]. This definition/classification has substantially increased our insights into the epidemiology of the AKI syndrome and has facilitated clinical and research communication in the field. In this article, we will address the intensive care medicine agenda on AKI.
Fig. 1.
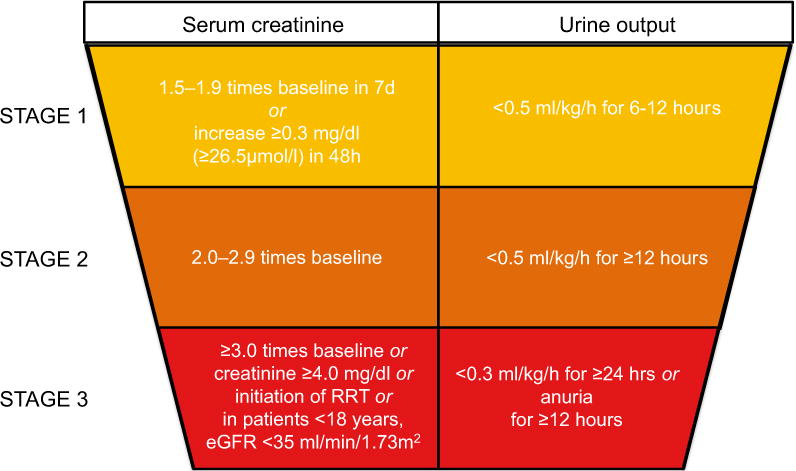
Kidney Disease Improving Global Outcomes (KDIGO) classification. Classification of AKI according to KDIGO criteria, defining three grades of severity of AKI on the basis of either the creatinine or urine output criteria
What is the current standard of care?
AKI is one of the most common complications of critical illness and is associated with serious short- and long-term complications. Over the last 10 years, research and quality improvement projects have focused on understanding the pathophysiology and epidemiology of AKI, identifying high-risk patients, diagnosing AKI earlier and developing strategies to prevent and treat AKI (Fig. 2).
Fig. 2.
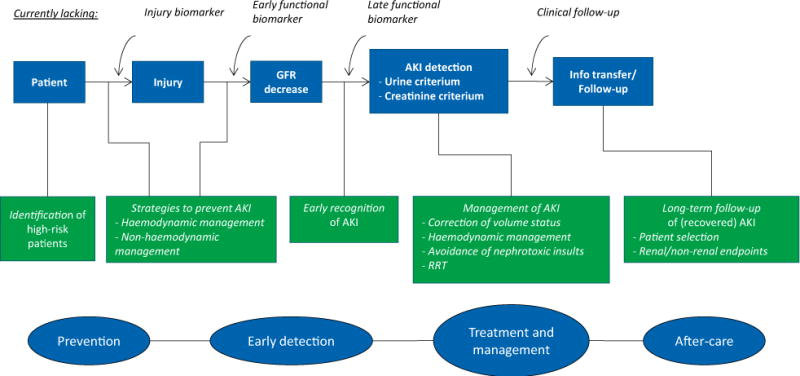
Current standard of care. This figure illustrates the absence of injury and functional biomarkers (other than creatinine) and clinical follow-up in current practice. Different tools that are available for prevention, early detection, treatment and aftercare are depicted
Identification of high-risk patients
Identifying patients who are at increased risk of AKI may result in earlier diagnosis, avoidance of potentially nephrotoxic exposures and better informed decision-making. Risk assessment tools and scores for AKI are relatively well established in patients undergoing cardiac surgery or receiving iodinated contrast [3, 4]. Despite their potential benefits, the danger of any risk assessment tool is their potential for deterring clinicians from deploying investigations or treatments which they consider potentially nephrotoxic, but may otherwise benefit the patient. The clinical benefits and potential dangers of the existing AKI scores have not been formally studied. Moreover, no established risk scores for AKI are available in more common patient groups, such as general surgical patients or acutely ill medical patients.
Strategies for prevention of AKI
-
Haemodynamic management to prevent septic AKI: sepsis is one of the most common causes of AKI. The main factors contributing to septic AKI are microcirculatory dysfunction, inflammation and bio-energetic adaptive responses to injury, including downregulated metabolism and cell-cycle arrest [5]. Under certain circumstances blood pressure may also directly influence kidney perfusion and glomerular filtration [6]. However, the exact mean arterial pressure (MAP) and perfusion targets to prevent AKI in individual patients are not known. In patients with septic shock a higher blood pressure in patients with pre-existing hypertension appears to be associated with less AKI [7].
The practice of fluid resuscitation to improve renal perfusion and function in sepsis is particularly controversial. Although intravascular fluid administration is important in volume-depleted patients, it can be counterproductive and harmful once intravascular volume has been restored. Restricted fluid management (i.e. fluid administration only in case of severe tissue hypoperfusion) resulted in less AKI progression compared to standard fluid administration (i.e. fluid boluses as long as circulation continued to improve) [8]. An ancillary study to the ProCESS (Protocolized Care for Early Septic Shock) trial also showed that the development of AKI in patients with septic shock was not influenced by protocolized resuscitation using fluid, blood and vasopressors [9].
Haemodynamic management in surgery-associated AKI: recent large studies confirmed an association between the severity and duration of intraoperative hypotension and the development of AKI [10]. Perioperative haemodynamic optimization may effectively protect renal function in surgical patients; however, the optimal haemodynamic targets and strategies are not known [11]. Several inotropic drugs have been studied in cardiac surgery patients [12], but no single agent can be recommended with regard to renoprotection.
Non-haemodynamic measures: several pharmacological and non-pharmacological interventions to prevent AKI have been studied, including selective renal vasodilators, adenosine, endocrine and anti-inflammatory strategies and remote ischaemic preconditioning (RIPC) [13, 14, 15]. None of them have shown consistent benefit or are routinely utilized in clinical practice.
Avoidance of nephrotoxicity: exposure to nephrotoxic drugs and agents is an important cause of AKI [16], especially when used in combination or in patients with other risk factors. It is evident that whenever possible nephrotoxic substances should be replaced by non-nephrotoxic equivalents. On the other hand, essential substances should not be withheld if important for the management of the patient [17].
Early recognition of AKI
Ideally, renal function should be measured and monitored in real time so that AKI is diagnosed as soon as it occurs, allowing adjustments of clinical management and drug dosing. However, the diagnosis of AKI is based on an Scr rise and/or fall in urine output, two markers which are not renal-specific and have important limitations. Automated electronic alerts (e-alerts) configured from electronic medical records and clinical information systems to warn healthcare providers of early or impending AKI have been evaluated [18]. The only randomised controlled trial (RCT) performed to date showed no improvement in clinical outcomes [19] but more research is necessary to evaluate the impact of these systems on care processes, patient outcomes and healthcare resources. A variety of functional and damage markers have been identified and validated in humans [20, 21]. Despite their potential to detect the development of AKI prior to an Scr rise, up to now no outcome benefit has been demonstrated. Progress has been made in the development of optical measurement and imaging techniques, but to date these techniques remain research tools and have not been incorporated into routine clinical practice [22]. Another potentially useful method is kinetic eGFR that allows detection of a changing GFR in non-steady state [23, 24]. However, this method does not account for changes in creatinine production (due to sepsis and reduced muscle mass) and distribution volume that occur in critically ill patients.
Management of AKI
The clinical care of patients with established AKI has shown wide variability. The mainstay of management consists of prevention of further damage by correcting hypovolaemia, individualized haemodynamic resuscitation and avoiding further nephrotoxic insults, but there are no agreed standards of care. There is also no effective pharmacological treatment to reverse AKI and induce repair. In the last few years, a major advance has been the recognition that “more” is not necessarily better. This is particularly relevant to fluid therapy where several studies have highlighted that not only the choice of fluid [25, 26] but also overzealous fluid administration is associated with harm to kidney function [27, 28]. To date, the optimal amount and preferred type of fluid for AKI patients remain unknown, but starches should no longer be used.
Renal replacement therapy (RRT) is applied to 10–15% of critically ill patients with AKI. Figure 3 gives an overview of the current standard of care in RRT in critically ill patients with AKI. Continuous RRT (CRRT) is the predominant form, being the initial modality in more than 75% of patients [16]. Despite data from RCTs and official guidelines, there is considerable variation in the delivery of CRRT. Even in areas where evidence exists [i.e. a target delivered dose of 20–25 ml/kg/h or the use of regional citrate anticoagulation (RCA)], practice varies. Some variation can be explained by local factors; others are due to existing knowledge gaps (i.e. optimal timing of RRT), but also the paucity of clearly defined high-quality indicators focused on the prescription, delivery and monitoring of the quality of CRRT care [29].
Fig. 3.
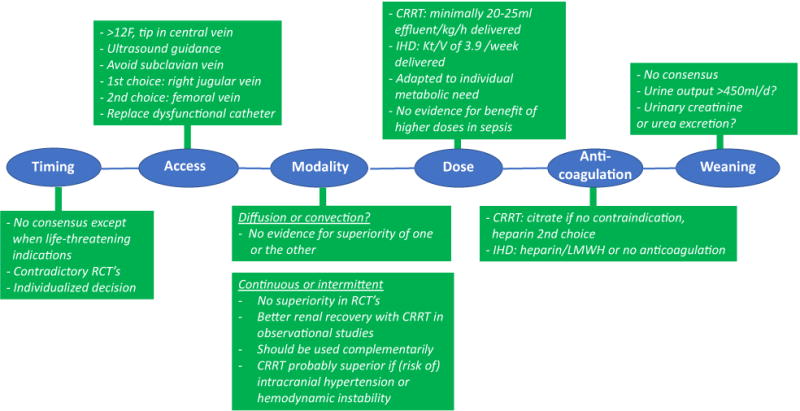
Current practice in RRT for AKI. The current standard of timing, vascular access, modality, dose, anticoagulation and weaning from renal replacement therapy. RCT randomised controlled trial, US ultrasound, CRRT continuous renal replacement therapy, IHD intermittent haemodialysis
Information transfer and follow-up
Survivors of AKI have a higher risk of long-term complications, including the development of chronic kidney disease (CKD) and dialysis-dependent end-stage renal disease (ESRD), cardiovascular complications and premature mortality. Nevertheless, kidney dysfunction is poorly documented in ICU discharge documents and nephrological follow-up is infrequent [30, 31]. Whether there is a role for robust pathways to monitor and screen AKI survivors to improve long-term outcomes has not been formally studied.
Major recent advantages
Defining and detecting acute kidney injury (AKI)
By relying on urine output and SCr changes, the KDIGO definition still limits timely and accurate AKI diagnosis and likely neglects subclinical forms of kidney dysfunction and damage [22, 32]. Biomarkers of structural kidney damage may be used to rule out AKI or to detect the presence of subclinical AKI, but may also provide a false positive AKI diagnosis especially in the setting of CKD [33]. At present two biomarkers are available for clinical use: neutrophil gelatinase-associated lipocalin (NGAL) and the combination of urinary insulin-like growth factor-binding protein (IGFBP) 7 and tissue inhibitor of metalloproteinases (TIMP) 2, two markers of cell-cycle arrest [20, 21]. These biomarkers appear specific and sensitive enough to be used in conjunction with existing markers of AKI for better stratifying renal injury. Whether the use of biomarkers impacts on clinical decisions and contributes to better prevention and management of AKI remains to be proven.
Long-term consequences of AKI
Increasing evidence suggests that the consequences of an episode of AKI are not limited to the acute hospitalization. Indeed, several observational trials, mainly based on analyses of databases, have shown an association of AKI with long-term mortality (mainly from cardiovascular diseases) and an increased risk for progression to CKD and need for chronic RRT [34]. Experimental data have established a pathophysiological basis for this AKI—CKD link [35].
Renal replacement therapy (RRT)
Anticoagulation
To maintain filter and circuit patency, anticoagulation is required. As the application of heparin is associated with several complications [mainly bleeding and heparin-induced thrombocytopenia (HIT)], the interest in RCA has increased [36, 37]. It has now become clear that compared with systemic heparin, RCA is associated with increased filter lifespan, reduced bleeding and transfusion rates and a lower incidence of HIT, however, without effect on mortality [38]. On the basis of these data, the KDIGO guidelines suggest using RCA as a first-line anticoagulant for CRRT when no contraindications are present.
Timing
The KDIGO guidelines recommend to commence RRT without delay in case of life-threatening complications [39]. In all other clinical scenarios, the guidelines leave it to the expert opinion of the treating physician [39]. The majority of previous observational studies suggest some beneficial effects of early initiation of RRT in critically ill patients with AKI [40]. However, the conclusions of several meta-analyses were based on heterogeneous studies with varying definitions and high risk of bias because the “early” group may have included patients that received RRT unnecessarily and patients not receiving RRT were not included. Two recently published RCTs were designed to identify the optimal timing for RRT. In the single-centre ELAIN study [41] the delayed strategy was associated with a significant increase in mortality in 231 KDIGO stage 2 patients. In the multicentre AKIKI trial [42], the delayed strategy could avoid RRT in 49% of the patients, but no effect on mortality was found in 620 KDIGO stage 3 patients. Apart from the differences in trial size and KDIGO stage, ELAIN predominantly enrolled (cardiac) surgery patients and AKIKI a mix of general ICU patients.
Remote ischaemic preconditioning (RIPC)
RIPC, inducing brief bouts of ischaemia at a remote site, is thought to diminish renal damage by releasing signaling molecules that activate Toll-like receptors in the proximal tubule epithelia, conditioning the epithelium to tolerate subsequent inflammatory or ischaemic stress [43, 44]. Recently, three multicentre RCTs were completed to test the hypothesis that RIPC would attenuate AKI following cardiac surgery. In 240 cardiac surgery patients at high risk for AKI, RIPC reduced the rate and severity of AKI, need for RRT, duration of ICU stay, and urinary concentrations of IGFBP-7 and TIMP2 compared with the sham procedure [45]. These results were not confirmed in two large multicentre double-blind RCTs [46, 47]. The discrepancy may be explained by a lower risk for development of AKI and the use of total intravenous anaesthesia with propofol that may abrogate the protective effects of RIPC [48]. Meta-analyses conclude that RIPC attenuates AKI following coronary angiography, but that the benefits after cardiac surgery are uncertain [15, 49, 50].
Common beliefs that have been contradicted by recent studies
ATN is the predominant histopathology of AKI
The misconception that ATN is the predominant histopathology of AKI results from the initial post-mortem and biopsy findings in patients with severe AKI treated from the 1950s to the 1970s, most of whom had trauma and haemorrhagic shock as the trigger for AKI. In modern ICUs, such causes of AKI are now uncommon with sepsis, cardiac surgery and other major-surgery-associated AKI accounting for the vast majority of cases. In these patients, rapid post-mortem studies suggest tubular injury to be local and patchy, and ultimately only one of the multiple factors leading to AKI in this setting [51].
Septic AKI is due to global renal ischaemia
Unsupported inferences drawn from analogy with other forms of shock such as cardiogenic shock and haemorrhagic shock [52] have led to the notion that septic AKI is predominantly due to global ischaemia. The limited data on renal blood flow (RBF) measurements in patients with established septic AKI show that global RBF is preserved or even increased [53]. Similar results are obtained in animal studies [54]. Nevertheless, in contrast to global ischaemia, compromised local blood flow resulting in proximal and distal tubule and medullary ischaemia may affect nephron integrity [5].
Aggressive fluid therapy is the standard of care in patients with AKI
The inferences based on analogy with true hypovolemic shock as may be seen with severe infections of the gastrointestinal tract (cholera, viral or bacterial gastroenteritis) and its associated AKI, which typically responds to rapid intravenous fluid therapy, have led clinicians to apply this paradigm to almost all other, now dominant, forms of AKI in developed countries (septic or post-operative AKI). In these patients, multiple studies confirmed an independent association between a positive fluid balance and mortality, likely due to interstitial oedema [55], and no evidence of benefit with fluid boluses on haemodynamics, renal function or patient outcomes [56]. There are no studies showing that aggressive fluid therapy or a positive fluid balance are beneficial to kidney function in any setting outside of major known and visible volume losses as seen with gastroenteritis or fistulae or other forms of body fluid losses and overwhelming evidence of possible harm [9].
Intensive vasopressor therapy worsens AKI severity
The misconception that intensive vasopressor therapy worsens the severity of AKI has a dual origin: animal models of AKI based on injection of high doses of norepinephrine directly into the renal artery and the wide belief that excessive renal vasoconstriction as a result of neurohumoral activation is the main pathophysiological mechanism underlying AKI in sepsis [52]. However, large animal data have clearly shown that hyperdynamic sepsis results in an increased, not decreased RBF [57]. The extrapolation of these data to humans is hindered by the absence of reliable tools to measure RBF in clinical practice. In the experimental setting, vasopressors improve kidney function [54] and several clinical studies with both norepinephrine and vasopressin have shown improvement, not deterioration, of kidney function with vasopressor-induced increases in MAP in patients with sepsis [58], hepatorenal syndrome and post cardiac surgery [6].
High dose RRT improves outcome
Two large multicentre RCTs [59, 60] did not show improved outcome with an almost doubling of the RRT dose. Using even higher doses in the setting of sepsis also had no effect on outcome [61]. This may have two reasons: (1) the artificial kidney is a limited-quality replacement of the natural kidney and (2) the potential benefit of RRT is neutralized by increased side effects. Compared with the natural kidney, the artificial kidney indeed only partially replaces excretory function (with limited and unselective clearance) and is very rudimentary in the regulation of water, acid—base and electrolyte balances, lacking the fine-tuning achieved by renal tubules. In addition, RRT lacks the blood pressure-regulating, metabolic and endocrine function of the kidney and does not correct the consequences of AKI on the immune system and remote organ dysfunction. RRT also has potential side effects related to the indiscriminate removal of both “good” (drugs, antibiotics, nutrients) and “bad” solutes, treatment-induced hypotension and electrolyte disturbances, exposure to the extracorporeal circuit (mainly the membrane) and the complications of dialysis catheter insertion, dwelling and circuit anticoagulation.
Remaining uncertainties (Table 1)
Table 1.
Remaining uncertainties in the field of AKI
| Is all AKI equal? Should management be individualized to specific aetiologies? Do different aetiologies have different prognosis? |
| Can we predict/detect AKI early enough to modify outcome? Should biomarkers be incorporated in the diagnosis? Can the use of biomarkers result in earlier intervention and thereby improve prognosis? |
| How do we optimize the use of RRT? Does RRT modality affect long-term renal outcome? What are the optimal parameters to guide timing of RRT? |
| What is the role of overt or subclinical AKI in the failure of other organs and can an AKI-specific therapy prevent or mitigate multiple organ failure? |
| How do we define renal recovery after AKI and how can it be optimized? |
Is all AKI equal? To what extent does the cause of AKI determine treatment and outcome?
AKI is a clinical syndrome encompassing multiple disease states with differing causes [62]. Similarly, septic AKI has been consistently associated with less severe histopathology than ischaemic or nephrotoxic ATN [63]. Thus, while the concept of AKI as a ‘disease’ represents a great stride forward in defining the condition, it may have shifted focus away from aspects of AKI specific to its aetiology that might permit better understanding of risk factors, treatment and prognosis. Future research should focus on the nature as well as severity of AKI to understand to what extent all AKI is broadly similar and to what extent management needs to be individualized to specific aetiologies of AKI and the clinical context.
Can we predict AKI early enough to modify outcome?
Therapeutic interventions aimed at limiting the risk of AKI or mitigating the consequences of renal insult have failed to demonstrate any benefit [39]. Most of the trials performed to date suffer from the unavoidable delay between renal injury and intervention. In addition to delayed biochemical diagnosis, over 80% of AKI complicating critical illness is present on ICU admission or develops within 2 days [64] precluding early preventative intervention. On the other hand, as 70% of patients with stage 1 AKI at admission go on to develop more severe AKI, further renal injury probably occurs during the ICU stay [64], providing the opportunity to mitigate AKI progression. Imprecise identification of the early tubular pathophysiology of AKI using conventional diagnostic criteria may be the key factor preventing early enough intervention. Consequently, numerous plasma and urinary biomarkers of tubular injury or more precise measures of glomerular function have been the subject of intense study [65]. However, while demonstrating statistical association with the development of AKI is relatively easy, proof that use of a biomarker translates into improved clinical outcomes is much harder [66]. As a consequence, there are currently no positive prospective RCTs of biomarker-driven interventions for AKI, nor are any novel biomarkers considered ready for incorporation into AKI diagnostic systems [67]. Combining biomarkers with other clinical parameters is an attractive way to improve the diagnostic ability [68]. However, a major problem in further refining prediction models is non-availability of an adequate gold standard for early AKI diagnosis [69], with candidate biomarkers judged against SCr-defined AKI—the imperfect standard we are seeking to improve. Future efforts may thus need to examine the clinical benefits of biomarker-driven interventions as a dual demonstration of diagnostic accuracy of the biomarker and clinical utility of the early intervention.
How do we optimize use of RRT to improve outcomes in AKI?
Despite extensive research and numerous adequately powered RCTs in thousands of patients, optimal modality, timing and dose of RRT remain controversial. The ongoing uncertainty with regard to the optimal modality relates to concerns such as subsequent risk of CKD [70], along with identification of specific subgroups (e.g. patients with fluid overload) in which a specific modality may be favoured [71]. In addition, the heterogeneity of patients, organizational and practical issues, along with the impact of variable prescription and execution [72] may have acted as unadjusted confounders and blurred evidence and conclusions. Future studies should account for this heterogeneity in carefully implementing multifaceted interventions while considering underlying risk factors.
What is the causative role of overt or subclinical AKI in the pathogenesis of multi-organ failure (MOF) and can any AKI-targeted treatment favourably affect patient outcomes in critical illness?
Experimental evidence suggests that AKI may promote or worsen extrarenal organ dysfunctions [73, 74]. Cardiorenal syndrome remains the best known and explored example of the close entanglement between renal dysfunction and other organs. Available data in animals suggest that AKI not only promotes fluid overload and its subsequent systemic consequences but is also associated with lung injury, hepatic dysfunction, small intestine epithelial cells apoptosis and neurological inflammation with behavioural changes [74]. Interestingly, these organ cross-talks are bidirectional, ventilation strategies in animals being associated with risk of further renal dysfunctions [75]. The clinical burden and implications of these experimental findings remain largely unknown. Exploring these interactions may clarify their clinical relevance, open fields of research of the underlying mechanisms and ultimately provide new opportunities for prevention not only of renal dysfunction but also of systemic consequences of AKI.
How do we define renal outcomes after AKI and how can we then optimize renal functional recovery?
While the short-term association between AKI and hospital mortality is well understood, the association between AKI and long-term adverse outcomes is now increasingly appreciated [76]. Development of CKD underpins this association as it is strongly associated with ongoing increased risk of death [77], particularly from cardiovascular causes [78]. However, assessment of long-term renal dysfunction and risk of progressive CKD after AKI can be very difficult. Loss of muscle mass associated with critical illness may significantly confound the use of SCr to assess renal functional recovery [79, 80]. Even when glomerular filtration rate (GFR) is more accurately assessed, loss of renal functional reserve [81] may increase long-term risk of CKD even when measured GFR is apparently normal. Importantly, proteinuria may be as important a risk factor for cardiovascular disease, recurrent AKI and progressive CKD as diminished GFR [82]. Overall, better methods are needed to determine completeness of renal functional recovery and prognosis after AKI. These may involve novel biomarkers or systematic measurement of renal function/functional reserve and traditional measures such as proteinuria. With better identification of those at risk, strategies to retard or prevent the development of CKD might be implemented to improve the long-term outcomes in AKI survivors.
The top ten trials to be performed in the next 10 years
As mentioned earlier, few clinical trials have demonstrated improvement in clinically important outcomes for critically ill patients with AKI. Where improved outcomes have been observed, data are often derived from trials prone to bias. Examples of bias include single-centre studies (limited generalizability), inadequate sample size (prone to type I error and exaggerated effect estimates), predominate use of surrogate endpoints (excretion of tubular injury markers, change in SCr), studies in which the primary exposure is heterogeneous (acute kidney injury is a complex and variably expressed syndrome), or the use of non-standardized endpoints (receipt of RRT where variability in practice may be influenced by provider bias), or short-term endpoints (28-day mortality or worsening of AKI stage). Such inadequacies are highlighted, for example, by the early studies on dosing of RRT where improved survival benefit was observed with higher dosing schedules, but this did not translate to similar benefits in larger RCTs [61, 62]. Interestingly, in general the few positive studies in ICU patients tend to follow a “less is more” philosophy that suggests that our drive to compensate for normal physiological responses may not always be appropriate.
The lack of trial success can be explained in part by the heterogeneity of AKI, by difficulties in early diagnosis with the current definition, as well as the lack of consistent clinically relevant endpoints. The inability to accurately phenotype the underlying cause of AKI in a timely manner has hindered appropriate patient selection and risk identification for interventions in clinical trials. The increasing availability of biomarkers (in tandem with clinical risk scoring) may assist in the early identification of patients at risk of or with early AKI [83]. Figures 2 and 4 illustrate how biomarkers may be integrated into trial design to better identify patients most likely to benefit from a particular intervention. Indeed, more recent trials have integrated biomarker use to guide eligibility [41, 84].
Fig. 4.
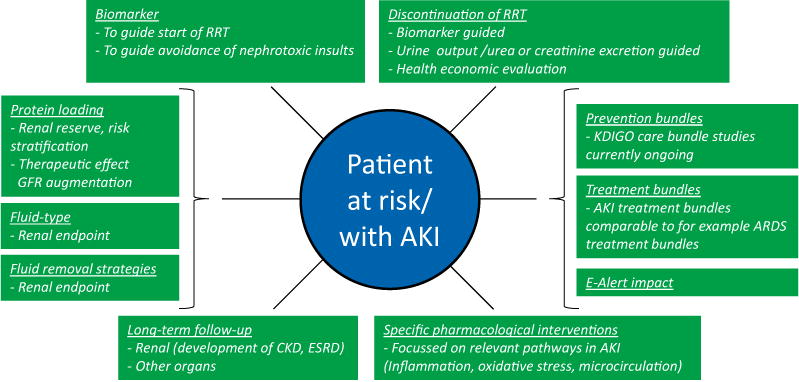
Research items to address. The use of potential diagnostic biomarkers could risk stratify those at highest risk of imminent kidney injury. These highest risk individuals could then be enrolled in RCTs evaluating novel therapeutics or processes of care. Also biomarkers could be used to guide the start and discontinuation of RRT. Clinical effects of prevention and treatment bundles as well as e-alert systems should be established. Protein loading to determine renal reserve may aid risk stratification and may also exert therapeutic effects by GFR augmentation. Effects of fluid types and fluid removal strategies on renal endpoints need to be determined. Clearly well-defined clinically relevant endpoints should be integrated. Naturally, as more relevant pathways in the development of AKI will be discovered, new pharmacological interventions that influence these pathways will need to be investigated. Finally, more attention should be paid to long-term follow-up, both for renal as well as other organ endpoints
Furthermore, future trials aimed at evaluating novel interventions for AKI should have clinically important endpoints which may include receipt of RRT (with caveats in terms of criteria) and/or development of or worsening CKD and mortality. Composites of this may be used such as MAKE30, MAKE60 and MAKE90 (major adverse kidney events) which include death, new dialysis and worsening kidney function [defined in this case as a sustained fall in estimated GFR (eGFR) of greater than 25%] at 30, 60 and 90 days after the diagnosis of AKI by KDIGO criteria [85]. Such patient-centred clinically important endpoints negate the arguments that the adverse outcomes after (particularly mild) AKI may simply be a result of the underlying phenotype and not related to AKI at all [86, 87]. More recently, it has been suggested that a better understanding of the risk for ESRD after AKI could inform the design and execution of clinical trials. For example, in clinical trials of CKD progression, a 30% decline in eGFR has been suggested as an alternative surrogate endpoint for ESRD, which may enable better-powered trials with a smaller sample size than the traditionally accepted surrogate endpoint of doubling of SCr [88,89].
Illustrated in Fig. 4 and Table 2, the following clinical trials would both inform and improve outcomes in AKI and advance the field over the next decade:
Biomarker-guided initiation of RRT: although the optimal strategy for starting RRT remains uncertain, several ongoing trials may provide some guidance for future practice. However, clarity of clinical phenotypes integrated with prognostic biomarkers that aid prediction of spontaneous renal recovery following insult may guide timing trials in a different direction to that of current practice.
Best practice studies: AKI treatment bundles: therapeutic (i.e. pharmacological) interventions for treatment of AKI associated with specific clinical conditions have traditionally been the focus of the majority of trials. Improving the outcomes of AKI patients may also primarily depend on the quality of various processes of care used to monitor, investigate and manage AKI. Such a study would parallel the management of patients with acute lung injury where protocol-driven ventilator management, fluid prescription and infection control measures have contributed to reduced mortality over time.
Best practice studies: AKI prevention bundles: following the same rationale, the application of targeted care bundles in patients at high risk of AKI such as high-risk surgery is needed. Studies on application of the KDIGO care bundles are currently being performed in cardiac surgery.
Best practice studies: e-alert impact: the impact of e-alerts on care processes/outcomes should be further examined. This could be tailored to specific at-risk populations (i.e. post-operative) and focus on care processes such as reducing unnecessary nephrotoxin exposure.
RCT fluid type focused on renal outcomes: although the balanced solutions versus saline argument rages, patient selection is paramount. Studies in patients at high risk for AKI are needed. Also, an adequately powered study comparing conservative fluid prescription titrated to physiological parameters to usual practice may provide evidence to better inform on best practice and harm avoidance in patients at risk.
RCT on discontinuation of CRRT: there has been a paucity of data to inform on when to ideally liberate patients from RRT. A kidney outcome and health economic evaluation-based study using conventional indicators of kidney recovery, such as urine output, creatinine excretion or rate of spontaneous decline in SCr, would advance the field considerably.
RCT of fluid removal strategies (non-extracorporeal) in preventing AKI: circumstantial evidence has accumulated to suggest volume overload is associated with worsening of AKI and non-renal major morbidity. An adequately powered study in at-risk patients using accepted therapeutic approaches should be performed.
RCT impact of AKI survivor follow-up on long-term kidney function: the long-term risks for CKD and ESRD following AKI are well established, as is the paucity of follow-up. No RCT has examined the effects of specific protocol-driven interventions on patients surviving AKI.
Protein loading to measure renal functional reserve: protein loading may be used to determine functional reserve, and thus improve risk stratification for future studies. Also, clinical effects of GFR augmentation, e.g. pre-operatively, need to be explored.
Specific pharmacological interventions: naturally, ongoing research will reveal new relevant pathways in the development of AKI and specific compounds that intervene in these pathways will need to be tested.
Table 2.
Clinical trials to be conducted in the field of AKI
| RCT comparing early versus late RRT initiation with inclusion guided by biomarker(s) of non-recovery |
| RCT assessing the impact of an AKI care bundle compared with standard of care on clinically important endpoints in patients with (early) AKI |
| RCT assessing the efficacy of an AKI care bundle compared with standard of care for prevention of AKI in specific populations at high risk |
| Cluster randomised trial evaluating the impact of e-alerts on the process of care and clinically important outcomes in specific at-risk populations (e.g. post-operative patients) |
| An adeguately powered RCT comparing the renal outcome of saline with balanced crystalloid in patients at high risk of AKI and presumed to reguire large volume resuscitation. The effects on renal function of conservative fluid prescription titrated to physiologic parameters compared with usual care should also be assessed |
| RCT assessing the impact on renal outcome and health economics of using specific indicators for RRT discontinuation versus usual care |
| RCT assessing the impact of a fluid removal strategy versus usual care on the development of AKI in patients with fluid overload |
| RCT assessing the impact of nephrological follow-up of AKI survivors on long-term kidney function and mortality |
| Analysis of the association between renal functional reserve (measured with acute protein loading) and short- and long-term renal outcome. RCT comparing the impact of preoperative GFR augmentation by acute protein loading on post-operative development of AKI |
| RCT(s) evaluating the efficacy of pharmacologic treatments intervening in (newly discovered) pathophysiological pathways in the development of AKI |
Although it is unlikely that all studies will be undertaken in the next 10 years, we hope that study design and particularly phenotypic identification will further our knowledge base and translate into improved outcomes for AKI patients.
Conclusion
AKI is frequently observed in critically ill patients and associated with impaired outcome. Consensus criteria facilitate to assess epidemiology more accurately. In the future, novel biomarkers and real-time assessment of GFR may possibly lead to adjustments of the definition of AKI, and to more swift recognition of AKI. This will facilitate novel strategies for prevention and treatment of AKI and guide our decision-making related to initiation and stopping of RRT as supportive treatment.
Acknowledgments
SMB is supported by a Canada Research Chair in Critical Care Nephrology.
PP has received travel reimbursement/honoraria from Baxter Healthcare Corp, Fresenius and AM-Pharma. MO has received honoraria and research funding from Fresenius Medical Care and Baxter Gambro. MJ has received honoraria or research support from Baxter Healthcare Corp, AM-Pharma, CLS Behring, Fresenius and Astute Medical. AZ received honoraria and research funding from Braun, Fresenius, Astellas and Astute Medical. EH received speakers fee from Alexion and Astute Medical, and a research grant from Bellco. RB has received honoraria and research support from Baxter Healthcare Corp, CSL Behring and BBraun. JP has received honoraria or research support for Nikkiso GmBH and Baxter Healthcare Corp. MD has received honoraria from MSD, Astellas and Bristol-Myers Sguibb, travel support from Gilead and Meditor Inc., a research grant from MSD, and funds to organize an educational meeting from MSD, Gilead, Jazz Pharmaceutical and Astellas. JB is co-inventor on KIM-1 patents that have been assigned to Partners Health Care. He has been a consultant for Lilly, Astellas and Mitobridge, and he/his family has equity in Medibeacon and Sentien. LF has received honoraria and research support from Astute medical, Fresenius, Baxter Gambro Renal and Orthoclinical Diagnostics. SB has received honoraria and research support from Baxter Healthcare Corp.
Footnotes
Conflicts of interest
MS declares no conflicts of interest.
References
- 1.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute renal failure—definition, outcome measures, animal models, fluid therapy and information technology needs: the second international consensus conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8:R204–R212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury: AKI definition. Kidney Intern Suppl. 2012;2:19–36. [Google Scholar]
- 3.Huen SC, Parikh CR. Predicting acute kidney injury following cardiac surgery: a systematic review. Ann Thorac Surg. 2013;93:337–347. doi: 10.1016/j.athoracsur.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehran R, Aymong ED, Nikolsky E, Lasic Z, Iakovou I, Fahy M, Mintz GS, Lansky AJ, Moses JW, Stone GW, Leon MB, Dangas G. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–1399. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 5.Zarbock A, Gomez H, Kellum JA. Sepsis-induced acute kidney injury revisited: pathophysiology, prevention and future therapies. Curr Opin Crit Care. 2014;20:588–595. doi: 10.1097/MCC.0000000000000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Redfors B, Bragadottir G, Sellgren J. Effects of norepinephrine on renal perfusion, filtration and oxygenation in vasodilatory shock and acute kidney injury. Intensive Care Med. 2011;37:60–67. doi: 10.1007/s00134-010-2057-4. [DOI] [PubMed] [Google Scholar]
- 7.Asfar P, Meziani F, Hamel JF, Grelon F, Megarbane B, Anguel N, Mira JP, Deguin PF. High versus low blood-pressure target in patients with septic shock. New Engl J Med. 2014;370:1583–1593. doi: 10.1056/NEJMoa1312173. [DOI] [PubMed] [Google Scholar]
- 8.Hjortrup PB, Haase N, Bundgaard H, Thomsen SL, Winding R, Petillä V, Aaen A, Lodahl D, Christensen H, Madsen MB, Winkel P, Wetterslev J, Perner A, CLASSIC Trial Group, Scandinavian Critical Care Trials Group Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the CLASSIC randomised, parallel-group, multicentre feasibility trial. Intensive Care Med. 2016;42:1695–1705. doi: 10.1007/s00134-016-4500-7. [DOI] [PubMed] [Google Scholar]
- 9.Kellum JA, Chawla LS, Keener C, Singbartl K, Palevsky PM, Pike FL, Yealy DM, Huang DT, Angus DC, ProCESS and ProGReSS-AKI Investigators The effects of alternative resuscitation strategies on acute kidney injury in patients with septic shock. Am J Respir Crit Care. 2016;193:281–287. doi: 10.1164/rccm.201505-0995OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun LY, Wijeysundera DN, Tait GA, Beattie WS. Association of intraoperative hypotension with acute kidney injury after elective noncardiac surgery. Anesthesiology. 2015;123:515–523. doi: 10.1097/ALN.0000000000000765. [DOI] [PubMed] [Google Scholar]
- 11.Brienza N, Giglio MT, Marucci M, Fiore T. Does perioperative hemodynamic optimization protect renal function in surgical patients? A meta-analytic study. Crit Care Med. 2009;37:2079–2090. doi: 10.1097/CCM.0b013e3181a00a43. [DOI] [PubMed] [Google Scholar]
- 12.Hou C, Gong J, Chen D, Wang W, Liu M, Liu B. Levosimendan for prevention of acute kidney injury after cardiac surgery: a meta-analysis of randomized controlled trials. Am J Kidney Dis. 2016;67:408–416. doi: 10.1053/j.ajkd.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Su X, Xie X, Liu L, Lv J, Song F, Perkovic V, Zhang H. Comparative effectiveness of 12 treatment strategies for preventing contrast-induced acute kidney injury: a systematic review and bayesian network metaanalysis. Am J Kidney Dis. 2016;16:30421–30428. doi: 10.1053/j.ajkd.2016.07.033. [DOI] [PubMed] [Google Scholar]
- 14.Lewicki M, Ng I, Schneider AG. HMG CoA reductase inhibitors (statins) for preventing acute kidney injury after surgical procedures reguiring cardiac bypass. Cochrane Database Syst Rev. 2015;3:CD010480. doi: 10.1002/14651858.CD010480.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou C, Jeon Y, Meybohm P, Zarbock A, Young PJ, Li L, Hausenloy DJ. Renoprotection by remote ischemic conditioning during elective coronary revascularization: a systematic review and meta-analysis of randomized controlled trials. Int J Cardiol. 2016;222:295–302. doi: 10.1016/j.ijcard.2016.07.176. [DOI] [PubMed] [Google Scholar]
- 16.Hoste EA, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, Edipidis K, Forni LG, Gomersall CD, Govil D, Honore PM, Joannes-Boyau O, Joanniidis M, Korhonen AM, Lavrentieva A, Mehta RL, Palevsky P, Roessler E, Ronco C, Uchino S, Vazguez JA, Vidal Andrade E, Webb S, Kellum JA. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med. 2015;41:1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 17.Ichai C, Vinsonneau C, Souweine B, Armando F, Canet E, Clec’h C, Constantin JM, Darmon M, Duranteau J, Gaillot T, Gamier A, Jacob L, Joannes-Boyau O, Juillard L, Journois D, Lautrette A, Muller L, Legrand M, Lerolle N, Rimmele T, Rondeau E, Tamion F, Walrave Y, Velly L. Acute kidney injury in the perioperative period and in intensive care units (excluding renal replacement therapies) Ann Intensive Care. 2016;6:48. doi: 10.1186/s13613-016-0145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colpaert K, Hoste EA, Steurbaut K, Benoit D, Van Hoecke S, De Turck F, Decruyenaere J. Impact of real-time electronic alerting of acute kidney injury on therapeutic intervention and progression of RIFLE class. Crit Care Med. 2012;40:1164–1170. doi: 10.1097/CCM.0b013e3182387a6b. [DOI] [PubMed] [Google Scholar]
- 19.Wilson FR, Shashaty M, Testani J, Ageel I, Borovskiy Y, Ellenberg SS, Feldman HI, Fernandez H, Gitelman Y, Lin J, Negoianu D, Parikh CR, Reese PR, Urbani R, Fuchs B. Automated, electronic alerts for acute kidney injury: a single-blind, parallel-group, randomised controlled trial. Lancet. 2015;385:1966–1974. doi: 10.1016/S0140-6736(15)60266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vijayan A, Faubel S, Askenazi DJ, Cerda J, Fissell WH, Heung M, Humphreys BD, Koyner JL, Liu KD, Mour G, Nolin TD, Bihorac A. Clinical use of the urine biomarker [TIMP-2] × [IGFBP7] for acute kidney injury risk assessment. Am J Kidney Dis. 2016;68:19–28. doi: 10.1053/j.ajkd.2015.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pickering JW, Endre ZH. Bench to bedside: the next steps for biomarkers in acute kidney injury. Am J Physiol Renal Physiol. 2016;311:F717–f721. doi: 10.1152/ajprenal.00268.2016. [DOI] [PubMed] [Google Scholar]
- 22.Molitoris BA, Reilly ES. Quantifying glomerular filtration rates in acute kidney injury: a reguirement for translational success. Semin Nephrol. 2016;36:31–41. doi: 10.1016/j.semnephrol.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen S. Retooling the creatinine clearance eguation to estimate kinetic GFR when the plasma creatinine is changing acutely. J Am Soc Nephrol. 2013;24:877–888. doi: 10.1681/ASN.2012070653. [DOI] [PubMed] [Google Scholar]
- 24.Dewitte A, Joannes-Boyau O, Sidobre C, Fleureau C, Bats ML, Derache P, Leuillet S, Ripoche J, Combe C, Ouattara A. Kinetic eGFR and novel AKI biomarkers to predict renal recovery. Clin J Am Soc Nephrol. 2015;10:1900–1910. doi: 10.2215/CJN.12651214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perner A, Haase N, Guttormsen AB, Tenhunen J, Klemenzson G, Aneman A, Madsen KR, Moller MH, Elkjaer JM, Poulsen LM, Bendtsen A, Winding R, Steensen M, Berezowicz P, Soe-Jensen R, Bestle M, Strand K, Wiis J, White JO, Thornberg KJ, Quist L, Nielsen J, Andersen LH, Hoist LB, Thormar K, Kjaeldgaard AL, Fabritius ML, Mondrup F, Pott FC, Moller TP, Winkel P, Wetterslev J. Hydroxyethyl starch 130/0.42 versus Ringer’s acetate in severe sepsis. N Engl J Med. 2012;367:124–134. doi: 10.1056/NEJMoa1204242. [DOI] [PubMed] [Google Scholar]
- 26.Myburgh JA, Finfer S, Bellomo R, Billot L, Cass A, Gattas D, Glass R, Lipman J, Liu B, McArthur C, McGuinness S, Rajbhandari D, Taylor CB, Webb SA. Hydroxyethyl starch or saline for fluid resuscitation in intensive care. N Engl J Med. 2012;367:1901–1911. doi: 10.1056/NEJMoa1209759. [DOI] [PubMed] [Google Scholar]
- 27.Raimundo M, Crichton S, Martin JR, Syed Y, Varrier M, Ostermann M. Increased fluid administration after early acute kidney injury is associated with less renal recovery. Shock. 2015;44:431–437. doi: 10.1097/SHK.0000000000000453. [DOI] [PubMed] [Google Scholar]
- 28.Prowle JR, Kirwan CJ, Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol. 2014;10:37–47. doi: 10.1038/nrneph.2013.232. [DOI] [PubMed] [Google Scholar]
- 29.Rewa OG, Villeneuvel PM, Lachance R, Eurich DT, Stelfox HT, Gibney RTN, Harling L, Featherstone R, Bagshaw SM. Quality indicators of continuous renal replacement therapy (CRRT) care in critically ill patients: a systematic review. Intensive Care Med. 2016;41:1411–1423. doi: 10.1007/s00134-016-4579-x. [DOI] [PubMed] [Google Scholar]
- 30.Sautenet B, Caille A, Giraudea B, Leger J, Vourc’h R, Buchler M, Halimi JM. Deficits in information transfer between hospital-based and primary-care physicians, the case of kidney disease: a cross-sectional study. J Nephrol. 2015;28:563–570. doi: 10.1007/s40620-015-0175-3. [DOI] [PubMed] [Google Scholar]
- 31.Kirwan CJ, Blunden MJ, Dobbie H, James A, Nedungadi A, Prowle JR. Critically ill patients reguiring acute renal replacement therapy are at an increased risk of long-term renal dysfunction, but barely receive nephrology follow-up. Nephron. 2015;129:164–170. doi: 10.1159/000371448. [DOI] [PubMed] [Google Scholar]
- 32.Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, Krawczeski CD, Koyner JL, Murray P, Zappitelli M, Goldstein SL, Makris K, Ronco C, Martensson J, Martling CR, Venge R, Siew E, Ware LB, Ikizler TA, Mertens PR. The outcome of neutrophil gelatinase-associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 201l;57:1752–1761. doi: 10.1016/j.jacc.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin J, Fernandez H, Shashaty MG, Negoianu D, Testani JM, Berns JS, Parikh CR, Wilson FP. False-positive rate of AKI using consensus creatinine-based criteria. Clin J Am Soc Nephrol. 2015;10:1723–1731. doi: 10.2215/CJN.02430315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parr SK, Siew ED. Delayed consequences of acute kidney injury. Adv Chronic Kidney Dis. 2016;23:186–194. doi: 10.1053/j.ackd.2016.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferenbach DA, Bonventre JV. Mechanisms of maladaptive repair after AKI leading to accelerated kidney ageing and CKD. Nat Reviews Nephrol. 2015;11:264–276. doi: 10.1038/nrneph.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oudemans-van Straaten HM, Kellum JA, Bellomo R. Clinical review: anticoagulation for continuous renal replacement therapy—heparin or citrate? Crit Care. 2011;15:202. doi: 10.1186/cc9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lanckohr C, Hahnenkamp K, Boschin M. Continuous renal replacement therapy with regional citrate anticoagulation: do we really know the details? Curr Opin Anaesthesiol. 2013;26:428–437. doi: 10.1097/ACO.0b013e3283620224. [DOI] [PubMed] [Google Scholar]
- 38.Bai M, Zhou M, He L, Ma F, Li Y, Yu Y, Wang P, Li L, Jing R, Zhao L, Sun S. Citrate versus heparin anticoagulation for continuous renal replacement therapy: an updated meta-analysis of RCTs. Intensive Care Med. 2015;41:2098–2110. doi: 10.1007/s00134-015-4099-0. [DOI] [PubMed] [Google Scholar]
- 39.Group KAW. KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 40.Karvellas CJ, Farhat MR, Sajjad I, Mogensen SS, Leung AA, Wald R, Bagshaw SM. A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: a systematic review and meta-analysis. Crit Care. 2011;15:R72. doi: 10.1186/cc10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zarbock A, Kellum JA, Schmidt C, Van Aken H, Wempe C, Pavenstadt H, Boanta A, Gerss J, Meersch M. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically III patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA. 2016;315:2190–2199. doi: 10.1001/jama.2016.5828. [DOI] [PubMed] [Google Scholar]
- 42.Gaudry S, Hajage D, Schortgen F, Martin-Lefevre L, Pons B, Boulet E, Boyer A, Chevrel G, Lerolle N, Carpentier D, de Prost N, Lautrette A, Bretagnol A, Mayaux J, Nseir S, Megarbane B, Thirion M, Forel JM, Maizel J, Yonis H, Markowicz P, Thiery G, Tubach F, Ricard JD, Dreyfuss D, Group AS Initiation strategies for renal-replacement therapy in the intensive care unit. New Engl J Med. 2016;375:122–133. doi: 10.1056/NEJMoa1603017. [DOI] [PubMed] [Google Scholar]
- 43.Gassanov N, Nia AM, Caglayan E, Er F. Remote ischemic preconditioning and renoprotection: from myth to a novel therapeutic option? J Am Soc Nephrol. 2014;25:216–224. doi: 10.1681/ASN.2013070708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kharbanda RK, Nielsen TT, Redington AN. Translation of remote ischaemic preconditioning into clinical practice. Lancet. 2009;374:1557–1565. doi: 10.1016/S0140-6736(09)61421-5. [DOI] [PubMed] [Google Scholar]
- 45.Zarbock A, Schmidt C, Van Aken H, Wempe C, Martens S, Zahn PK, Wolf B, Goebel U, Schwer CI, Rosenberger P, Haeberle H, Gorlich D, Kellum JA, Meersch M. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA. 2015;313:2133–2141. doi: 10.1001/jama.2015.4189. [DOI] [PubMed] [Google Scholar]
- 46.Meybohm R, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, Coburn M, Schaelte G, Boning A, Niemann B, Roesner J, Kletzin F, Strouhal U, Reyher C, Laufenberg-Feldmann R, Ferner M, Brandes IF, Bauer M, Stehr SN, Kortgen A, Wittmann M, Baumgarten G, Meyer-Treschan T, Kienbaum P, Heringlake M, Schon J, Sander M, Treskatsch S, Smul T, Wolwender E, Schilling T, Fuernau G, Hasenclever D, Zacharowski K, Collaborators RIS A multicenter trial of remote ischemic preconditioning for heart surgery. N Engl J Med. 2015;373:1397–1407. doi: 10.1056/NEJMoa1413579. [DOI] [PubMed] [Google Scholar]
- 47.Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, Knight R, Kunst G, Laing C, Nicholas J, Pepper J, Robertson S, Xenou M, Clayton T, Yellon DM, Investigators ET Remote ischemic preconditioning and outcomes of cardiac surgery. N Engl J Med. 2015;373:1408–1417. doi: 10.1056/NEJMoa1413534. [DOI] [PubMed] [Google Scholar]
- 48.Kottenberg E, Thielmann M, Bergmann L, Heine T, Jakob H, Heusch G, Peters J. Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol—a clinical trial. Acta Anaesthesiol Scand. 2012;56:30–38. doi: 10.1111/j.1399-6576.2011.02585.x. [DOI] [PubMed] [Google Scholar]
- 49.Bei WJ, Duan CY, Chen JY, Wang K, Liu YH, Liu Y, Tan N. Remote ischemic conditioning for preventing contrast-induced acute kidney injury in patients undergoing percutaneous coronary interventions/coronary angiography: a meta-analysis of randomized controlled trials. J Cardiovasc Pharmacol Ther. 2016;21:53–63. doi: 10.1177/1074248415590197. [DOI] [PubMed] [Google Scholar]
- 50.Zhang L, Diao Y, Chen G, Tanaka A, Eastwood GM, Bellomo R. Remote ischemic conditioning for kidney protection: a meta-analysis. J Crit Care. 2016;33:224–232. doi: 10.1016/j.jcrc.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 51.Lerolle N, Nochy D, Guerot E, Bruneval P, Fagon JY, Diehl JL, Hill G. Histopathology of septic shock induced acute kidney injury: apoptosis and leukocytic infiltration. Intensive Care Med. 2010;36:471–478. doi: 10.1007/s00134-009-1723-x. [DOI] [PubMed] [Google Scholar]
- 52.Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351:159–169. doi: 10.1056/NEJMra032401. [DOI] [PubMed] [Google Scholar]
- 53.Prowle JR, Bellomo R. Sepsis-associated acute kidney injury: macrohemodynamic and microhemodynamic alterations in the renal circulation. Semin Nephrol. 2015;35:64–74. doi: 10.1016/j.semnephrol.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 54.Di Giantomasso D, May CN, Bellomo R. Norepinephrine and vital organ blood flow. Intensive Care Med. 2002;28:1804–1809. doi: 10.1007/s00134-002-1444-x. [DOI] [PubMed] [Google Scholar]
- 55.Vaara ST, Korhonen AM, Kaukonen KM, Nisula S, Inkinen O, Hoppu S, Laurila JJ, Mildh L, Reinikainen M, Lund V, Parviainen I, Petillä V, FINNAKI Study Group Fluid overload is associated with an increased risk for 90-day mortality in critically ill patients with renal replacement therapy: data from the prospective FINNAKI study. Grit Care. 2012;16:R197. doi: 10.1186/cc11682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wheeler AP, Bernard GR, Thompson BT, Schoenfeld D, Wiedemann HP, DeBoisblanc BP, Connors AF, Hite RD, Harabin AL, National Heart Lung and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network Pulmonary-artery versus central venous catheter to guide treatment of acute lung injury. N Engl J Med. 2006;354:2213–2224. doi: 10.1056/NEJMoa061895. [DOI] [PubMed] [Google Scholar]
- 57.Langenberg C, Wan L, Egi M, May CN, Bellomo R. Renal blood flow in experimental septic acute renal failure. Kidney Int Suppl. 2006;69:1996–2002. doi: 10.1038/sj.ki.5000440. [DOI] [PubMed] [Google Scholar]
- 58.Redl-Wenzl EM, Armbruster C, Edelmann G, Fischl E, Kolacny M, Wechsler-Fordos A, Sporn P. The effects of norepinephrine on hemodynamics and renal function in severe septic shock states. Intensive Care Med. 1993;19:151–154. doi: 10.1007/BF01720530. [DOI] [PubMed] [Google Scholar]
- 59.Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, Finkel K, Kellum JA, VA/NIH Acute Renal Failure Trial Network Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med. 2008;359:7–20. doi: 10.1056/NEJMoa0802639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S, McArthur C, McGuin-ness S, Myburgh J, Norton R, Scheinkestel C, Su S, RENAL Replacement Therapy Study Investigators Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med. 2009;361:1627–1638. doi: 10.1056/NEJMoa0902413. [DOI] [PubMed] [Google Scholar]
- 61.Joannes-Boyau O, Honoré PM, Perez P, Bagshaw SM, Grand H, Canivet JL, Dewitte A, Flamens C, Pujol W, Grandoulier AS, Fleureau C, Jacobs R, Broux C, Floch H, Branchard O, Franck S, Rose H, Collin V, Boer W, Calderon J, Gauche B, Spapen HD, Janvier G, Ouattara A. High-volume versus standard-volume haemofiltration for septic shock patients with acute kidney injury (IVOIRE study): a multicentre randomized controlled trial. Intensive Care Med. 2013;39:1535–1546. doi: 10.1007/s00134-013-2967-z. [DOI] [PubMed] [Google Scholar]
- 62.Oliver J, Mac DM, Tracy A. The pathogenesis of acute renal failure associated with traumatic and toxic injury; renal ischemia, nephrotoxic damage and the ischemic episode. J Clin Invest. 1951;30:1307–1439. doi: 10.1172/JCI102550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kosaka J, Lankadeva YR, May CN, Bellomo R. Histopathology of septic acute kidney injury: a systematic review of experimental data. Crit Care Med. 2016;44:e897–e903. doi: 10.1097/CCM.0000000000001735. [DOI] [PubMed] [Google Scholar]
- 64.Nisula S, Kaukonen KM, Vaara ST. Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: the FINNAKI study. Intensive Care Med. 2013;39:420–428. doi: 10.1007/s00134-012-2796-5. [DOI] [PubMed] [Google Scholar]
- 65.Vanmassenhove J, Vanholder R, Nagler E, Van Biesen W. Urinary and serum biomarkers for the diagnosis of acute kidney injury: an in-depth review of the literature. Nephrol Dial Transplant. 2013;28:254–273. doi: 10.1093/ndt/gfs380. [DOI] [PubMed] [Google Scholar]
- 66.Ostermann M, Joannidis M. Biomarkers for AKI improve clinical practice: no. Intensive Care Med. 2015;41:618–622. doi: 10.1007/s00134-014-3540-0. [DOI] [PubMed] [Google Scholar]
- 67.McCullough PA, Shaw AD, Haase M, Bouchard J, Waikar SS, Siew ED, Murray PT, Mehta RL, Ronco C. Diagnosis of acute kidney injury using functional and injury biomarkers: workgroup statements from the tenth Acute Dialysis Quality Initiative Consensus Conference. Contrib Nephrol. 2013;182:13–29. doi: 10.1159/000349963. [DOI] [PubMed] [Google Scholar]
- 68.Pickering JW, Endre ZH. New metrics for assessing diagnostic potential of candidate biomarkers. Clin J Am Soc Nephrol. 2012;7:1355–1364. doi: 10.2215/CJN.09590911. [DOI] [PubMed] [Google Scholar]
- 69.Waikar SS, Betensky RA, Emerson SC, Bonventre JV. Imperfect gold standards for kidney injury biomarker evaluation. J Am Soc Nephrol. 2012;23:13–21. doi: 10.1681/ASN.2010111124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schneider AG, Bellomo R, Bagshaw SM, et al. Choice of renal replacement therapy modality and dialysis dependence after acute kidney injury: a systematic review and meta-analysis. Intensive Care Med. 2013;39(6):987–997. doi: 10.1007/s00134-013-2864-5. [DOI] [PubMed] [Google Scholar]
- 71.Truche AS, Darmon M, Bailly S, et al. Continuous renal replacement therapy versus intermittent hemodialysis in intensive care patients: impact on mortality and renal recovery. Intensive Care Med. 2016;42:1408–1417. doi: 10.1007/s00134-016-4404-6. [DOI] [PubMed] [Google Scholar]
- 72.Schortgen F, Soubrier N, Delclaux C, et al. Hemodynamic tolerance of intermittent hemodialysis in critically ill patients: usefulness of practice guidelines. Am J Respir Crit Care Med. 2000;162:197–202. doi: 10.1164/ajrccm.162.1.9907098. [DOI] [PubMed] [Google Scholar]
- 73.Faubel S, Shah PB. Immediate consequences of acute kidney injury: the impact of traditional and nontraditional complications on mortality in acute kidney injury. Adv Chronic Kidney Dis. 2016;23:179–185. doi: 10.1053/j.ackd.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 74.Doi K, Rabb H. Impact of acute kidney injury on distant organ function: recent findings and potential therapeutic targets. Kidney Int. 2016;89:555–564. doi: 10.1016/j.kint.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 75.Imai Y, Parodo J, Kajikawa O, et al. Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA. 2003;289:2104–2112. doi: 10.1001/jama.289.16.2104. [DOI] [PubMed] [Google Scholar]
- 76.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371:58–66. doi: 10.1056/NEJMra1214243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lai CF, Wu VC, Huang TM, Yeh YC, Wang KC, Han YY, Lin YF, Jhuang YJ, Chao CT, Shiao CC, Tsai PR, Hu FC, Chou NK, Ko WJ, Wu KD, National Taiwan University Hospital Study Group on Acute Renal Failure (NSARF) Kidney function decline after a non-dialysis-reguiring acute kidney injury is associated with higher long-term mortality in critically ill survivors. Crit Care. 2012;16:R123. doi: 10.1186/cc11419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu VC, Wu CH, Huang TM, Wang CY, Lai CF, Shiao CC, Chang CH, Lin SL, Chen YY, Chen YM, Chu TS, Chiang WC, Wu KD, Tsai PR, Chen L, Ko WJ, NSARF Group Long-term risk of coronary events after AKI. J Am Soc Nephrol. 2014;25:595–605. doi: 10.1681/ASN.2013060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Prowle JR, Kolic I, Purdell-Lewis J, Taylor R, Pearse RM, Kirwan CJ. Serum creatinine changes associated with critical illness and detection of persistent renal dysfunction after AKI. Clin J Am Soc Nephrol. 2014;9:1015–1023. doi: 10.2215/CJN.11141113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schetz M, Gunst J, Van den Berghe G. The impact of using estimated GFR versus creatinine clearance on the evaluation of recovery from acute kidney injury in the ICU. Intensive Care Med. 2014;40:1709–1717. doi: 10.1007/s00134-014-3487-1. [DOI] [PubMed] [Google Scholar]
- 81.Sharma A, Mucino MJ, Ronco C. Renal functional reserve and renal recovery after acute kidney injury. Nephron Clin Pract. 2014;127:94–100. doi: 10.1159/000363721. [DOI] [PubMed] [Google Scholar]
- 82.Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. doi: 10.1038/ki.2010.483. [DOI] [PubMed] [Google Scholar]
- 83.Sutherland SM, Chawla LS, Kane-Gill SL, Hsu RK, Kramer AA, Goldstein SL, Kellum JA, Ronco C, Bagshaw SM. Utilizing electronic health records to predict acute kidney injury risk and outcomes: workgroup statements from the 15th ADQI consensus conference. Can J Kidney Health Dis. 2016;3:11. doi: 10.1186/s40697-016-0099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zarbock A, Schmidt C, Van Aken H, Wempe C, Martens S, Zahn PK, Wolf B, Goebel U, Schwer CI, Rosenberger P, Haeberle H, Gorlich D, Kellum JA, Meersch M, Renal Rl. Effect of remote ischemic preconditioning on kidney injury among high-risk patients undergoing cardiac surgery: a randomized clinical trial. JAMA. 2015;313:2133–2141. doi: 10.1001/jama.2015.4189. [DOI] [PubMed] [Google Scholar]
- 85.Billings FT, Shaw AD. Clinical trial endpoints in acute kidney injury. Nephron Clin Pract. 2014;127:89–93. doi: 10.1159/000363725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rifkin DE, Coca SG, Kalantar-Zadeh K. Does AKI truly lead to CKD? J Am Soc Nephrol. 2012;23:979–984. doi: 10.1681/ASN.2011121185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Coca SG. Is it AKI or nonrecovery of renal function that is important for long-term outcomes? Clin J Am Soc Nephrol. 2013;8:173–176. doi: 10.2215/CJN.12621212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, Arima H, Chadban SJ, Cirillo M, Djurdjev O, Green JA, Heine GH, Inker LA, Irie F, Ishani A, Ix JH, Kovesdy CP, Marks A, Ohkubo T, Shalev V, Shankar A, Wen CP, de Jong PE, Iseki K, Stengel B, Gansevoort RT, Levey AS, CKD Prognosis Consortium Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311:2518–2531. doi: 10.1001/jama.2014.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Thompson A, Lawrence J, Stockbridge N. GFR decline as an end point in trials of CKD: a viewpoint from the FDA. Am J Kidney Dis. 2014;64:836–837. doi: 10.1053/j.ajkd.2014.09.006. [DOI] [PubMed] [Google Scholar]


