Abstract
Background
Risk stratification and early detection of cardiac allograft vasculopathy (CAV) is essential in orthotopic heart transplantation (OHT) patients. This study assesses the changes in myocardial blood flow (MBF) noninvasively in OHT patients using quantitative cardiac PET with regadenoson.
Methods
Twelve patients (Group 1) (8 males, 4 females, mean age 55 ± 7 y) with no history of post OHT myocardial ischemia were enrolled after 5.4± 2.0 y after OHT. Fifteen patients (Group 2) (9 males, 6 females, mean age 71 ± 9 y) with intermediate pretest probability but not documented evidence for coronary artery disease (CAD) were also included to serve as control. Global and regional MBFs were assessed using dynamic 13N-NH3 PET at rest and during regadenoson-induced hyperemia. The coronary flow reserve (CFR) was also calculated as the ratio of hyperemic to resting MBF.
Results
Mean regadenoson-induced rate-pressure products were similar in both groups, while there was an increase in resting rate-pressure product in Group 1 patients. Both mean and median values of resting MBF were higher in Group 1 than Group 2 patients (1.33±0.31 and 1.01±0.21 mL/min/g for Groups 1 and 2, respectively, P<.001), while mean hyperemic MBF values were similar in both Groups (2.68±0.84 and 2.64±0.94 mL/min/g, P=NS) but median hyperemic MBF values were lower in Group 1 than Group 2 patients (2.0 vs. 2.60 mL/min/g, P=.018). Both mean and median CFR values demonstrated a significant reduction toward the Group 1 compared to Group 2 patients (2.07±0.74 vs. 2.63±0.48, P = .025).
Conclusions
This study suggests that the MBF in OHT patients may be abnormal at resting state with diminished CFR. This hints that the epicardial and microvascular coronary subsystem may be exacerbated after OHT leading to the gradual progression of CAV.
Keywords: Dynamic PET, regadenoson, coronary flow reserve, orthotopic heart transplant, cardiac allograft vasculopathy
INTRODUCTION
Cardiac allograft vasculopathy (CAV) remains one of the major causes of morbidity and mortality after orthotopic heart transplantation (OHT).1–3 Accurate definition of its prevalence and long-term prognosis are crucial for the evaluation of allograft dysfunction that may lead to silent myocardial infarction, or sudden death.4, 5 CAV often presents a diffuse vascular pattern, and has been difficult to diagnose noninvasively by the lack of a sensitive method to detect developing vascular pathology in the allograft.
Coronary angiography (CA)6, 7 and intravascular ultrasonography (IVUS)8–10 are commonly accepted methods that have been used for detecting CAV. These structural imaging modalities are best equipped for identifying focal, eccentric narrowing of the vessel lumen11 rather than the more diffuse pathology of CAV that evolves from an initial intimal thickening into concentric and longitudinal lesions, involving epicardial coronary vessels and the intramyocardial microvascular system. The impaired endocardial perfusion caused by sustained microvascular dysfunction will alter subendocardial layers of the myocardium, which will translate into early endothelial vasoreactive functional abnormalities, reducing CFR, representative of the capacity of the coronary circulation to dilate, following increasing metabolic demands.
A noninvasive test of coronary function for assessing the endothelial-dependent and -independent coronary function abnormalities associated with vasculopathy in the allograft would then be pivotal for potentially predicting the course of the disease. Different from standard noninvasive imaging modalities that lack the capability of measuring absolute changes in myocardial blood flow, cardiac PET is a clinically well-established quantitative imaging technique that allows assessment of myocardial blood flow in the physical unit of ml/g/min. When performed during vasodilator stress testing, these measurements provide indices of endothelial-dependent and -independent coronary function.12
Adenosine and dipyridamole have been the cornerstone of vasodilator stress testing in myocardial perfusion imaging (MPI). Recently, regadenoson, a selective adenosine 2A receptor agonist, has been introduced as a new vasodilator stress agent, with reported better patient tolerability and reduced side effects.13 While regadenoson does not depend on the endothelial function for vasodilation at the level of the coronary vessels, it has been demonstrated in several trials that it is not inferior to adenosine for detection of myocardial ischemia.14, 15 There are some preliminary studies in an animal model16 that suggest regadenoson increases the blood-brain barrier permeability by endothelial cells signaling, and facilitates CNS entry of macromolecules. Its use as a tool to assess early CFR abnormalities in OHT patients with suspected CAV remains to be fully understood. This is one of the main purposes of this pilot project to see if this relatively new MPI stress agent would be suitable to assess CAV in the OHT patients.
In this paper, we report our initial experience with regadenoson PET MPI in OHT patients with suspected CAV. We aimed to use the quantitative PET to determine the global and regional blood flows in response to regadenoson, and how it differs from another cohort of subjects with no documented evidence of ischemic heart disease.
METHODS
Twenty seven patients (17 males and 10 females) were enrolled in this study. The study was approved by the Institutional Review Board (IRB) of the University of California, San Francisco (UCSF). Patients received full information about the potential risk and benefit of their participation in the study.
Twelve OHT patients (Group 1) (8 males, 4 females, mean age 55 ± 14 years) with an average left ventricular ejection fraction (LVEF) (51±12)% and 15 patients (Group 2) (9 males, 6 females, mean age 72 ± 8 years) with preserved LVEF (57± 13)% and an intermediate pretest probability of CAD that served as controls (Table 1) were enrolled.
Table 1.
Patient characteristics
| OHT (Group 1) | Control (Group 2) | |
|---|---|---|
| Gender | 8 M, 4 F | 9 M, 6 F |
| Age (y) | 55±14 | 72 ± 8 |
| HR Rest (bpm) | 76±14 | 66±11 |
| Systolic BP Rest (mmHg) | 147±23 | 141±19 |
| Diastolic BP Rest (mmHg) | 76±13 | 73±9 |
| HR Stress (bpm) | 89±17 | 94±27 |
| Systolic BP Stress (mmHg) | 117±18 | 127±15 |
| Diastolic BP Stress (mmHg) | 61±12 | 60±5 |
| Total Cholesterol (mg/dL) | 158±57 | 156±18 |
| HDL (mg/dL) | 49±15 | 52±14 |
| LDL (mg/dL) | 70±26 | 88±17 |
| Weight (lb) | 182±69 | 189±52 |
| Beta Blockers | 7 | 7 |
| Diuretic | 6 | 7 |
| Calcium Channel Blocker | 2 | 3 |
| Smoking | 5 | 5 |
The group 2 patients, who might be abnormal or asymptotic, were regarded as control for the baseline of CFR measurement since these patients did not have evidence of perfusion abnormalities at the time of the PET study.
The average duration between OHT and PET MPI in Group 1 patients was (5.4± 2.0 years). None of these patients had any coronary intervention after the OHT.
Relevant clinical indicators such as blood pressure (BP), heart rate (HR), LVEF at rest and with stress, lipid profile as well as a history of hypertension, hyperlipidemia, smoking, beta blocker, calcium channel blocker and diuretic therapy were recorded for each patient (Table 1).
Imaging protocol
PET imaging protocol
A Discovery VCT PET/CT scanner (GE Healthcare, Waukesha, WI) was used for all PET imaging studies. All patients fasted for at least 4 hours and refrained from caffeine-containing beverages for 24 hours.
The protocol began with a dynamic rest scan with 740 MBq (20 mCi) of 13N-NH3 IV (10 to 20 sec duration) bolus injection administered at the start of the dynamic scan. Beginning with the intravenous 13N-NH3 (ammonia) injection, 2D prospective dynamic image sets were acquired for 15 minutes (36 × 5 s, 2 × 60 s, and 2 × 300 s). Between resting and stress acquisition, about 40 minutes duration were allowed for physical decay of 13N.
For the stress scan, regadenoson (Lexiscan®, Astellas Pharma; 0.4 mg regadenoson in 5 ml solution) was administered over approximately 15 to 20 sec into a peripheral vein, followed by a 5 ml saline flush. A same amount of dose of 740 MBq (20 mCi) of 13N-NH3 as rest was injected approximately 30 sec after the regadenoson administration. Antidote was administered as necessary, depending on the patient’s response. Data acquisition began at the beginning of the 13N-NH3 injection and continued for 15 minutes. An X-ray CT transmission scan was performed at the end of the rest/stress PET study.
Twelve-lead ECG, heart rate and blood pressure were monitored continuously and recorded at baseline and during and after regadenoson administration. Peak stress heart rate was defined as the highest heart rate at any time after regadenoson administration. Stress systolic, diastolic and mean blood pressure was defined as the values at the time of peak heart rate.
Image reconstruction
The dynamic PET data for each patient were reconstructed using 2D filtered back projection (FBP) algorithm provided by the scanner manufacturer. All CT-based attenuation-corrected PET images were sampled at a 5-second duration for the first 3 minutes, 60 seconds for the next two minutes, and 5 minutes thereafter with a total of 40 dynamic frames for further kinetic analysis. The registration accuracy between the attenuation map derived from CT and reconstructed PET volume was verified for each study. When there was a mismatch between the attenuation map and PET volume, re-alignment was manually performed, and the reconstruction was repeated using the aligned attenuation map.
PET data analysis and estimation of MBF
The myocardium was oriented along the long-axis and short-axis and subdivided into 17 segments following the recommendation from American Heart Association (AHA) from base through mid-cavity to apical regions. The time activity curves (TACs) for 17 myocardial segments as well as activity concentrations of left and right ventricular blood pools for each rest-stress pair were extracted. The myocardial blood flow (MBF) and coronary flow reserve (CFR) values were determined using the PMOD Cardiac PET Modeling Tool (PCARDP) (PMOD Technologies, Zurich, Switzerland).
The regional TAC for each segment was fitted with an irreversible two-tissue compartment model based on the work of Hutchins et al.17 and the corresponding uptake and washout rates were estimated. An exponential metabolite correction was included as described by van den Hoff et al.18, with a delay time t0=0.48 min and half-life T1/2=6.69 min. The double spillover correction19 for the activity in the myocardium from the left and right ventricle was also incorporated.
For the quantification of regional MBF with dynamic PET, the measured K1-values for 13N-NH3 PET were considered to be equal to the flow. This is due to the fact that K1-values bear a linear relationship with the flow over a reasonable normal range of MBF since the first pass extraction for 13N-NH3 is nearly equal to one.20
The segments related to the conventional regions supplied by the three major coronary arteries in the territory of the left anterior descending artery (LAD), the right coronary artery (RCA), the left circumflex artery (LCX), respectively were combined to calculate territorial MBF both at rest and stress. CFR was defined as the ratio of peak hyperemic to resting MBF.
Statistical analysis
All estimated values were expressed as mean ± SD. Median and quartile values were also calculated. P-values were calculated using two-tailed t-test to draw statistical significance. Any P-value less than 0.05 were considered statistically significant.
While calculating P-value we implicitly assumed that the distribution of the measured/estimated parameters is Gaussian in nature. Noting the fact that the distribution is partially skewed and small sample size, we also tested the results with non-parametric Wilcoxon analysis for unpaired data, and compared the results with parametric statistics.
All statistical calculations were performed using the open-source statistical package R (http://www.R-project.org/).
RESULTS
Clinical characteristics
Clinical characteristics of the patients in both groups are shown in Table 1. Based on clinical assessment, none of the Group 1 patients who received OHT had evidence of rejection. None of the Group 2 patients demonstrated evidence of ischemia or myocardial infarction at the time of PET studies.
Resting heart rate (78±13 versus 66±9 bpm, P<.001), average blood pressure (108±18 versus 105±14 mm Hg; P=.10) and rate-pressure product (10 824±1892 versus 7424±1353 bpm·mm Hg; P<.001) were higher in patients with OHT (Group 1) than control (Group 2) patients. However, there were no significant differences in heart rates (94±20 versus 91±16 bpm, P=NS), average blood pressure (99±15 versus 100±9 mm Hg, P=NS) and rate-pressure product (10824±1892 versus 7424±1353 bpm.mmHg) between patients in Group 1 and Group 2 during regadenoson-induced hyperemia.
Estimation of MBF and CFR with 13N-NH3 PET
The mean territorial MBF values at rest in controls (Group 2) were 1.01±0.21, 1.0±0.11, and 0.91±0.11 mL·g−1·min−1 in the LAD, RCA, and LCX territory, respectively, and served as a baseline. Mean global MBF was higher in the OHT patients (Group 1) than in controls (Group 2) (1.33±0.31 versus 1.01±0.21 mL·min−1·g−1; P<.001). There was no significant difference in mean MBF during stress between Group 1 and 2 patients (2.68±0.84 versus 2.64±0.94 mL·g−1·min−1, P=NS). However, the median value of the stress MBF was lower in Group 1 (OHT) patients (2.07±0.74 versus 2.63±0.48 mL·g−1·min−1, P=.018), indicating a majority of the OHT patients had lower MBF during stress (Table 2). Within the premise of parametric statistics, the CFR values were significantly lower in OHT than controls (2.07±0.74 vs. 2.63±0.48, P = .025).
Table 2.
Group 1 (OHT)
| Min. | 1st Qu. | Median | Mean | 3rd Qu. | Max. | ||
|---|---|---|---|---|---|---|---|
| MBF Stress | LAD | 1.322 | 1.938 | 2.408 | 2.661 | 3.239 | 5.004 |
| RCA | 1.482 | 1.904 | 2.306 | 2.525 | 3.061 | 4.254 | |
| LCX | 1.558 | 1.968 | 2.361 | 2.719 | 3.343 | 4.858 | |
| Global | 1.673 | 2.017 | 2.074 | 2.637 | 3.218 | 4.162 | |
| MBF Rest | LAD | 0.8671 | 1.189 | 1.351 | 1.403 | 1.659 | 1.927 |
| RCA | 0.6143 | 1.054 | 1.319 | 1.34 | 1.467 | 2.388 | |
| LCX | 0.8505 | 0.9336 | 1.268 | 1.215 | 1.369 | 1.748 | |
| Global | 0.9181 | 1.165 | 1.334 | 1.33 | 1.455 | 1.774 | |
| CFR | LAD | 0.686 | 1.479 | 1.881 | 1.954 | 2.304 | 3.331 |
| RCA | 0.79 | 1.536 | 1.729 | 2.114 | 2.601 | 4.034 | |
| LCX | 1.229 | 1.654 | 2.024 | 2.307 | 2.842 | 3.885 | |
| Global | 1.069 | 1.514 | 1.822 | 2.053 | 2.624 | 3.252 |
Due to a wide variability of MBF and small sample size, we also performed a nonparametric Wilcoxon analysis to confirm the results from parametric statistics. Mean global MBF was higher in the OHT patients (1.33±0.31 versus 1.01±0.21 mL·min−1·g−1; P=.011). There was again no significant difference in mean MBF during stress between Group 1 and 2 patients (2.68±0.84 versus 2.64±0.94 mL·g−1·min−1, P=NS). The CFR values were significantly lower in OHT than controls (2.07±0.74 vs. 2.63±0.48, P = .047) as found previously with two-tailed t-test.
In Fig 1, we show bar plots of regional CFR of OHT (Group 1) and control patient (Group 2) averaged over each patient cohort. The CFR<2 were considered abnormal while CFR>2.5 were normal. The gray value of the CFR range was 2.0<CFR<2.50. The bars in bold represent the CFRs in major vascular territories (LAD, RCA, and LCX) and global myocardium.
Fig. 1.
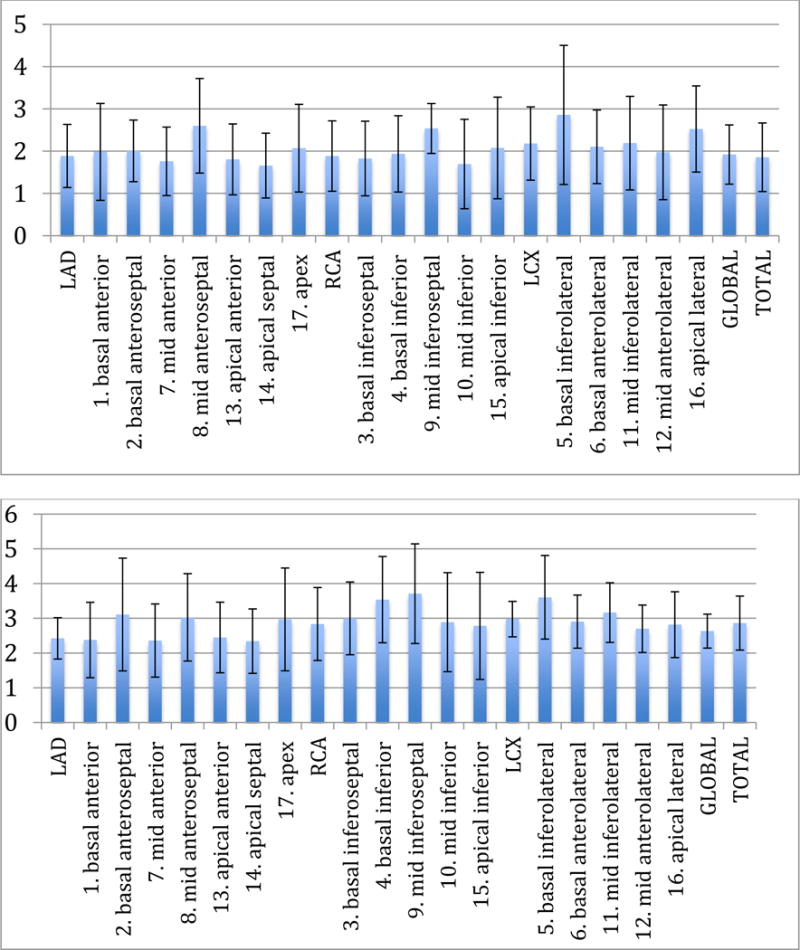
Regional CFR (17-segments) averaged over patient cohorts of OHT (Top) and Control (Bottom). Error bars represent the standard deviation of the mean.
Out of 12 OHT patients, 7 (58%) had CFR values below 2 and 3 patients (25%) had values in the grey zone (2–2.5) while 2 patients (17%) had CFR values greater than 2.5. Similarly, out of 15 undocumented patients 0 (0%) had a CFR below 2, 5 patients (33%) had values in the grey zone while 10 patients (67%) had CFR values greater than 2.5.
A qualitative assessment and a statistical summary of mean and median MBF values at rest and stress along with corresponding CFR values for the OHT patients (Group 1) and the control group (Group 2) are shown in table 2 and 3.
Table 3.
Group 2 (Control)
| Min. | 1st Qu. | Median | Mean | 3rd Qu. | Max. | ||
|---|---|---|---|---|---|---|---|
| MBF Stress | LAD | 1.488 | 2.19 | 2.335 | 2.509 | 2.803 | 4.173 |
| RCA | 1.431 | 2.306 | 2.509 | 2.873 | 3.492 | 4.788 | |
| LCX | 1.221 | 2.336 | 2.636 | 2.729 | 2.865 | 5.422 | |
| Global | 1.395 | 2.333 | 2.607 | 2.68 | 3.008 | 4.112 | |
| MBF Rest | LAD | 0.7009 | 0.8856 | 0.9855 | 1.015 | 1.136 | 1.407 |
| RCA | 0.6404 | 0.8836 | 0.9574 | 1.007 | 1.177 | 1.404 | |
| LCX | 0.4549 | 0.758 | 0.8853 | 0.9169 | 1.012 | 1.54 | |
| Global | 0.7056 | 0.918 | 0.9942 | 1.016 | 1.064 | 1.444 | |
| CFR | LAD | 1.515 | 2.165 | 2.388 | 2.45 | 2.736 | 3.493 |
| RCA | 1.714 | 2.323 | 2.732 | 2.887 | 3.192 | 5.782 | |
| LCX | 2.424 | 2.641 | 2.747 | 2.959 | 3.152 | 4.015 | |
| Global | 1.976 | 2.256 | 2.535 | 2.631 | 2.854 | 3.507 |
Figure 2 depicts a comparison between CFRs for the OHT patients (Group 1) and the control group (Group 2).
Fig. 2.
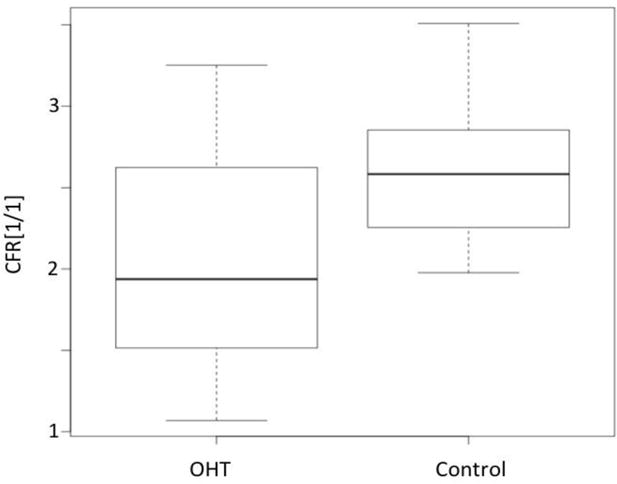
Boxplot of global CFR values for OHT (Group 1) and Control (Group 2).
DISCUSSION
The main conclusions that can be derived from this pilot study are: (1) Regadenoson may be a valid alternative to the more commonly used vasodilators – adenosine and dipyridamole – for measuring coronary flow reserve with PET imaging, and (2) reduced hyperemic CFR assessed noninvasively may represent the early microvascular abnormalities, in absence of qualitative myocardial perfusion defects, that characterize progression of CAV after OHT.
Since 2009 regadenoson has been used safely and successfully as a pharmacological myocardial stress perfusion agent both in SPECT and PET MPI, including OHT patients for assessing the development of diffuse cardiac allograft vasculopathy (CAV).21 However, to date, no study has been reported to use PET imaging and regadenoson for assessing MBF and CFR changes in the post OHT patients, as a tool to disclose the early vasoreactive abnormalities that could unravel the development of CAV. In recent years, regadenoson has become widely used in the nuclear cardiology laboratory, given its advantages for patient tolerability, reported overall safety, and mechanism of action.22 Additional advantages of regadenoson may include: quick response, no need for an infusion or weight-based dosing, a potentially useful alternative for OHT patients to achieve a targeted heart rate without physical exercise; in addition to rapid onset of maximal hyperemia (within 1 minute of regadenoson injection) and the short-lived nature of its hyperemic response (less than 5 minutes) without significant additional monitoring time compared to a standard stress test of about 20 minutes. However, the limited data evaluating the safety and feasibility in MPI PET for OHT patients may be one of the major disadvantages of its common use.
Typically, myocardial blood flow (MBF) in normal volunteers averages 1 ml/min/g at rest and increases up to 5 ml/min/g during peak hyperemia. In a study that compared quantitatively the efficacy of intravenous adenosine and dipyridamole for pharmacologic induction of myocardial hyperemia found that the average MBF was 1.1 ± 0.2 ml/min/g and increased to a peak value to 4.4 ± 0.9 ml/min/g during adenosine and 4.3 ± 1.3 ml/min/g after dipyridamole administration.23 Similarly, hyperemia to baseline flow ratios (CFR) averaged 4.3 ± 1.6 for adenosine and 4.0 ± 1.3 for dipyridamole. Our values of MBF in controls were 1.01±0.21 mL/min/g at rest and 2.68±0.94 mL/min/g at stress with a CFR equal to 2.63±0.48. The relatively lower MBF (2.68 ml/min/g) in controls during hyperemia with regadenoson compared to adenosine and dipyridamole may be due to patients advanced age (mean age of 72± 8).
In this study, although mean hyperemic myocardial blood flows were similar for both Groups (2.63 versus 2.68 ml/min/g respectively, P =NS), but median values of hyperemic MBF were significantly lower (2.01 vs. 2.60 mL/min/g, P=0.018) indicating a bias due to a small sample size. However, both the mean and median resting myocardial blood flows were higher in Group 1 (1.34 versus 1.01 ml/min/g, respectively P<.001).
The elevated resting MBF after transplant found in our study is consistent with previous studies, and can be explained in terms of a higher rate-pressure product in OHT patients. For example, Seneff et al.24 reported that the resting MBF averaged 1.63±0.51 ml/g/min in a group of 35 allograft recipients. The general trend of increased resting MBF may be due to coronary-hypersensitivity in OHT patients with known reduced coronary vascular resistance.25
There was a positive correlation between rate-pressure product (10728±2178 bpm·mmHg) and resting myocardial blood flow in OHT patients (Fig. 3). Both higher systolic blood pressure (147±26 mmHg) and heart rate (77±16 bpm) were associated with higher cardiac work that may result from intimal thickening of the arteries, a precursor of CAV with consequently increased MBF. The positive correlation (r =0.23, P=0.038) may relate to the fact that the rate-pressure product does not take into account other variables such as resting coronary vasomotor tone and vasoregulation in these functionally denervated hearts and ventricular stroke.26
Fig. 3.
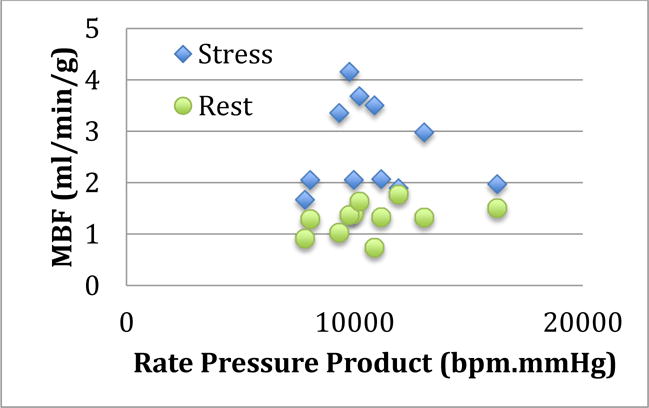
MBF (ml/min/g) at rest and stress in Group 1 (OHT) patients as a function of rate-pressure product.
The coronary vascular resistance index (CVRI) was calculated as the ratio of mean arterial blood pressure (mmHg) to myocardial blood flow (ml/g/min). It has been routinely used as a parameter to correlate homogeneity in changes in MBF, related to variations of hemodynamics. At rest, this index was lower for OHT than Control (86.8 ±22 Vs. 104±28, P=0.041). However, it remained unchanged during hyperemia (36.5±13 versus 31.0±11.0, P=NS). This reduced vascular resistance likely relates to the limited sympathetic reinnervation known to prevail after OHT.27
One may wonder how the results derived in this work could be different if the MBF is normalized to the mean rate pressure product (MRPP). We therefore calculated MBF normalized to the MRPP which was defined as the ratio of MBF [ml/g/min] to MRPP [bpm.mmHg] times 10 000. Table 4 depicts the normalized mean MBF and CFR for both OHT and Controls.
Table 4.
MBF and CFR normalized to mean-rate-pressure-product (MRPP)
| OHT | Control | |||
|---|---|---|---|---|
| Rest | Stress | Rest | Stress | |
| Mean Pressure | 115.5 | 96 | 105 | 83 |
| HR | 76.6 | 89 | 66 | 95 |
| MRPP | 8893 | 8544 | 6930 | 7885 |
| Mean MBF | 1.33 | 2.63 | 1.01 | 2.68 |
| Normalized MBF | 1.49 | 3.07 | 1.45 | 3.4 |
| Normalized CFR | 2.01 | 2.34 | ||
The hyperemic response to regadenoson in normal patients would preserve endothelium-independent coronary vascular smooth muscle relaxation, while the abnormal response in OHT patients could suggest a vasculopathy likely located at the level of the coronary endothelium. When transplant recipients are compared to a control group, it is difficult to take into account factors such as the enhanced rate-pressure product induced by immunosuppressive treatment or the influence of denervation on coronary blood flow. Although the pathogenesis of cardiac graft vasculopathy remains to be established, endothelial injury may be the early stage of cardiac graft vasculopathy, probably related to immunologic mechanisms. In the early months after transplantation, we are very likely faced with an endothelial dysfunction without morphologic changes allowing an increase in the uptake of cytokines. These cytokines produced by macrophage and T-cell activations have been identified in the plasma of cardiac transplant recipients, and may promote the release of endothelin, which is correlated with serotonin-induced coronary vasoconstriction. Moreover, cytokines also act directly on vascular smooth muscle and produce inflammation-related hyperemia, probably through activation of inducible nitric oxide synthase. This inflammation-induced hyperemia, probably mediated by an enhanced nitric oxide formation, is likely responsible of the increased MBF.
There was a considerable variation in hyperemic MBF values both in OHT and control patients. This may be attributed to various confounding factors such as patient’s age, body mass index, state of vasoreactivity, individual effect of regadenoson due to polymorphism, as well as the time duration since OHT (5.4± 2.0 years). The correlation between hyperemic rate-pressure product and MBF was not significant (P=NS). There was an increase in heart rate, but a decrease in systolic blood pressure during hyperemia.
Our findings that there was no significant global change in mean hyperemic MBF in the OHT (Group 1) compared with the control group (Group 2) is also consistent with other similar reports28, 29 despite other studies suggested that there was a moderate decrease in MBF observed in hyperemia in patients with maximal intimal thickening possibly related to CAV.30 Some studies also indicated that the MBF could be impaired in the early stage after OHT and partially restored usually within one year in those free of allograft rejection and/or CAV.31 The average enrollment duration in our study was 5.4 ± 2.0 years after OHT, which might hint at the possibility of restoration of normal MBF in some patients free from CAV, which, in turn, blunted the rest of the other MBF values. However, it should be noted that the global median value of hyperemic MBF in the OHT group was significantly lower in comparison to that of the control group, which again implies that a number of allograft recipients may have comparatively lower hyperemic MBF. The CFR demonstrated a diminished trend either as reduction in the hyperemic MBF or elevated resting MBF.
Prognostic implications and limitations
There are several prognostic implications related to an abnormal CFR in patients who underwent OHT. Abnormalities in vasodilatory capacity, reflected by a blunted coronary flow reserve have been associated with subsequent changes in intimal thickening observed in intravascular ultrasound.32
In addition, an abnormal coronary flow reserve seems to inversely correlate with plaque volume in patients with history of OHT by IVUS, and angiographically normal coronary arteries and normal LV function. OHT patients with normal CFR have myocardial deformation response during exercise comparable to that of healthy controls, whereas patients with reduced CFR failed to improve their longitudinal myocardial deformation during exercise, which led to lower peak exercise workload and lower peak CO. This is likely linked to abnormalities in diastolic function, which is known to play a pivotal role in the development of ischemic heart disease.
Full prognostic implications of reduced CFR in OHT is clinically valuable and important, but beyond the scope of this pilot study. This will be a future extension of the current study.
Due to very low OHT patients our inclusion criteria were not specific to particular patient characteristics. Our institution (UCSF) has an average of 15 heart-transplant surgeries per year. Due to many logistic reasons we could not recruit them all. So, all patients who had history of OHT surgery but no evidence of perfusion abnormalities were included and assessed in this study.
Because of nonspecific inclusion criteria, the results presented in this study should be considered as indicative rather than conclusive. The conclusions derived from this pilot project, though important, may be limited due to small sample size.
NEW KNOWLEDGE GAINED
Prognostic implications of reduced CFR in OHT patients are clinically important and can be assessed by using quantitative PET with regadenoson. The hyperemic MBF on OHT patients may be less affected while resting MBF may be elevated due to possible vasculopathy resulting a net reduced CFR. The general trend of increased resting MBF may be due to coronary-hypersensitivity in OHT patients with known reduced coronary vascular resistance.
CONCLUSIONS
Our preliminary study suggests that the quantitative cardiac PET with regadenoson may be a useful tool for assessing early changes in CAV after OHT. Our study employed PET with regadenoson for the assessment of MBF and CFR, which in turn, provides information on impairment of epicardial and microvascular coronary system to evaluate for the presence and progression of CAV in the post OHT patients.
Acknowledgments
Authors would like to thank nuclear medicine technologists and cyclotron and clinical staff at UCSF China Basin imaging facility for conducting patient scans. The study was supported in part by the National Institutes of Health under grant R01 HL050663.
Abbreviations
- CA
Coronary angiography
- CABG
Coronary bypass graft surgery
- CAD
Coronary artery disease
- CAV
Cardiac allograft vasculopathy
- CFR
Coronary flow reserve
- IVUS
Intravascular ultrasonography
- LAD
Left anterior descending artery
- LCX
Left circumflex artery
- MBF
Myocardial blood flow
- OHT
Orthotopic heart transplantation
- RCA
Right coronary artery
Footnotes
Journal Subject Code: Diagnostic testing [60] – PET
Disclosure
The authors have no conflict of interest.
References
- 1.Gao SZ, Schroeder JS, Alderman EL, Hunt SA, Valantine HA, Wiederhold V, Stinson EB. Prevalence of accelerated coronary artery disease in heart transplant survivors. Comparison of cyclosporine and azathioprine regimens. Circulation. 1989;80:III100–105. [PubMed] [Google Scholar]
- 2.Schmauss D, Weis M. Cardiac allograft vasculopathy: Recent developments. Circulation. 2008;117:2131–2141. doi: 10.1161/CIRCULATIONAHA.107.711911. [DOI] [PubMed] [Google Scholar]
- 3.Zimmer RJ, Lee MS. Transplant coronary artery disease. JACC Cardiovascular interventions. 2010;3:367–377. doi: 10.1016/j.jcin.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 4.Hollenberg SM, Klein LW, Parrillo JE, Scherer M, Burns D, Tamburro P, Oberoi M, Johnson MR, Costanzo MR. Coronary endothelial dysfunction after heart transplantation predicts allograft vasculopathy and cardiac death. Circulation. 2001;104:3091–3096. doi: 10.1161/hc5001.100796. [DOI] [PubMed] [Google Scholar]
- 5.Lund LH, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, Dobbels F, Goldfarb SB, Levvey BJ, Meiser B, Yusen RD, Stehlik J. The registry of the international society for heart and lung transplantation: Thirty-first official adult heart transplant report–2014; focus theme: Retransplantation. J Heart Lung Transplant. 2014;33:996–1008. doi: 10.1016/j.healun.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Wellnhofer E, Stypmann J, Bara CL, Stadlbauer T, Heidt MC, Kreider-Stempfle HU, Sohn HY, Zeh W, Comberg T, Eckert S, Dengler T, Ensminger SM, Hiemann NE. Angiographic assessment of cardiac allograft vasculopathy: Results of a consensus conference of the task force for thoracic organ transplantation of the german cardiac society. Transplant international: official journal of the European Society for Organ Transplantation. 2010;23:1094–1104. doi: 10.1111/j.1432-2277.2010.01096.x. [DOI] [PubMed] [Google Scholar]
- 7.Costanzo MR, Naftel DC, Pritzker MR, Heilman JK, 3rd, Boehmer JP, Brozena SC, Dec GW, Ventura HO, Kirklin JK, Bourge RC, Miller LW. Heart transplant coronary artery disease detected by coronary angiography: A multiinstitutional study of preoperative donor and recipient risk factors. Cardiac transplant research database. J Heart Lung Transplant. 1998;17:744–753. [PubMed] [Google Scholar]
- 8.Kobashigawa JA, Tobis JM, Starling RC, Tuzcu EM, Smith AL, Valantine HA, Yeung AC, Mehra MR, Anzai H, Oeser BT, Abeywickrama KH, Murphy J, Cretin N. Multicenter intravascular ultrasound validation study among heart transplant recipients: Outcomes after five years. J Am Coll Cardiol. 2005;45:1532–1537. doi: 10.1016/j.jacc.2005.02.035. [DOI] [PubMed] [Google Scholar]
- 9.Tuzcu EM, Kapadia SR, Sachar R, Ziada KM, Crowe TD, Feng J, Magyar WA, Hobbs RE, Starling RC, Young JB, McCarthy P, Nissen SE. Intravascular ultrasound evidence of angiographically silent progression in coronary atherosclerosis predicts long-term morbidity and mortality after cardiac transplantation. J Am Coll Cardiol. 2005;45:1538–1542. doi: 10.1016/j.jacc.2004.12.076. [DOI] [PubMed] [Google Scholar]
- 10.Kobashigawa JA, Pauly DF, Starling RC, Eisen H, Ross H, Wang SS, Cantin B, Hill JA, Lopez P, Dong G, Nicholls SJ. Cardiac allograft vasculopathy by intravascular ultrasound in heart transplant patients: Substudy from the everolimus versus mycophenolate mofetil randomized, multicenter trial. JACC Heart failure. 2013;1:389–399. doi: 10.1016/j.jchf.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Gao SZ, Alderman EL, Schroeder JS, Hunt SA, Wiederhold V, Stinson EB. Progressive coronary luminal narrowing after cardiac transplantation. Circulation. 1990;82:IV269–275. [PubMed] [Google Scholar]
- 12.Schindler TH, Schelbert HR, Quercioli A, Dilsizian V. Cardiac pet imaging for the detection and monitoring of coronary artery disease and microvascular health. JACC Cardiovascular imaging. 2010;3:623–640. doi: 10.1016/j.jcmg.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Lieu HD, Shryock JC, von Mering GO, Gordi T, Blackburn B, Olmsted AW, Belardinelli L, Kerensky RA. Regadenoson, a selective a2a adenosine receptor agonist, causes dose-dependent increases in coronary blood flow velocity in humans. J Nucl Cardiol. 2007;14:514–520. doi: 10.1016/j.nuclcard.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Cerqueira MD, Nguyen P, Staehr P, Underwood SR, Iskandrian AE. Effects of age, gender, obesity, and diabetes on the efficacy and safety of the selective a2a agonist regadenoson versus adenosine in myocardial perfusion imaging integrated advance-mpi trial results. JACC Cardiovasc Imaging. 2008;1:307–316. doi: 10.1016/j.jcmg.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Mahmarian JJ, Cerqueira MD, Iskandrian AE, Bateman TM, Thomas GS, Hendel RC, Moye LA, Olmsted AW. Regadenoson induces comparable left ventricular perfusion defects as adenosine: A quantitative analysis from the advance mpi 2 trial. JACC Cardiovasc Imaging. 2009;2:959–968. doi: 10.1016/j.jcmg.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Jackson S, Anders NM, Mangraviti A, Wanjiku TM, Sankey EW, Liu A, Brem H, Tyler B, Rudek MA, Grossman SA. The effect of regadenoson-induced transient disruption of the blood-brain barrier on temozolomide delivery to normal rat brain. J Neurooncol. 2016;126:433–439. doi: 10.1007/s11060-015-1998-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hutchins GD, Schwaiger M, Rosenspire KC, Krivokapich J, Schelbert H, Kuhl DE. Noninvasive quantification of regional blood flow in the human heart using n-13 ammonia and dynamic positron emission tomographic imaging. J Am Coll Cardiol. 1990;15:1032–1042. doi: 10.1016/0735-1097(90)90237-j. [DOI] [PubMed] [Google Scholar]
- 18.van den Hoff J, Burchert W, Borner AR, Fricke H, Kuhnel G, Meyer GJ, Otto D, Weckesser E, Wolpers HG, Knapp WH. [1-(11)c]acetate as a quantitative perfusion tracer in myocardial pet. J Nucl Med. 2001;42:1174–1182. [PubMed] [Google Scholar]
- 19.Fang YH, Muzic RF., Jr Spillover and partial-volume correction for image-derived input functions for small-animal 18f-fdg pet studies. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2008;49:606–614. doi: 10.2967/jnumed.107.047613. [DOI] [PubMed] [Google Scholar]
- 20.Schelbert HR, Phelps ME, Huang SC, Macdonald NS, Hansen H, Kuhl DE. N-13 ammonia as an indicator of myocardial blood-flow. Circulation. 1981;63:1259–1272. doi: 10.1161/01.cir.63.6.1259. [DOI] [PubMed] [Google Scholar]
- 21.Cavalcante JL, Barboza J, Ananthasubramaniam K. Regadenoson is a safe and well-tolerated pharmacological stress agent for myocardial perfusion imaging in post-heart transplant patients. J Nucl Cardiol. 2011;18:628–633. doi: 10.1007/s12350-011-9399-3. [DOI] [PubMed] [Google Scholar]
- 22.Stolker JM, Lim MJ, Shavelle DM, Morris DL, Angiolillo DJ, Guzman LA, Kennedy KF, Weber E, Zareh M, Neumayr RH, Zenni MM. Pooled comparison of regadenoson versus adenosine for measuring fractional flow reserve and coronary flow in the catheterization laboratory. Cardiovascular revascularization medicine: including molecular interventions. 2015;16:266–271. doi: 10.1016/j.carrev.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 23.Chan SY, Brunken RC, Czernin J, Porenta G, Kuhle W, Krivokapich J, Phelps ME, Schelbert HR. Comparison of maximal myocardial blood flow during adenosine infusion with that of intravenous dipyridamole in normal men. J Am Coll Cardiol. 1992;20:979–985. doi: 10.1016/0735-1097(92)90201-w. [DOI] [PubMed] [Google Scholar]
- 24.Senneff MJ, Hartman J, Sobel BE, Geltman EM, Bergmann SR. Persistence of coronary vasodilator responsivity after cardiac transplantation. Am J Cardiol. 1993;71:333–338. doi: 10.1016/0002-9149(93)90801-i. [DOI] [PubMed] [Google Scholar]
- 25.Chilian WM, Eastham CL, Marcus ML. Microvascular distribution of coronary vascular resistance in beating left ventricle. Am J Physiol. 1986;251:H779–788. doi: 10.1152/ajpheart.1986.251.4.H779. [DOI] [PubMed] [Google Scholar]
- 26.Di Carli MF, Tobes MC, Mangner T, Levine AB, Muzik O, Chakroborty P, Levine TB. Effects of cardiac sympathetic innervation on coronary blood flow. The New England journal of medicine. 1997;336:1208–1215. doi: 10.1056/NEJM199704243361703. [DOI] [PubMed] [Google Scholar]
- 27.Wilson RF, Christensen BV, Olivari MT, Simon A, White CW, Laxson DD. Evidence for structural sympathetic reinnervation after orthotopic cardiac transplantation in humans. Circulation. 1991;83:1210–1220. doi: 10.1161/01.cir.83.4.1210. [DOI] [PubMed] [Google Scholar]
- 28.Preumont N, Lenaers A, Goldman S, Vachiery JL, Wikler D, Damhaut P, Degre S, Berkenboom G. Coronary vasomotility and myocardial blood flow early after heart transplantation. Am J Cardiol. 1996;78:550–554. doi: 10.1016/s0002-9149(96)00363-3. [DOI] [PubMed] [Google Scholar]
- 29.Chan SY, Kobashigawa J, Stevenson LW, Brownfield E, Brunken RC, Schelbert HR. Myocardial blood flow at rest and during pharmacological vasodilation in cardiac transplants during and after successful treatment of rejection. Circulation. 1994;90:204–212. doi: 10.1161/01.cir.90.1.204. [DOI] [PubMed] [Google Scholar]
- 30.Kofoed KF, Czernin J, Johnson J, Kobashigawa J, Phelps ME, Laks H, Schelbert HR. Effects of cardiac allograft vasculopathy on myocardial blood flow, vasodilatory capacity, and coronary vasomotion. Circulation. 1997;95:600–606. doi: 10.1161/01.cir.95.3.600. [DOI] [PubMed] [Google Scholar]
- 31.Preumont N, Berkenboom G, Vachiery J, Jansens J, Antoine M, Wikler D, Damhaut P, Degre S, Lenaers A, Goldman S. Early alterations of myocardial blood flow reserve in heart transplant recipients with angiographically normal coronary arteries. J Heart Lung Transplant. 2000;19:538–545. doi: 10.1016/s1053-2498(00)00093-0. [DOI] [PubMed] [Google Scholar]
- 32.Allen-Auerbach M, Schoder H, Johnson J, Kofoed K, Einhorn K, Phelps ME, Kobashigawa J, Czernin J. Relationship between coronary function by positron emission tomography and temporal changes in morphology by intravascular ultrasound (ivus) in transplant recipients. J Heart Lung Transplant. 1999;18:211–219. doi: 10.1016/s1053-2498(98)00037-0. [DOI] [PubMed] [Google Scholar]


