Abstract
iNKT cells and mast cells have both been implicated in the syndrome of allergic asthma through their activation-induced release of Th2 type cytokines and secretion of histamine and other mediators, respectively, which can promote airways hyperresponsiveness (AHR) to agents such as methacholine. However, a mechanistic link between iNKT cells and mast cell recruitment or activation has never been explored. Our objective was to determine whether iNKT cells are necessary for the recruitment of mast cells and if iNKT cells can influence the acute allergen induced bronchoconstriction (AIB) caused by mast cell mediator release. To do so, we pharmacologically eliminated iNKT cells using a specific antibody (NKT-14) and examined its impact on airway inflammation and physiological phenotype. In mice treated with NKT-14, the elimination of iNKT cells was sufficient to prevent AHR and pulmonary eosinophilic inflammation elicited by administration of the iNKT cell agonist, αGalCer. In mice treated with NKT-14 and then sensitized and challenged with house dust mite extract (HDM), eliminating the iNKT cells significantly reduced both AHR and AIB but did not affect pulmonary inflammation, the mast cell population, nor the release of the mast cell mediators mast cell protease-1 and prostaglandin D2. We conclude that while iNKT cells contribute to the phenotype of allergic airways disease through the manifestation of AIB and AHR, their presence is not required for mast cell recruitment and activation, or to generate the characteristic inflammatory response subsequent to allergen challenge.
Keywords: iNKT cell, airway hyperresponsiveness, allergen induced bronchoconstriction, antibody treatment
1. INTRODUCTION
The pathophysiology of asthmatic airways depends to a significant degree on bronchoconstriction, mast cell expansion and mast cell activation [1, 2]. There are two distinct physiological hallmark reactions in allergic asthma: allergen induced bronchoconstriction (AIB) of the airway smooth muscles is the manifestation of an immediate allergen-induced activation of mast cells in the asthmatic lung within minutes of exposure, whereas airways hyperresponsiveness (AHR) is a clinical indicator provoked by challenge with agonists such as methacholine that is dominated by peripheral airway closure and that can linger for many days subsequent to allergen exposure or even be a stable phenotype of disease [3–5]. Hence, targeting the expansion and activation of mast cells to achieve adequate treatment of allergic asthma is an important research and therapeutic objective.
Invariant natural killer T (iNKT) cells share characteristics of both the adaptive and innate immune system. iNKT cells are also unique in that they respond to glycolipids, but not protein derived peptide antigens, presented by CD1d–expressing antigen-presenting cells [6, 7]. iNKT cell activation is followed, within minutes, by the release of cytokines e.g. IL-4, IL-13, IL-17 and IL-22, which are implicated in asthma [8–10]. However, their release of Th1, Th2, and Th17 type cytokines promotes the function of several leukocyte populations bridging the innate and the adaptive immune responses [7, 11], making it difficult to predict their role in every circumstance. Previous reports suggest that iNKT cells could function as important cellular mediators in asthma-like inflammatory models, namely the inflammatory response and the process of allergic sensitization suggesting that iNKT cells are required for induction of airway inflammation [6, 12, 13]. It has also been proposed that iNKT cells might play an important role in human asthma as well as in a mouse model of asthma and indeed iNKT cells have been shown to be increased in asthmatics and absence of iNKT cells can be protective against AHR in mice [6, 14, 15]. Furthermore, signaling from iNKT cells attracts mast cells [16–18], which has led to the suggestion that interfering with the iNKT cell – mast cell axis might have therapeutic benefits in human asthma [16]. Both mast cells and iNKT cells are stimulated by IL-33, leading to maturation and expansion of mast cells [19–21]. iNKT cells might contribute to the development and maintenance of innate immune responses via the ability of IL-33 to expand iNKT cell numbers and increase their release of IFNγ [22]. In addition, iNKT cells can release IL-9 that has been suggested to be involved in the recruitment and maturation of mast cells [23, 24]. On the other hand, it seems like iNKT cell activation by the specific ligand α-GalactosylCeramide (αGalCer) can suppress allergically induced cytokine release and alterations in the breathing pattern [25]. It is, however, not known if iNKT cells are involved in the allergen-driven mast cell expansion.
Studies in animals have suggested that iNKT cells might be important in allergic asthma, which has been supported by findings of their presence in the lung of patients with asthma [6, 21, 26–29], although some human studies do not support this conclusion [30–32]. In addition, crosstalk between iNKT cells and mast cells has been suggested to regulate inflammation [33]. Uncertainties still remain, however, regarding the specific role of iNKT cells in the context of allergic airways disease, in particular with respect to their relationship to mast cell expansion in asthma. Rather than relying on iNKT cell knock-out mice, some of which have been shown to have issues with respect to their T cell receptor repertoire [34], we used an antibody targeting iNKT cells, allowing for specific depletion of this cell type [35].
We hypothesized that eliminating iNKT cells might reduce AIB, AHR, and mast cell numbers in the allergic lung. This hypothesis is supported by our recently published data showing that mice allergic to ovalbumin display protection against AHR if iNKT cells are eliminated [35]. Because AIB is mast cell dependent we used our house dust mite (HDM) model of asthma [36] to test whether iNKT cells are necessary for the recruitment of mast cells to the lung during allergy and if eliminating iNKT cells would have any effects on the phenotype of AIB in addition to AHR.
2. METHODS
2.1. Animals
Female BALB/cJ 6–8 weeks old, were purchased from The Jackson Laboratory (Bar Harbor, ME) and were used beginning at 7–9 weeks of age. Housed in a specific pathogen free environment, the mice had free access to standard laboratory chow and water ad libitum, and were exposed to 12-hour light/dark cycles. All experimental procedures were approved by the University of Vermont Institutional Animal Care and Use Committee.
2.2. Reagents
Pancuronium bromide was purchased from Fisher Scientific (Hampton, NH), HDM extract was purchased from Greer Laboratories (Lenoir, NC), phosphate buffered saline (PBS) was obtained from Life Technologies (Carlsbad, CA), isoflurane was obtained from Vedco Inc. (St Joseph, MO), and Sodium-pentobarbital was purchased from Penro Specialty Compounding (Colchester, VT). Anti-CD16/CD32, anti-CD3-FITC, and anti-CD45-Pacific Orange were purchased from BD Pharmingen (San Jose, CA), anti-cKit-APC and anti-FcεRI-PE were obtained from eBioscience (San Diego, CA). CD1d tetramer loaded with PBS57 was provided by the NIH tetramer facility. The monoclonal antibody targeting iNKT cells, NKT-14 was provided by NKT Therapeutics (Waltham, MA) and the corresponding isotype-control IgG2a antibody was obtained from BioXcell (West Lebanon, NH), α-GalactosylCeramide (αGalCer) was purchased from Funakoshi Co. Ltd., Japan.
2.3. Sensitization with HDM
Mice were sensitized with house dust mite (HDM) Dermatophagoides pteronyssinus extract (Greer, Lenoir, NC). HDM was re-suspended in PBS at a protein concentration of 2.5 mg/ml. Our previous study demonstrated that this regimen produced not only AHR but also a mast cell-dependent AIB concomitant with lung mast cell expansion and mast cell degranulation products being released in the lung upon allergen exposure [36]. Mice were divided into two groups, experimental and control. Both groups of mice were anesthetized with isoflurane. While the experimental group was sensitized with intranasal instillation of 50 µl of the HDM suspension for 15 days over three consecutive weeks, the control group was sham sensitized with PBS. The animals showed no visible reactions relating to the HDM administration. The amount of lipopolysaccharide (LPS) in the HDM extract varies from batch to batch and we have calculated that the dose of LPS delivered to the mice in our study varied from 4.73 – 31.25 EU at each administration.
2.4. Treatment with NKT-14
Animals were treated with intra peritoneal (i.p.) injections once with NKT-14 or isotype control antibody, a non-specific IgG2a antibody, at a dose of 5 mg/kg. The antibodies were dissolved in PBS and administered three days before the start of HDM sensitization.
2.5. Assessment of acute bronchoconstriction and airways hyperresponsiveness
72 hours after the last HDM intra nasal exposure, the mice were anaesthetized with i.p. sodium pentobarbital (90mg/kg), the trachea cannulated and connected to a computer controlled small animal ventilator (flexiVent™, SCIREQ, Montreal, Canada), and ventilated at 200 breaths/minute with a stroke volume of 0.25 ml, adjusted for gas compression in the tubing resulting in a delivered tidal volume of about 0.20 ml. Next the mice were paralyzed with pancuronium bromide i.p. (0.8mg/kg). The depth of anesthesia was monitored with EKG throughout the experiment as previously described [3, 37, 38]. The animals were stabilized over about ten minutes of regular ventilation at a positive end-expiratory pressure (PEEP) of 3 cmH2O. A standard lung volume history was then established by delivering two total lung capacity maneuvers (TLC) to a pressure limit of 30 cmH2O and holding for three seconds. Next, two baseline measurements of respiratory input impedance (Zrs) were obtained. The mice were then exposed to either nebulized saline, methacholine or HDM via an in-line piezo-electric nebulizer (Aeroneb®) as described previously [36] and detailed in 2.7 and 2.8 below.
2.6. αGalCer induced AHR
Mice were treated with a single 2µg αGalCer in 50µl PBS + 0.05% Tween-20 or 50µl control PBS + 0.05% Tween-20 via oropharyngeal inhalation. Mice were either treated with i.p. NKT-14 or isotype control IgG2a three days before the αGalCer administration. The mice were tested for AHR and BALF was collected one day after the αGalCer challenge.
2.7. Assessment of HDM induced bronchoconstriction (AIB)
Seventy-two hours after the last HDM exposure, the mice were connected to the flexiVent as described above and were then tested for AIB. Following stabilization and standard lung volume history as described above, the mice were either exposed to an inhalation of vehicle control dose of PBS over 40 seconds followed by a Zrs measurements every 10 seconds for three minutes; or the mice received 40 seconds of aerosolized HDM (2.5 mg/ml) delivered via the in-line piezoelectric nebulizer. The inhalation was immediately followed by Zrs measurements every 10 seconds for three minutes. A Zrs measurement takes three seconds; one second for exhalation to PEEP and two seconds to deliver the measurement oscillation leaving seven seconds for regular ventilation before the next measurement cycle starts. Lungs were harvested and snap-frozen in liquid nitrogen after the AHR assessment and saved for later cytokine measurements.
2.8. Assessment of AHR
Mice were sensitized with HDM and surgically prepared as described above in 2.5. Following stabilization and standard lung volume history as described above, mice were exposed to an inhalation of aerosolized PBS (vehicle control) for 10 s. Zrs was then measured every 10 s for 3 minutes. This complete sequence of maneuvers and measurements was then repeated for aerosol exposures to three incremental doses of methacholine (3, 12 and 25 mg/ml) [39]. Lungs were harvested and prepared for flow cytometry.
2.9. Calculation of respiratory mechanics
Zrs was determined using the forced oscillation technique described previously [3, 39]. Briefly: Zrs over the frequency range 1–20.5 Hz was determined using a two second broadband perturbation in volume applied by the flexiVent™. Each determination of Zrs was fit with the constant phase model of impedance [40] given by
where Rn is a frequency independent Newtonian resistance reflecting that of the conducting airways, I is airway gas inertance (in the mouse I is negligible), G characterizes tissue dampening, H characterizes tissue stiffness or elastance, i is the imaginary unit, and f is frequency in Hz [40–42].
2.10. Collection of bronchoalveolar lavage fluid and lung specimens
After the AIB measurement was completed broncho-alveolar lavage fluid (BALF) was collected. Lungs were lavaged with 1 ml of 1X Dulbecco’s PBS, and the volume of bronchoalveolar lavage fluid (BALF) collected was measured for each sample, and the total number of leukocytes was counted using an automated cell counter (Advia, Siemens Healthcare Inc.). The cell pellet was separated from the supernatant, re-suspended in 400 µl of PBS, and counted. Differential cell counts were determined by spinning the cells onto glass slides with a Cytospin and staining with hematoxylin and eosin.
2.11. Protein extraction protocol
Frozen lung tissue (from lungs after AIB measurement) was pulverized to a fine powder using a liquid nitrogen-chilled mortar and pestle, transferred to the 100-µl mark of a liquid nitrogen-chilled 1.5-ml Eppendorf tube, and lysed by adding 400 µl of cell extraction buffer [PBS containing 0.5% Triton X-100 and 1× protease inhibitor cocktail (Sigma, St. Louis, MO)] and allowing the tubes to warm to the temperature of wet ice. The mixture was vortexed and incubated on ice for 30 min with occasional vortexing. The cell lysates were clarified by centrifugation at 13,000 g at 4°C for 10 min. The clarified cell extracts were transferred to clean microcentrifuge tubes, which were stored at −80°C until ready for analysis. Protein concentration was determined using Bradford assay (Bio-Rad, Hercules, CA), protein concentration was equilibrated using cell extraction buffer, and cell extracts were diluted at least 1:10 in Standard Diluent Buffer before analysis by Luminex. mMCP-1 was analyzed using ELISA (eBioscinece, San Diego, CA). The concentration of mMCP-1 in the BALF was related to a standard curve made by pooling samples from four different lungs and diluting in steps creating a standard curve of arbitrary values. The sample concentrations are thus reported as arbitrary units (AU). BALF concentration of PGD2 was analyzed by ELISA (Cayman, Ann Arbor, MI).
2.12 Enzymatic digestion and mast cell enumeration
Lungs were harvested post AHR and lung cells in freshly prepared enzyme mix (Miltenyi Biotec, Auburn, CA) were mechanically digested using a GentleMACs dissociator (Miltenyi Biotec) using the factory provided onboard programs: four rounds of program m_lung_01 (8 seconds and 300 total rounds per run), followed by a 15m incubation at 37°, then two rounds of program “B”, a second 15m incubation at 37° (38 seconds and 2083 total rounds per run), and, finally, a single round of m_lung_02 (31 seconds and 553 total rounds per run). A short centrifugation was performed to collect cells, which were then re-suspended in PBS and filtered through a 70 micron filter. After washing the cells in PBS, red blood cells were removed with ACK lysing solution (8,024 mg/l NH4Cl, 1,001 mg/l KHCO3, 7.722 mg/l EDTA•Na2•2H2O). Cells were washed, counted with an Advia 120 (Siemens, Munich, Germany) and re-suspended in PBS in preparation for antibody staining and flow cytometric analysis.
2.12.1 Surface staining and flow cytometric analysis
2×106 lung cells were stained with UV live/dead discriminator (Invitrogen, Grand Island, NY) and then resuspended in FACS staining buffer (PBS + 2% FCS, 0.2% sodium azide). Fc receptors were blocked using TruStain FcX (Biolegend), followed by staining with anti-CD3-FITC (Biolegend, San Diego, CA), anti-CD45-Pacific Orange (Invitrogen, Grand Island, NY), anti-ckit-APC and anti-FcεRI-PE (eBioscience, San Diego, CA), anti-TCR beta, and CD1d tetramer\PBS57. Data were collected on an LSR II (BD Biosciences), and analysis was performed using FlowJo (Tree Star, Ashland, OR) software. Mast cells were defined as CD3−ckit+FcεRI+ cells [43]. Backgating demonstrated that these cells were of intermediate side scatter.
2.13 Data analysis
Origin 8.0 (Origin Lab, Northampton, MA) and Graph Pad Prism 7.01 (GraphPad Software, Inc, LaJolla, CA) were used for analysis, and data are expressed as means ± SEM. ANOVA followed by Tukey’s post-hoc test was used to determine statistical significance. Differences were considered to be significant at P < 0.05.
3. RESULTS
3.1 Direct stimulation of iNKT cells
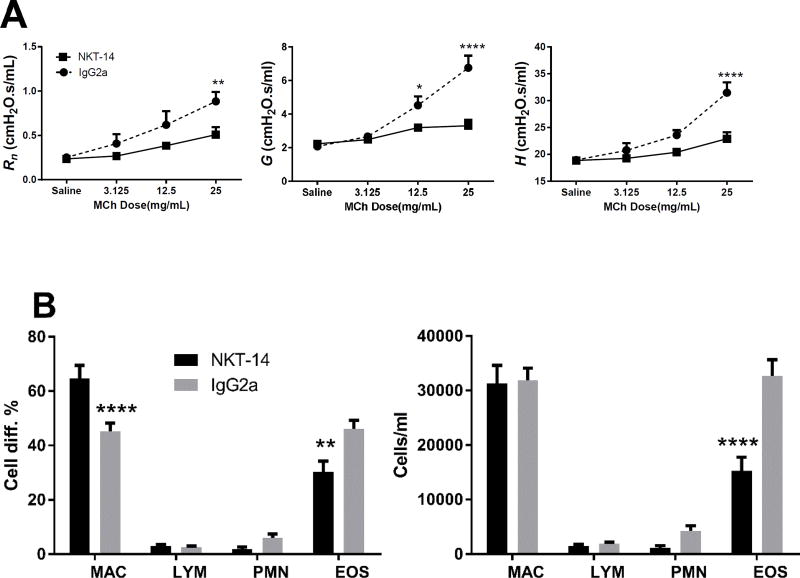
iNKT cells can be stimulated by the specific CD1d ligand αGalCer, thus we decided to first examine whether αGalCer and the iNKT targeting antibody, NKT-14, would have effect on the respiratory phenotype. BALB/c mice pretreated with the isotype control antibody IgG2a or NKT-14 were challenged o.p. with the iNKT cell stimulating glycosphingolipid αGalCer. In Fig 1A it is shown that αGalCer induced a robust increase of AHR that was reminiscent of the AHR produced by allergen sensitization, i.e. significant increases of Rn, G and H in the IgG2a control treated mice. In the NKT-14 treated mice, the lung mechanics parameters were significantly reduced and comparable to the levels seen in control mice (see Fig 3A). The BALF displayed an increase of eosinophils that was significantly reduced by NKT-14 (Fig 1B), although the levels of eosinophils after NKT-14 treatment appeared higher than in non-sensitized mice (see Fig 3B for comparison). Thus, eliminating the iNKT cells with NKT14 was sufficient to significantly diminish the AHR and inflammation resulting from the αGalCer challenge.
Figure 1. Effects of NKT-14 on iNKT dependent AHR.
Mice were treated with NKT-14 or control IgG2a and three days later challenged with a single dose of oropharyngeal inhalation of αGalCer (2 µg in 50µl PBS) and 24 hours later AHR was assessed with incrementing doses of inhaled methacholine. A) NKT-14 significantly inhibited the AHR all lung function parameters Rn, G and H induced by iNKT cell activation with the specific ligand αGalCer. B) Cell differentials in BALF demonstrated elevated numbers of eosinophils that were partially reversed by NKT-14. *p<0.05, **p<0.01, ****p<0.0001
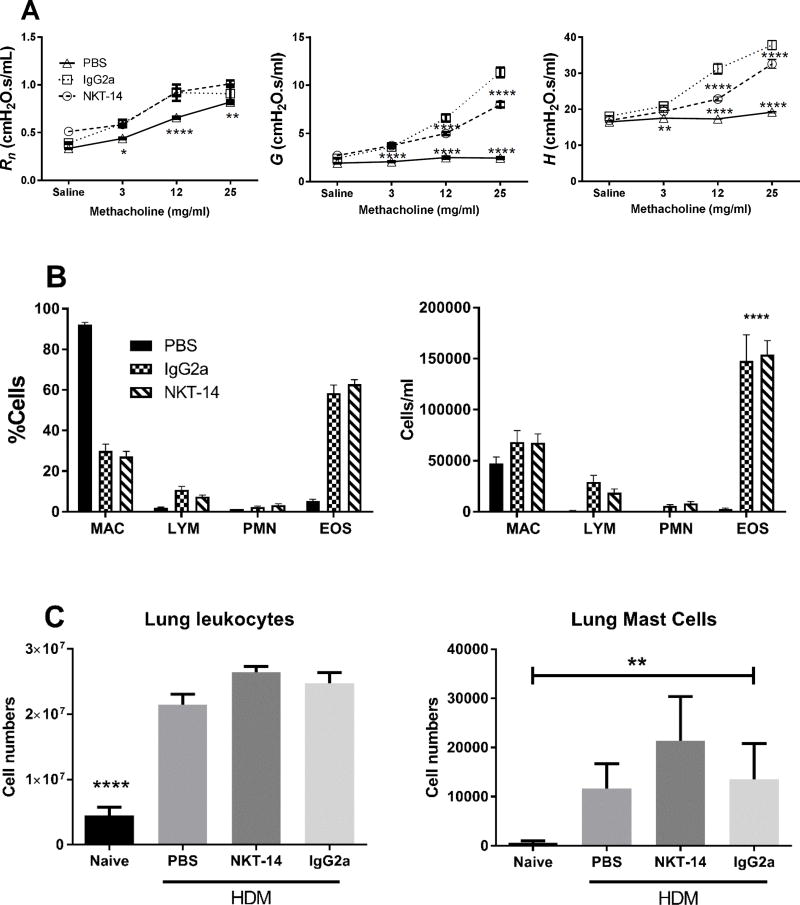
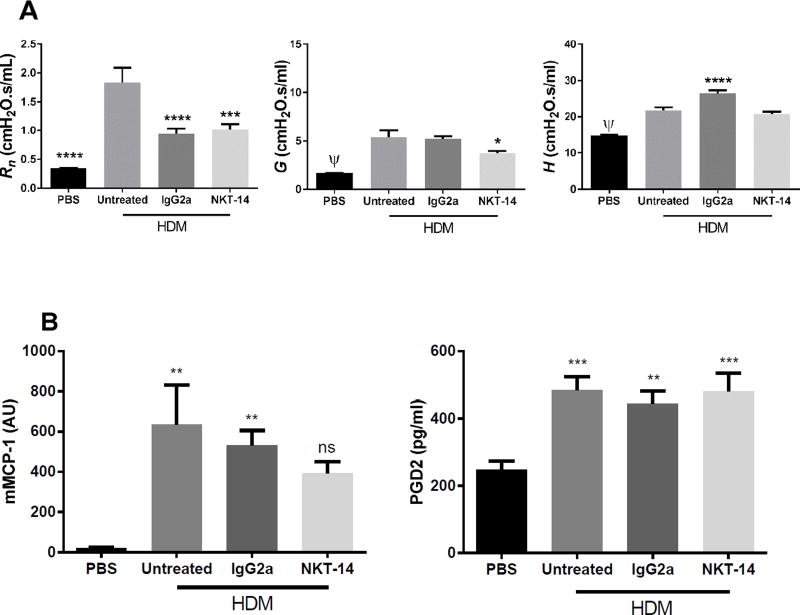
Figure 3. Effects of NKT-14 on HDM induced AHR and BALF cells.
Mice were treated i.p. with NKT-14, IgG2a (control Ab) or no treatment and then sensitized with i.n. HDM; one group received sham sensitization with i.n. PBS. 72 hours later, AHR was assessed with incrementing doses of inhaled methacholine. A) Both groups treated with IgG2a and NKT-14 had significantly increased responses compared with control PBS, however NKT-14 treated mice had significantly lower responses in G and H compared with IgG2a treated mice. B) After euthanasia, mice were lavaged with PBS. HDM sensitization significantly increased the number of eosinophils (EOS) but this was not affected by neither NKT-14 nor IgG2a treatment. *p<0.05, **p<0.01, ****p<0.0001. C: Following the AHR assessment lung tissue was harvested and prepared for flow cytometry. Total lung leukocytes were significantly increased by sensitization with HDM and neither NKT-14 nor IgG2a had any significant effects on lung tissue leukocyte numbers. Lung tissue mast cells were increased by sensitization with HDM but neither NKT-14 nor IgG2a had any effect on the mast cell numbers. A totally naïve group of mice, not exposed to methacholine, was also included to determine baseline lung leukocytes and mast cells. **p<0.01, ****p<0.0001.
3.2. iNKT cells depletion
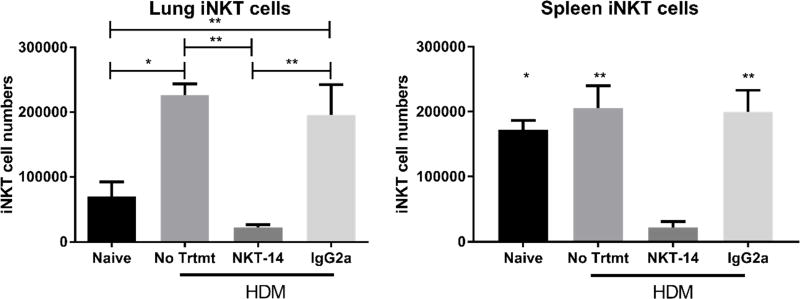
Following sensitization with HDM, we harvested lung tissue to quantify iNKT cells using flow cytometry (Fig 2). We found that HDM sensitization caused a significant increase in lung iNKT cells and that NKT-14 treatment practically eliminated the lung iNKT cell population while the isotype control antibody had only a partial effect. While NKT-14 significantly reduced the iNKT cell population, we discovered about 10% of tissue-associated iNKT cells remained in the lungs. As a control, we harvested spleens and found that spleen iNKT cells were eliminated to an extent similar to that observed in the lung; the control isotype IgG2a only partially reduced the frequency of iNKT cells in the spleen. These data indicate that the NKT-14 antibody eliminated about 90% of the lung and spleen iNKT cells from HDM-sensitized mice.
Figure 2. iNKT cells in lungs and spleen.
Mice were treated i.p. with NKT-14, IgG2a (control Ab) or no treatment and then sensitized with HDM. Lungs and spleens were harvested and processed for flow cytometry. NKT-14 significantly reduced the iNKT in both lung and spleen. *p<0.05, **p<0.01.
3.3. Airways hyperresponsiveness and inflammation
The assessment of AHR was performed using incremental doses of inhaled nebulized methacholine. In Fig 3A we show that the Rn of PBS control mice increased with methacholine, whereas G and H were virtually unaffected. All mice sensitized with HDM demonstrated a dose dependent response that was elevated compared with PBS controls, but mice pretreated with NKT-14 were significantly less sensitive to methacholine in terms of G and H; hence, NKT-14 reduced the HDM induced AHR.
After the mechanics measurement, BALF was obtained. In Fig 3B it is shown that cell totals were significantly increased in sensitized mice whether treated with NKT-14, isotype control, or not. Mice that had been sham sensitized or were naïve did not have increased numbers of leukocytes and differential analysis of BALF cells revealed significant increases of eosinophils that were not affected by NKT-14 treatment.
3.4. Mast cell population and lung cytokines
Mast cells are key players in generating the physiological lung phenotype as they are rapid responders to inhaled allergens. We hypothesized that the lung mast cell population would be dependent on iNKT cells; hence, we determined the number of mast cells using flow cytometry in mice sensitized with HDM following NKT-14 treatment (Fig 3C). We found, however, no evidence of reduced mast cell numbers in NKT-14 treated mice. The isotype control IgG2a was also without effect on the mast cell number. Analysis of the total numbers of lung tissue leukocyte showed that mice sensitized with HDM had increased numbers of lung tissue leukocytes but that NKT-14 was without effect on the cell numbers (Fig 3C). We confirmed our previous findings [36] that naïve mice have almost no lung mast cells.
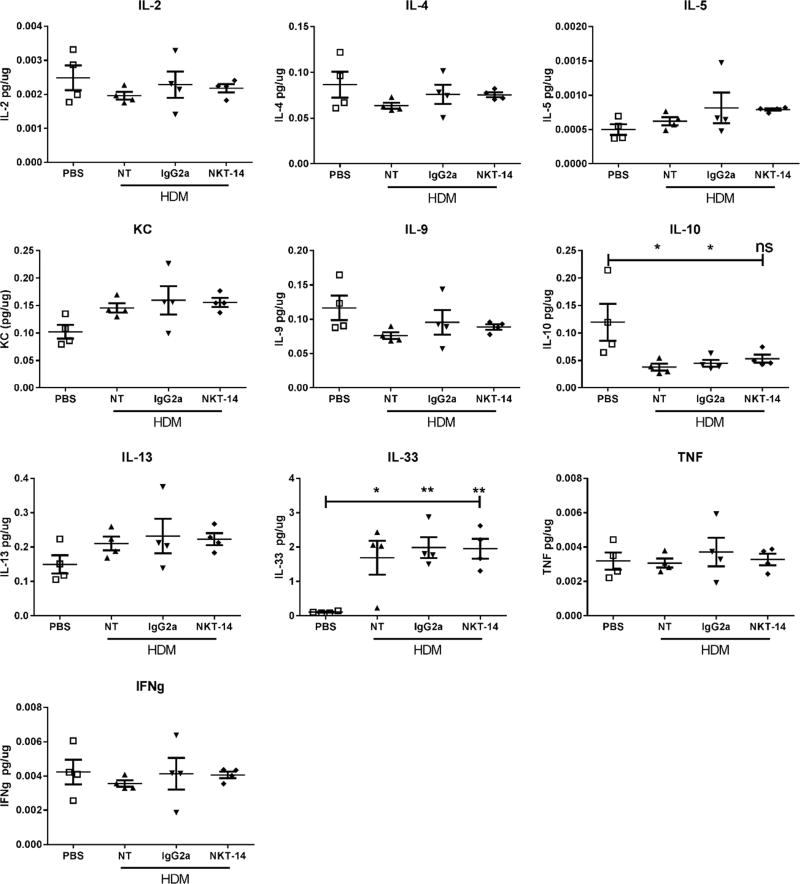
Following euthanasia, the upper right-hand lobe of the lung was collected, snap-frozen in liquid nitrogen, and later analyzed for selected cytokines. In Fig 4 it is shown that only IL-33 was significantly increased by HDM exposure whereas IL-10 was significantly decreased. However, neither NKT-14 nor the isotype control IgG2a antibody had any detectable effects on these or any other lung tissue cytokine.
Figure 4. Effects of NKT-14 on lung tissue cytokines.
Mice were treated i.p. with NKT-14, IgG2a or no treatment (NT) and then sensitized with i.n. HDM; one group received sham sensitization with i.n. PBS. 72 hours later mice were challenged with HDM, AIB measured and then lungs were harvested and cytokines measured. HDM sensitization and challenge significantly increased tissue IL-33 and also decreased IL-10. All other changes in cytokine levels were insignificant. *p<0.05, **p<0.01, non-significant (ns).
3.5. Allergen induced bronchoconstriction
Next, we determined the effects of NKT-14 on AIB during allergy. These studies were performed in HDM sensitized mice who received an inhalation of nebulized HDM while being ventilated and whose lung mechanics immediately measured. As presented in Fig 5A HDM sensitized mice responded with an increase in Rn that we interpret as a constriction of the conducting airways caused by mast cell mediator release, as previously demonstrated [36]. NKT-14 limited the Rn increase; however, we saw a similar effect with the isotype control antibody. The negative control mice (sham sensitized with PBS) did not respond to inhaled HDM. We also observed an increase in H, reflecting the stiffness of the lung. We found an increase in G in sensitized mice that was partially inhibited by NKT-14 but not by the isotype control antibody. The BALF from mice tested for AIB was also analyzed for mMCP-1 and PGD2 as indicators of mast cell mediator release. Both mMCP-1 and PGD2 were significantly increased by HDM inhalation but neither were significantly affected by iNKT cell elimination (Fig 5B).
Figure 5. Effects of NKT-14 on AIB and mast cell mediators.
(A) Mice were treated i.p. with NKT-14, IgG2a (control Ab) or no treatment and then sensitized with i.n. HDM; one group received sham sensitization with i.n. PBS. 72 hours later, mice were given inhaled nebulized HDM and the resulting AIB was measured. The HDM challenge induced a significant Rn increase in HDM sensitized mice over control PBS, interpreted as a bronchoconstriction, this was significantly reduced by NKT-14 and IgG2a. Likewise HDM induced significant increases in G and H. NKT-14 partly but significantly reduced the response in G, but did not affect H. (B) The levels of mMCP-1 and PGD2 in BALF were measured with Elisa. Mice sensitized with HDM expressed significantly elevated release of mMCP-1 in untreated and control treated (IgG2a) mice but not in NKT-14 treated animals. Elimination of the iNKT cells with NKT-14 was without significant effect. PGD2 levels were significantly increased in all groups and NKT-14 was without effect *p<0.05, ***p<0.001, ****p<0.0001, ψp<0.0001 (PBS vs. Untreated).
4. DISCUSSION
We have recently developed a mast cell dependent model of allergic asthma in mice that in addition to the commonly studied AHR, Th2 cytokine profile, and cellular airway inflammation, also displays allergen-inducible mast cell-dependent bronchoconstriction [36], emulating what might be observed in the airways of a patient with allergic asthma. In the present study, we show that NKT-14, an antibody specifically targeting iNKT cells, effectively eliminated iNKT cells from the lung. In our previous study we demonstrated that the HDM induced bronchoconstriction was indeed mast cells dependent because mast cell deficient mice did not demonstrate any AIB [36].
We have also demonstrated that the elimination of iNKT cells using NKT-14 was effective, and that it did not affect the total number of leukocytes in lung tissue, supporting the notion that NKT-14 is specific for iNKT cells [35, 44]. In this study, we show that targeting iNKT cells with NKT-14 also translated to an elimination of αGalCer induced AHR, further illustrating the specificity and impact of NKT-14. In this context we noticed that αGalCer induced an increase of eosinophils in BALF which was significantly reduced by NKT-14, further supporting the notion that iNKT cells could be responsible for recruitment of other inflammatory cells. NKT-14 did not return the eosinophil numbers back to baseline and we speculate that this could be due to incomplete elimination of iNKT cells. iNKT cells have previously been suggested to be important for AHR in the context of allergy [35], and our experiment using αGalCer illustrates that iNKT cells are capable of generating an AHR with strong similarities with allergically generated AHR in terms of the pattern of the parameters Rn, G and H. This observation of similarity should, however, not be interpreted to mean that iNKT cells are critical for generating AHR or asthma. We found that while eliminating iNKT cells reduced AHR in HDM allergic mice (G and H, but not Rn), AHR did not return to baseline (Fig 3). Hence, iNKT cells might play a partial role for AHR during allergy, or this observation could suggests that the small number of iNKT cells that appeared to remain after NKT-14 treatment (about 10%) were sufficient to induce AHR. In a similar manner, we tested allergic mice for AIB (Fig 5), and again, elimination of iNKT cells offered partial protection. In this model, the AIB response is mast cell driven and typically dominated by a response of the conducting airways, i.e. Rn, as we previously demonstrated [36]. The AIB is significant for being primarily a response in the conducting airways and as such demonstrated by an increase in Rn upon stimulation with an allergen. Our data demonstrate that this happens with HDM and is reduced by elimination of the iNKT cells. Depletion of 90% of iNKT cells using NKT14 did not influence mast cell recruitment to the lung, suggesting that iNKT cells exhibit little influence over mast cell recruitment. However, we cannot rule out the possibility that the small number of iNKT cells remaining after depletion was sufficient to mediate an effect on mast cell recruitment. Additional experiments would be needed to rule out this possibility.
The cytokine release in allergic models often displays a Th2 profile, i.e. characterized by a release of IL-4, IL-5 and IL-13, all of which have been connected with the development of AHR. In contrast with other studies using B6.Jα18−/− mice (deficient in type I NKT cells but not type II NKT cells) or CD1d−/− mice [14, 28], we did not detect any effects of iNKT depletion on lung cytokines. It should be noted, however, that there has been issues identified with the B6.Jα18−/− mice because of their impaired TCRα diversity, suggesting that results obtained with this particular strain of mice must be interpreted with a great deal of caution or even reevaluated as suggested by Bedel et al. [34]. Bedel et al. showed that the genetic modifications in the B6.Jα18−/− mice were not limited to the iNKT cells but had widespread consequences on the T cell population in general. In this context, it is important to note that NKT-14 is specific for iNKT cells but does not affect the type II NKT cells [44]. The possibility to use a specific agent like NKT-14 therapeutically to deplete a cell type in a model is significantly different from studying a model in which the cell type never existed due to a gene knockout. By using a specific antibody targeting iNKT cells we avoided the issues of life-long depletion and also avoided the non-specific effects that could arise from using a genetically modified mouse strain. In this context we also note that our model using high doses of HDM produced a very different phenotype characterized by the physiological response (AIB) and mast cell recruitment, but also the conspicuous lack of several cytokines compared with the traditional OVA model, as exemplified by Akbari et al. [14]. While we do not know what might be the mechanism behind these differences, one could speculate that iNKT cells could work via a mechanism that is independent of e.g. IL-5 and IL-13.
We did observe significant increases of IL-33 in the lung lysates from all sensitized groups. We analyzed the cytokines in homogenized lung tissue and we speculate that the IL-33 levels reflect both secreted as well as internally stored IL-33 that was released during tissue processing. Nevertheless, eliminating iNKT cells did not have any effect on the production of IL-33 illustrating that iNKT cells are likely not essential for the production of this cytokine in the lung, at least not as a consequence of allergic sensitization and challenge. In this study, we did not observe any significant changes in the traditional Th2 cytokines. It is, however, possible that the harvest at 72 hours post the last allergen challenge could be too late to recover any of these induced cytokines.
We analyzed mMCP-1 and PGD2 to determine if HDM inhalation acutely released mast cell products into the airways. While mMCP-1 and PGD2 were significantly elevated in sensitized mice, we conclude that the influence of iNKT cells was minimal. We have previously shown that HDM triggers the release of mMCP-1 and histamine in lungs with expanded mast cells and that this coincided with bronchoconstriction [36]. Hence this finding illustrates that our model behaved as expected with the additional observation that PGD2 release is similarly affected by HDM sensitization and challenge. What appears to be a contrast between the physiological effect (reduced AIB) and mast cell mediator release (mMCP-1 and PGD2) might be a reflection of the fact that NKT-14 did only partially, albeit significantly, reverse the HDM induced AIB. It is also possible that iNKT signaling to the mast cells only affects a limited set of mast cell derived mediators and that mMCP-1 and PGD2 are not among those affected.
We acknowledge the limitations with our study. The mice received a single injection of NKT-14 and while the flow cytometry analysis showed a significant reduction of iNKT cells, there was a remaining small population that could potentially be iNKT cells, suggesting that NKT-14 did not fully eliminate these cells. In our previous study [35], we administered a second dose at day 14 but this did not further diminish lung iNKT cell numbers. Consequently, we decided to commence with a single dose of NKT-14 in this study. Whatever the nature of these remaining 10% of cells, it is unlikely that such a small remnant population would be responsible for the lack of efficacy in regard to IL-33 and mast cell response observed in this study. In other studies of inflammatory models, we have found reduction of iNKT cells in target tissue as low as 70% has had therapeutic efficacy (unpublished observations).
In this study, we also found that the isotype IgG2a had some effects on the outcome variables. While we do not have any explanation for this, we feel that it would be beyond the scope of the present study to further elucidate the IgG2a effects. That being said, our observations raise a general question about how to best identify a proper control antibody in in vivo models. Interestingly, the effects of the IgG2a were limited to the respiratory mechanics but did not affect the lung lymphocyte population, iNKT cells or the cytokines.
We recently published that iNKT cell elimination with NKT-14, in a traditional model using ovalbumin (OVA) as the sensitizing allergen, can reduce AHR if NKT-14 was administered before allergen exposure. However, we also found that iNKT cells were not essential for AHR in mice with an established allergy. Briefly, when the mice were first sensitized and then later had iNKT cells eliminated, the AHR was largely unaffected [35]. Despite the effects on AHR in pretreated mice, we did not observe any effects on cellular inflammation nor did the treatment with NKT-14 affect lung cytokines. The effects of NKT-14 on the lung function parameters in the present study were not as pronounced as observed in the OVA-model, raising a couple of possibilities on the role of iNKT cells. One possibility is that allergy is not the only factor affecting the development of AHR. It has been shown that LPS can induce AHR in the context of allergic sensitization [45] and because LPS is present in HDM extract, this is certainly a possibility, although the LPS levels are relatively low. Another possibility is that the robustness of the HDM induced inflammation (long-term and high dose) overrides any effects that removal of iNKT cells might have had. HDM extract is a complex cocktail from the entire house dust mite and its fecal matter, which includes not only protein epitopes but also contains various enzymes, chitin and lipopolysaccharides, all with the potential to affect the airways; whereas OVA is a purified protein antigen that has been shown to produce a well-defined Th2 response [42]. Indeed, it has been shown that HDM can facilitate OVA sensitization, probably involving a GM-CSF mechanism [46–48]. Thus, it is possible that the effects of iNKT cell elimination on the physiological outcomes were varied due on the sensitization model used (e.g. HDM or OVA) and a possible effect of adjuvant cannot be excluded either. One could speculate that AHR caused by HDM extract and similar complex allergens depends on mechanisms partly unrelated to allergy and Th2, e.g. by affecting the epithelial lining leading to increased permeability, which we have shown can lead to increased AHR by simply altering the kinetics of the methacholine-smooth muscle interaction [49]. Indeed, the primary source for IL-33 in the lung is likely the epithelial cells [50] and HDM extract has been shown to induce an asthma-like phenotype via Toll-like receptor 4 [51]. If the same is true in human asthma, it could help in our understanding of why some asthma is refractory to steroid treatment, because steroids would likely have limited effects on epithelial integrity damage not caused by an immunological response.
In summary, we have demonstrated that NKT-14 effectively eliminated iNKT cells in the lung and that this significantly reduced AHR and AIB. However, we also conclude that iNKT cells do not influence mast cell recruitment to the lung. Consequently, the effects of iNKT cells on the AIB is likely via either direct effects on the smooth muscle or via modifying signaling to the mast cells.
Acknowledgments
This study was funded by grants from NIH/NIAID 1R21AI112804-01, NIH/NHLBI 2R01HL085646-05A1, 1R01HL107291-A1, University of Vermont IGP grant, NIH/NCRR P30 GM103532 and NKT Therapeutics Inc. who also donated NKT-14 and IgG2a.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gomez E, Corrado OJ, Baldwin DL, Swanston AR, Davies RJ. Direct in vivo evidence for mast cell degranulation during allergen-induced reactions in man. J Allergy Clin Immunol. 1986;78(4 Pt 1):637–45. doi: 10.1016/0091-6749(86)90082-5. [DOI] [PubMed] [Google Scholar]
- 2.Virk H, Arthur G, Bradding P. Mast cells and their activation in lung disease. Transl Res. 2016 doi: 10.1016/j.trsl.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Lundblad LKA, Thompson-Figueroa J, Allen GB, Rinaldi L, Norton RJ, Irvin CG, Bates JH. Airway hyperresponsiveness in allergically inflamed mice: the role of airway closure. Am J Respir Crit Care Med. 2007;175(8):768–74. doi: 10.1164/rccm.200610-1410OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wagers S, Lundblad LKA, Ekman M, Irvin CG, Bates JHT. The allergic mouse model of asthma: normal smooth muscle in an abnormal lung? J Appl Physiol. 2004;96(6):2019–2027. doi: 10.1152/japplphysiol.00924.2003. [DOI] [PubMed] [Google Scholar]
- 5.Fuchs B, Sjöberg L, Westerberg CM, Ekoff M, Swedin L, Dahlén S-E, Adner M, Nilsson GP. Mast cell engraftment of the peripheral lung enhances airway hyperresponsiveness in a mouse asthma model. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2012;303(12):L1027–L1036. doi: 10.1152/ajplung.00227.2012. [DOI] [PubMed] [Google Scholar]
- 6.Matangkasombut P, Pichavant M, Dekruyff RH, Umetsu DT. Natural killer T cells and the regulation of asthma. Mucosal immunology. 2009;2(5):383–92. doi: 10.1038/mi.2009.96. [DOI] [PubMed] [Google Scholar]
- 7.Van Kaer L, Parekh VV, Wu L. Invariant natural killer T cells: bridging innate and adaptive immunity. Cell and tissue research. 2011;343(1):43–55. doi: 10.1007/s00441-010-1023-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DeKruyff RH, Yu S, Kim HY, Umetsu DT. Innate immunity in the lung regulates the development of asthma. Immunological reviews. 2014;260(1):235–48. doi: 10.1111/imr.12187. [DOI] [PubMed] [Google Scholar]
- 9.Stott B, Lavender P, Lehmann S, Pennino D, Durham S, Schmidt-Weber CB. Human IL-31 is induced by IL-4 and promotes TH2-driven inflammation. J Allergy Clin Immunol. 2013;132(2):446–54. e5. doi: 10.1016/j.jaci.2013.03.050. [DOI] [PubMed] [Google Scholar]
- 10.Souwer Y, Szegedi K, Kapsenberg ML, de Jong EC. IL-17 and IL-22 in atopic allergic disease. Curr Opin Immunol. 2010;22(6):821–6. doi: 10.1016/j.coi.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 11.Guan P, Bassiri H, Patel NP, Nichols KE, Das R. Invariant natural killer T cells in hematopoietic stem cell transplantation: killer choice for natural suppression. Bone Marrow Transplant. 2016 doi: 10.1038/bmt.2015.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wingender G, Rogers P, Batzer G, Lee MS, Bai D, Pei B, Khurana A, Kronenberg M, Horner AA. Invariant NKT cells are required for airway inflammation induced by environmental antigens. The Journal of experimental medicine. 2011;208(6):1151–62. doi: 10.1084/jem.20102229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akbari O. The role of iNKT cells in development of bronchial asthma: a translational approach from animal models to human. Allergy. 2006;61(8):962–8. doi: 10.1111/j.1398-9995.2006.01124.x. [DOI] [PubMed] [Google Scholar]
- 14.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, Taniguchi M, Grusby MJ, DeKruyff RH, Umetsu DT. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nature medicine. 2003;9(5):582–8. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 15.Akbari O, Faul JL, Hoyte EG, Berry GJ, Wahlstrom J, Kronenberg M, DeKruyff RH, Umetsu DT. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. The New England journal of medicine. 2006;354(11):1117–29. doi: 10.1056/NEJMoa053614. [DOI] [PubMed] [Google Scholar]
- 16.Kearley J, Erjefalt JS, Andersson C, Benjamin E, Jones CP, Robichaud A, Pegorier S, Brewah Y, Burwell TJ, Bjermer L, Kiener PA, Kolbeck R, Lloyd CM, Coyle AJ, Humbles AA. IL-9 governs allergen-induced mast cell numbers in the lung and chronic remodeling of the airways. Am J Respir Crit Care Med. 2011;183(7):865–75. doi: 10.1164/rccm.200909-1462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kearley J, Buckland KF, Mathie SA, Lloyd CM. Resolution of allergic inflammation and airway hyperreactivity is dependent upon disruption of the T1/ST2-IL-33 pathway. Am J Respir Crit Care Med. 2009;179(9):772–81. doi: 10.1164/rccm.200805-666OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones TG, Hallgren J, Humbles A, Burwell T, Finkelman FD, Alcaide P, Austen KF, Gurish MF. Antigen-induced increases in pulmonary mast cell progenitor numbers depend on IL-9 and CD1d–restricted NKT cells. J Immunol. 2009;183(8):5251–60. doi: 10.4049/jimmunol.0901471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iikura M, Suto H, Kajiwara N, Oboki K, Ohno T, Okayama Y, Saito H, Galli SJ, Nakae S. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Laboratory investigation; a journal of technical methods and pathology. 2007;87(10):971–8. doi: 10.1038/labinvest.3700663. [DOI] [PubMed] [Google Scholar]
- 20.Kim HY, Chang Y-J, Subramanian S, Lee H-H, Albacker LA, Matangkasombut P, Savage PB, McKenzie ANJ, Smith DE, Rottman JB, DeKruyff RH, Umetsu DT. Innate lymphoid cells responding to IL-33 mediate airway hyperreactivity independently of adaptive immunity. Journal of Allergy and Clinical Immunology. 2012;129(1):216–227. e6. doi: 10.1016/j.jaci.2011.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Albacker LA, Chaudhary V, Chang YJ, Kim HY, Chuang YT, Pichavant M, Dekruyff RH, Savage PB, Umetsu DT. Invariant natural killer T cells recognize a fungal glycosphingolipid that can induce airway hyperreactivity. Nature medicine. 2013;19(10):1297–304. doi: 10.1038/nm.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bourgeois E, Van LP, Samson M, Diem S, Barra A, Roga S, Gombert JM, Schneider E, Dy M, Gourdy P, Girard JP, Herbelin A. The pro-Th2 cytokine IL-33 directly interacts with invariant NKT and NK cells to induce IFN-gamma production. European journal of immunology. 2009;39(4):1046–55. doi: 10.1002/eji.200838575. [DOI] [PubMed] [Google Scholar]
- 23.Sabatino G, Nicoletti M, Neri G, Saggini A, Rosati M, Conti F, Cianchetti E, Toniato E, Fulcheri M, Caraffa A, Antinolfi P, Frydas S, Pandolfi F, Potalivo G, Galzio R, Conti P, Theoharides TC. Impact of IL −9 and IL-33 in mast cells. Journal of biological regulators and homeostatic agents. 2012;26(4):577–86. [PubMed] [Google Scholar]
- 24.Monteiro M, Agua-Doce A, Almeida CF, Fonseca-Pereira D, Veiga-Fernandes H, Graca L. IL-9 Expression by Invariant NKT Cells Is Not Imprinted during Thymic Development. J Immunol. 2015;195(7):3463–71. doi: 10.4049/jimmunol.1403170. [DOI] [PubMed] [Google Scholar]
- 25.Hachem P, Lisbonne M, Michel ML, Diem S, Roongapinun S, Lefort J, Marchal G, Herbelin A, Askenase PW, Dy M, Leite-de-Moraes MC. Alpha-galactosylceramide-induced iNKT cells suppress experimental allergic asthma in sensitized mice: role of IFN-gamma. European journal of immunology. 2005;35(10):2793–802. doi: 10.1002/eji.200535268. [DOI] [PubMed] [Google Scholar]
- 26.Nambiar J, Clarke AW, Shim D, Mabon D, Tian C, Windloch K, Buhmann C, Corazon B, Lindgren M, Pollard M, Domagala T, Poulton L, Doyle AG. Potent neutralizing anti-CD1d antibody reduces lung cytokine release in primate asthma model. MAbs. 2015;7(3):638–50. doi: 10.1080/19420862.2015.1016693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nie H, Yang Q, Zhang G, Wang A, He Q, Liu M, Li P, Yang J, Huang Y, Ding X, Yu H, Hu S. Invariant NKT cells act as an adjuvant to enhance Th2 inflammatory response in an OVA-induced mouse model of asthma. PLoS ONE. 2015;10(4):e0119901. doi: 10.1371/journal.pone.0119901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer EH, Goya S, Akbari O, Berry GJ, Savage PB, Kronenberg M, Nakayama T, DeKruyff RH, Umetsu DT. Glycolipid activation of invariant T cell receptor+ NK T cells is sufficient to induce airway hyperreactivity independent of conventional CD4+ T cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(8):2782–7. doi: 10.1073/pnas.0510282103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reynolds C, Barkans J, Clark P, Kariyawasam H, Altmann D, Kay B, Boyton R. Natural killer T cells in bronchial biopsies from human allergen challenge model of allergic asthma. Journal of Allergy and Clinical Immunology. 2009;124(4):860–862. doi: 10.1016/j.jaci.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 30.Mutalithas K, Croudace J, Guillen C, Siddiqui S, Thickett D, Wardlaw A, Lammas D, Brightling C. Bronchoalveolar lavage invariant natural killer T cells are not increased in asthma. Journal of Allergy and Clinical Immunology. 2007;119(5):1274–1276. doi: 10.1016/j.jaci.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 31.Vijayanand P, Seumois G, Pickard C, Powell RM, Angco G, Sammut D, Gadola SD, Friedmann PS, Djukanović R. Invariant Natural Killer T Cells in Asthma and Chronic Obstructive Pulmonary Disease. New England Journal of Medicine. 2007;356(14):1410–1422. doi: 10.1056/NEJMoa064691. [DOI] [PubMed] [Google Scholar]
- 32.Thomas SY, Lilly CM, Luster AD. Invariant natural killer T cells in bronchial asthma. The New England journal of medicine. 2006;354(24):2613–6. doi: 10.1056/NEJMc066189. author reply 2613-6. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez Roldan N, Orinska Z, Ewers H, Bulfone-Paus S. CD252 regulates mast cell mediated, CD1d–restricted NKT-cell activation in mice. European journal of immunology. 2016;46(2):432–9. doi: 10.1002/eji.201545879. [DOI] [PubMed] [Google Scholar]
- 34.Bedel R, Matsuda JL, Brigl M, White J, Kappler J, Marrack P, Gapin L. Lower TCR repertoire diversity in Traj18-deficient mice. Nature immunology. 2012;13(8):705–6. doi: 10.1038/ni.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheuplein F, Lamont DJ, Poynter ME, Boyson JE, Serreze D, Lundblad LK, Mashal R, Schaub R. Mouse Invariant Monoclonal Antibody NKT14: A Novel Tool to Manipulate iNKT Cell Function In Vivo. PLoS ONE. 2015;10(10):e0140729. doi: 10.1371/journal.pone.0140729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li S, Aliyeva M, Daphtary N, Martin RA, Poynter ME, Kostin SF, van der Velden JL, Hyman AM, Stevenson CS, Phillips JE, Lundblad LK. Antigen-induced mast cell expansion and bronchoconstriction in a mouse model of asthma. Am J Physiol Lung Cell Mol Physiol. 2014;306(2):L196–206. doi: 10.1152/ajplung.00055.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lundblad LK, Irvin CG, Adler A, Bates JH. A reevaluation of the validity of unrestrained plethysmography in mice. J Appl Physiol (1985) 2002;93(4):1198–207. doi: 10.1152/japplphysiol.00080.2002. [DOI] [PubMed] [Google Scholar]
- 38.Lundblad LK, Rinaldi LM, Poynter ME, Riesenfeld EP, Wu M, Aimi S, Barone LM, Bates JH, Irvin CG. Detrimental effects of albuterol on airway responsiveness requires airway inflammation and is independent of beta-receptor affinity in murine models of asthma. Respiratory research. 2011;12:27. doi: 10.1186/1465-9921-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erik Riesenfeld GBA, Bates Jason HT, Poynter Matthew E, Wu Min, Aimi Steven, Lundblad Lennartk KA. The Temporal Evolution of Airways Hyperresponsiveness and Inflammation. J Aller Ther. 2012;(SI: 005) doi: 10.4172/2155-6121.S1-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schuessler T, Bates J. A computer-controlled research ventilator for small animals: design and evaluation. IEEE transactions on bio-medical engineering. 1995;42(9):860–866. doi: 10.1109/10.412653. [DOI] [PubMed] [Google Scholar]
- 41.Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol. 1992;72(1):168–78. doi: 10.1152/jappl.1992.72.1.168. [DOI] [PubMed] [Google Scholar]
- 42.Riesenfeld E, Allen GB, Bates JH, Poynter ME, Wu M, Aimiand S, Lundblad LK. The Temporal Evolution of Airways Hyperresponsiveness and Inflammation. Journal of allergy & therapy. 2012;1(5):1–7. doi: 10.4172/2155-6121.S1-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moore ML, Newcomb DC, Parekh VV, Van Kaer L, Collins RD, Zhou W, Goleniewska K, Chi MH, Mitchell D, Boyce JA, Durbin JE, Sturkie C, Peebles RS., Jr STAT1 negatively regulates lung basophil IL-4 expression induced by respiratory syncytial virus infection. J Immunol. 2009;183(3):2016–26. doi: 10.4049/jimmunol.0803167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scheuplein F, Thariath A, Macdonald S, Truneh A, Mashal R, Schaub R. A Humanized Monoclonal Antibody Specific for Invariant Natural Killer T (iNKT) Cells for In Vivo Depletion. PLoS ONE. 2013;8(9):e76692. doi: 10.1371/journal.pone.0076692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Starkhammar M, Larsson O, Kumlien Georen S, Leino M, Dahlen SE, Adner M, Cardell LO. Toll-like receptor ligands LPS and poly (I:C) exacerbate airway hyperresponsiveness in a model of airway allergy in mice, independently of inflammation. PLoS ONE. 2014;9(8):e104114. doi: 10.1371/journal.pone.0104114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Rijt LS, Logiantara A, Utsch L, Canbaz D, Boon L, van Ree R. House dust mite allergic airway inflammation facilitates neosensitization to inhaled allergen in mice. Allergy. 2012;67(11):1383–91. doi: 10.1111/all.12017. [DOI] [PubMed] [Google Scholar]
- 47.Fattouh R, Pouladi MA, Alvarez D, Johnson JR, Walker TD, Goncharova S, Inman MD, Jordana M. House dust mite facilitates ovalbumin-specific allergic sensitization and airway inflammation. Am J Respir Crit Care Med. 2005;172(3):314–21. doi: 10.1164/rccm.200502-198OC. [DOI] [PubMed] [Google Scholar]
- 48.Cates EC, Fattouh R, Wattie J, Inman MD, Goncharova S, Coyle AJ, Gutierrez-Ramos JC, Jordana M. Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. J Immunol. 2004;173(10):6384–92. doi: 10.4049/jimmunol.173.10.6384. [DOI] [PubMed] [Google Scholar]
- 49.Bates JH, Stevenson CA, Aliyeva M, Lundblad LK. Airway responsiveness depends on the diffusion rate of methacholine across the airway wall. J Appl Physiol (1985) 2012;112(10):1670–7. doi: 10.1152/japplphysiol.00703.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prefontaine D, Nadigel J, Chouiali F, Audusseau S, Semlali A, Chakir J, Martin JG, Hamid Q. Increased IL-33 expression by epithelial cells in bronchial asthma. J Allergy Clin Immunol. 2010;125(3):752–4. doi: 10.1016/j.jaci.2009.12.935. [DOI] [PubMed] [Google Scholar]
- 51.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nature medicine. 2009;15(4):410–6. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]