Abstract
Background
We aimed to investigate the association of serum cathepsin D levels with in-hospital mortality and Syntax scores (SXscore) in non-ST elevation myocardial infarction (NSTEMI) patients.
Methods
A total of 88 patients were included in the study. The patients were divided into two groups: those with in-hospital mortality (-), and those with in-hospital mortality (+). The receiver operating characteristics curve was used to show the sensitivity and specificity of serum cathepsin D levels, and the optimal cut-off value for predicting in-hospital mortality and high SXscore.
Results
Patients with (+) in-hospital mortality and high SXscore had lower serum cathepsin D levels compared to the patients with (-) in-hospital mortality and low SXscore. Using a cutoff score of < 16 for the cathepsin D level, in-hospital mortality was predicted with a sensitivity and specificity of 73.4% and 77.6%, respectively, and also predicted high SXscore with a sensitivity and specificity of 72.4% and 67.6%, respectively.
Conclusions
Serum cathepsin D levels established upon admission were significantly and independently lower in NSTEMI patients with high rate of mortality, high SXscores, and low left ventricular ejection fraction.
Keywords: Non-ST elevation myocardial infarction, Serum cathepsin D, Syntax score
INTRODUCTION
Cathepsin D is a soluble lysosomal aspartic endopeptidase.1 Generally, it is localized within lysosomes and plays a significant role in protein degradation and the processing of precursor proteins.2 Cathepsin D typically degrades the proteins contained within lysosomes in acidic pH.3 It has been suggested that lysosomal enzymes, including pre procathepsin D, are released from monocyte-derived macrophages in atherosclerotic lesions. These modify low density lipoprotein cholesterol (LDL-C) particles, macrophages, and smooth muscle cells to induce atherosclerosis.3 Furthermore, it has also been shown that cathepsin D plays a role in cardiomyocyte autophagy, which protects against the progression of post-infarction cardiac.4,5 Recent studies have explored serum cathepsin D activity in ST elevation myocardial infarction (STEMI), demonstrating that activityis significantly higher in patients with STEMI compared to age-matched controls.6
The syntax score (SXscore) assesses the angiographic severity of coronary arteries,7 and can predict morbidity and mortality irrespective of disease severity in different clinical conditions, including non-ST elevation myocardial infarction (NSTEMI).8-14
In this study, we investigated the relationship between serum cathepsin D levels and in-hospital mortality and SXscore in NSTEMI patients, and analyzed the correlation between cathepsin D levels and angiographic and clinical risk scores. This is the first study of its kind documented in the literature.
MATERIALS AND METHODS
A total of 88 patients who were admitted to our clinic with NSTEMI and underwent coronary angiography (CA) between March and November 2015 were included in this study. The diagnosis of NSTEMI was based on increased troponin levels and the presence of at least one of the following: ischemic symptoms: 1) ischemic electrocardiographic changes other than acute ST segment elevations; and 2) new wall motion abnormalities/new loss of viable myocardium as assessed with echocardiography.15 Patients with elevated troponin due to causes other than acute coronary events were excluded from the study, such as acute heart failure, pulmonary embolism, active infection or sepsis, chronic kidney disease, stroke, arrhythmias or aortic dissection. Patients with hematologic disorders, chronic inflammatory diseases, previous stroke, liver disease, malignancy, rheumatologic diseases, STEMI and myocardial infarction (MI) were also excluded.
Transthoracic echocardiography was performed within 72 hours of each patient’s hospital admission. Left ventricular ejection fraction (LVEF) was calculated using Simpson’s method, and low LVEF was defined as < 50%.
Patient blood samples were collected after a 12-hour fasting period. These samples were stored in plain tubes, and serum was separated after centrifugation at 1500 rpm for 10 minutes and stored at -80 °C until subsequent analysis. Blood samples from calcium EDTA tubes were analyzed using an auto-analyzer. Complete blood count and differentials were obtained from the peripheral venous samples taken upon admission.
Cathepsin D activity in EDTA probes was determined using the Sensolyte TM 520 Cathepsin D Assay Kit (AnaSpec, Inc., San Jose, CA, USA), as previously described,16 which was performed during coronary angiography. The enzymatic activity was presented in relative fluorescence units.
At the time of diagnosis, and before coronary angiography, all patients were given 300 mg acetyl salicylic acid po and either 180 mg ticagrelor, 300 mg clopidogrel po (patients < 75 years of age), 75 mg clopidogrel po (patients ≥ 75 years of age) or70 U/kg heparin iv.
The standard Judkins technique and 6 or 7F catheters (Massachusetts Expo; Boston Scientific Corporation, Natick, MA, USA) were used to perform a baseline angiography through the radial or femoral artery, using a Siemens Axiom Sensis XP device.
Two experienced and independent interventional cardiologists, who were unaware of the clinical data of the patients, calculated the SXscores. No discrepancies were identified by the interventional cardiologists who assessed the SXscores. Each lesion of ≥ 1.5 mm in diameter that had ≥ 50% stenosis was scored using version 2.1 of an online scoring system (www.syntaxscore.com). A SXscore ≥ 23 was regarded as severe coronary artery disease. Following this process, the patients were divided into two groups: those with low SXscores (< 23) and those with high SXscores (≥ 23).
SPSS 22.0 statistical software (SPSS Inc. Chicago, IL, USA) was used to analyze the data. The Kolmogorov-Smirnov test was employed to analyze the distribution pattern. Continuous data was presented as median and interquartile range (IQR), or mean ± standard deviation (SD). Spearman’s correlation coefficient was calculated to examine the association between two continuous variables. Multiple linear regression analysis was performed to investigate the independent predictors of LVEF. The effects of different variables on SXscore and in-hospital mortality were determined through univariate analysis. Variables with unadjusted p values (< 0.15 in logistic regression analysis) were identified as potential risk factors and included in the full model. Potential risk factors were eliminated through likelihood ratio tests with a reduced model, using a stepwise multivariate logistic regression analysis. A p value < 0.05 was considered statistically significant. The receiver operating characteristics (ROC) curve was adopted to demonstrate the sensitivity and specificity of serum cathepsin D levels, in addition to optimal cut-off value for predicting in-hospital mortality and high SXscore.
The Ankara Numune Education and Research Hospital’s local ethics committee approved the study protocol, and all patients provided informed written consent.
RESULTS
The study group comprised 88 patients, nine of whom (10.2%) had in-hospital mortality (+). Gender, age, smoking status, rates of diabetes mellitus and hypertension, levels of total cholesterol, low density lipoprotein, high density lipoprotein, creatinine, and B-type natriuretic peptide, as well as neutrophil and lymphocyte counts, were similar in (-) and (+) in-hospital mortality groups. The baseline clinical characteristics of the study patients and results of the univariate analysis (p values) are presented in Table 1.
Table 1. Clinical and laboratory characteristics of the study population according to in-hospital mortality.
| Variables | Non-STEMI | p value | ||
| Overall (n = 88) (100%) | In-hospital mortality (-) (n = 79) (89.8%) | In-hospital mortality (+) (n = 9) (10.2%) | ||
| Male n (%) | 64 (72.7%) | 58 (73.4%) | 6 (66.7%) | 0.253 |
| Age, mean ± SD | 61 ± 13 | 58 ± 13 | 62 ± 14 | 0.415 |
| Diabetes mellitus, n (%) | 18 (20.4%) | 16 (20.3%) | 2 (22.2%) | 0.830 |
| Hypertension, n (%) | 22 (25%) | 19 (24.1%) | 3 (33.3%) | 0.605 |
| Current Smoker, n (%) | 27 (30.6%) | 23 (29.1%) | 4 (44.4%) | 0.160 |
| White blood cell count, mean ± SD, (103/μL) | 12.6 ± 3.6 | 11.1 ± 3.7 | 14.5 ± 3.5 | 0.002 |
| Hemoglobin, median (IQR), (g/dL) | 14.1 ± 1.7 | 14.1 ± 1.6 | 14.0 ± 1.7 | 0.862 |
| Neutrophil count, mean ± SD, (103/μL) | 9.4 ± 3.6 | 9.3 ± 3.7 | 9.6 ± 3.5 | 0.155 |
| Lymphocyte count, median (IQR), (103/μL) | 2.1 ± 1.1 | 2.1 ± 1.1 | 2.1 ± 1.1 | 0.880 |
| Platelet count, mean ± SD, (103 cells/mm3) | 251 ± 82 | 243 ± 70 | 275 ± 92 | < 0.001 |
| Total cholesterol, mean ± SD, (mg/dL) | 189 ± 60 | 187 ± 57 | 193 ± 65 | 0.355 |
| Low density lipoprotein, mean ± SD, (mg/dL) | 126 ± 48 | 125 ± 46 | 129 ± 49 | 0.913 |
| High density lipoprotein, mean ± SD, (mg/dL) | 38 ± 9 | 38 ± 9 | 38 ± 9 | 0.895 |
| Peak troponin I, median (IQR), (mg/dL) | 12 (5-37) | 8 (4-32) | 27 (8-52) | < 0.001 |
| Creatinine, mean ± SD, (mg/dL) | 0.9 ± 0.2 | 0.9 ± 0.2 | 1.0 ± 0.2 | 0.560 |
| C-reactive protein, median (IQR), (mg/dL) | 8 (3-17) | 6 (2-13) | 14 (7-35) | 0.040 |
| B-type natriuretic peptide, median (IQR), (pg/mL) | 101 (47-250) | 88 (30-197) | 105 (52-255) | 0.235 |
| Left ventricular ejection fraction, mean ± SD, (%) | 48.9 ± 10.0 | 51.3 ± 8.7 | 44.4 ± 10.9 | < 0.001 |
| Cathepsin D, mean ± SD, (mg/L) | 19.0 ± 8.8 | 21.8 ± 9.1 | 12.1 ± 6.9 | < 0.001 |
| Syntax score, mean ± SD | 22.4 ± 6.7 | 19.2 ± 6.9 | 26.5 ± 5.9 | < 0.001 |
IQR, interquartile range; SD, standard deviation.
As outlined in Table 2, white blood cell, platelet count, C-reactive protein, peak troponin I level, LVEF, SXscore and serum cathepsin D level correlated with in-hospital mortality in univariate analyses. When the seven variables (white blood cell count, platelet count, C-reactive protein, peak troponin I level, LVEF, SXscore and serum cathepsin D level) were incorporated in the multivariate analysis, the independent predictors of in-hospital mortality were peak troponin I, LVEF, serum cathepsin D level and SXscore.
Table 2. Results of univariate and multivariate logistic regression analysis (predictors of in-hospital mortality).
| Variables | Univariate analysis | Multivariate analysis | ||
| p value | Adjusted OR (95% CI) | p value | Adjusted OR (95% CI) | |
| White blood cell count | 0.095 | 1.352 (0.910-1.792) | ||
| Platelet count | 0.293 | 1.150 (0.818-1.382) | ||
| C-reactive protein | 0.065 | 0.950 (0.715-1.085) | ||
| Peak troponin I | 0.005 | 1.103 (1.013-1.193) | 0.017 | 1.075 (1.065-1.085) |
| Left ventricular ejection fraction | < 0.001 | 0.605 (0.415-0.795) | 0.002 | 0.672 (0.430-0.815) |
| Syntax score | < 0.001 | 1.950 (1.360-2.540) | < 0.001 | 1.760 (1.345-2.175) |
| Cathepsin D | < 0.001 | 0.585 (0.380-0.790) | < 0.001 | 0.655 (0.475-0.835) |
CI, confidence interval; OR, odds ratio.
The univariate analysis (shown in Table 3) found that platelet count, C-reactive protein, peak troponin I level, LVEF and serum cathepsin D level were significantly related to a high SXscore in NSTEMI patients. The in-hospital mortality rate was higher in patients with higher SXscores. When the six variables (platelet count, C-reactive protein, peak troponin I level, LVEF, serum cathepsin D level, and in-hospital mortality) were incorporated into the multivariate analysis, the independent predictors of high SXscore were serum cathepsin D level, LVEF, C-reactive protein and in-hospital mortality.
Table 3. Multivariate logistic regression analysis showing independent predictors of high Syntax scores.
| Variables | Univariate analysis | Multivariate analysis | ||
| p value | Adjusted OR (95% CI) | p value | Adjusted OR (95% CI) | |
| Platelet | 0.757 | 1.095 (0.980-1.210) | ||
| Peak troponin I | 0.130 | 1.150 (0.818-1.382) | ||
| C-reactive protein | 0.008 | 1.053 (1.015-1.091) | 0.025 | 1.035 (1.015-1.050) |
| Left ventricular ejection fraction | 0.005 | 0.613 (0.410-0.816) | 0.022 | 0.660 (0.410-0.890) |
| Cathepsin D | < 0.001 | 0.605 (0.415-0.795) | < 0.001 | 0.672 (0.430-0.815) |
| In-hospital mortality | < 0.001 | 1.905 (1.200-2.610) | < 0.001 | 1.720 (1.305-2.135) |
CI, confidence interval; OR, odds ratio.
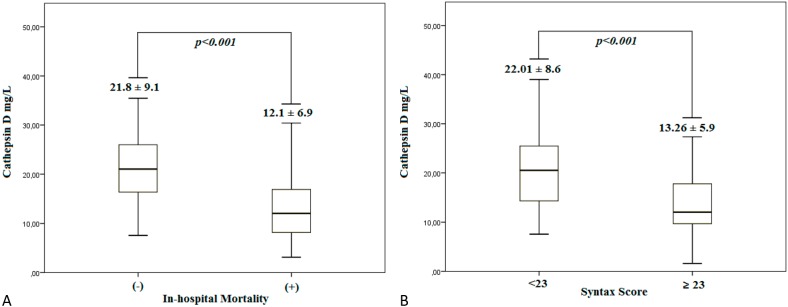
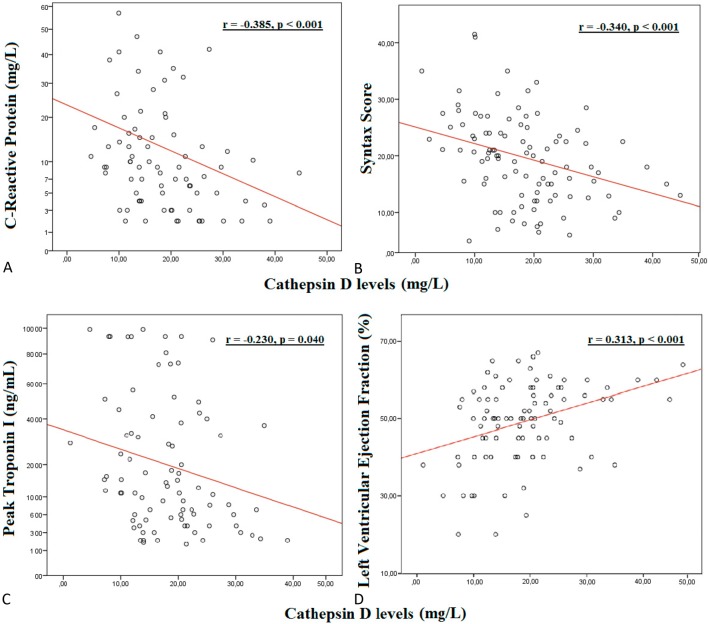
Patients with (+) in-hospital mortality and high SXscores had lower serum cathepsin D levels when compared to patients with (-) in-hospital mortality and low SXscores (Figure 1A-B). As shown in Figure 2, there were negative correlations between serum cathepsin D levels and C-reactive protein (2A), and SXscore (2B) and peak troponin-I (2C) levels in patients with NSTEMI (r = -0.385, p < 0.001; r = -0.340, p < 0.001; r = -0.230, p = 0.040, respectively). Figure 2(D) demonstrates the positive correlation between serum cathepsin D levels and LVEF (r = 0.313, p < 0.001). Multiple linear regression analysis (Table 4) found that high cathepsin D is an independent predictor of high LVEF (OR: 1.675 95% CI: 1.533-1.812).
Figure 1.
(A, B) Comparison of serum cathepsin D level in (-) and (+) in-hospital mortality and -low and -high Syntax score groups, respectively.
Figure 2.
Correlation of serum cathepsin D levels and CRP values (A), Syntax scores (B), peak troponin I levels (C) and LVEF (D).
Table 4. Results of univariate and multivariate linear regression analysis for predicting LVEF.
| Variables | Univariate analysis | Multivariate analysis | ||
| p value | Adjusted OR (95% CI) | p value | Adjusted OR (95% CI) | |
| White blood cell count | 0.233 | 0.930 (0.810-1.050) | ||
| Platelet count | 0.105 | 0.965 (0.910-1.010) | ||
| Syntax score | < 0.001 | 0.452 (0.210-0.792) | < 0.001 | 0.676 (0.360-0.912) |
| Cathepsin D | < 0.001 | 1.705 (1.421-1.989) | < 0.001 | 1.675 (1.533-1.812) |
CI, confidence interval; OR, odds ratio.
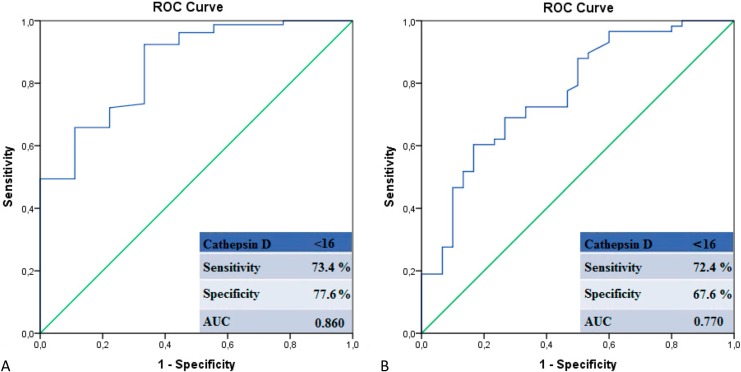
ROC analysis was also performed to determine the cut-off value of serum cathepsin D to predict in-hospital mortality and a high SXscore. Using a cut-off score of < 16 for cathepsin D level, in-hospital mortality was predicted with a sensitivity of 73.4%, and specificity of 77.6%. Also predicted was a high SXscore with a sensitivity of 72.4% and specificity of 67.6% (Figure 3A-B).
Figure 3.
(A, B) Receiver-operating characteristic (ROC) analysis of serum cathepsin D levels in patients with in-hospital mortality and high Syntax scores.
Finally, the patients were divided into two groups based on a serum cathepsin D cut-off value of 16. White blood cell, neutrophil, platelet, C-reactive protein, peak troponin I, SXscores and in-hospital mortality were higher; however, LVEF was lower in the low serum cathepsin D group (Table 5).
Table 5. Clinical and laboratory characteristics of the study population according to serum Cathepsin D levels.
| Variables | Cathepsin D < 16 (n = 36) (40.9%) | Cathepsin ≥ 16 (n = 52) (59.1%) | p value |
| White blood cell count, mean ± SD, (μL) | 14.9 ± 5.6 | 12.1 ± 4.7 | < 0.001 |
| Hemoglobin, mean ± SD, (g/dL) | 14.1 ± 1.5 | 14.1 ± 1.6 | 0.875 |
| Neutrophil count, mean ± SD, (103 μL) | 9.6 ± 3.5 | 7.1 ± 2.6 | 0.001 |
| Lymphocyte count, mean ± SD, (103 μL) | 2.0 ± 1.1 | 2.1 ± 1.2 | 0.458 |
| Platelet count, mean ± SD, (103 cells/mm3) | 255 ± 95 | 217 ± 76 | < 0.001 |
| Total cholesterol, mean ± SD, (mg/dL) | 185 ± 60 | 180 ± 57 | 0.54 |
| Low density lipoprotein, mean ± SD, (mg/dL) | 128 ± 50 | 125 ± 44 | 0.355 |
| High density lipoprotein, mean ± SD, (mg/dL) | 36 ± 10 | 41 ± 9 | 0.067 |
| Peak troponin I, median (IQR), (mg/dL) | 23 (9-44) | 9 (4-31) | < 0.001 |
| Creatinine, mean ± SD, (mg/dL) | 0.9 ± 0.2 | 1 ± 0.1 | 0.677 |
| C-reactive protein, median (IQR), (mg/dL) | 17 (5-37)0 | 7 (1-10)0 | < 0.001 |
| B-type natriuretic peptide, median (IQR), (pg/mL) | 123 (25-308) | 78 (34-175) | 0.165 |
| Left ventricular ejection fraction, mean ± SD, (%) | 43.5 ± 13.3 | 51.3 ± 9.1 | 0.001 |
| In hospital mortality, n (%) | 8 (22.2%) | 1 (0.2%) | < 0.001 |
| Syntax score, mean ± SD | 28 ± 9 | 17 ± 10 | < 0.001 |
Abbreviations are in Table 1.
DISCUSSION
To the best of our knowledge, the present study is the first to investigate the role of serum cathepsin D levels in NSTEMI patients. Our results indicated that serum cathepsin D levels significantly and independently correlated with angiographic, clinical risk scores and in-hospital mortality.
The SXscore is an anatomic scoring system based on coronary angiography that evaluates lesion severity. It also predicts poor cardiovascular outcomes, including morbidity and mortality in NSTEMI patients.17,18 Palmerini et al.17reported that SXscore was an independent predictor of one-year death rate, MI, cardiac death and target vessel revascularization in patients with NSTEMI. Scherff et al.18found that the SXscore anticipated short-term adverse clinical events in elderly patients with MI and those who underwent primary percutaneous coronary intervention (PCI). A separate study found that high SXscore is an independent factor for stent thrombosis in patients with STEMI, and a predictor of late mortality. In this study, Magro and colleagues demonstrated a relationship between SXscore and the development of no reflow in patients treated with primary PCI for STEMI.19
Yadav et al. demonstrated a strong correlation between the severity and complexity of coronary artery disease as assessed by the SXscore, and the occurrence of stent thrombosis at 30-day and one-year follow-up in patients with NSTEMI who underwent PCI.20 This research confirms that SXscore predicts in-hospital mortality rate in patients with NSTEMI. A SXscore ≥ 23 is associated with a higher in-hospital mortality rate. Our findings demonstrate that serum cathepsin D level meaningfully correlates with SXscore, peak troponin levels and C-reactive protein. These findings suggest that serum cathepsin D levels can be beneficial for clinical and angiographic risk assessment in NSTEMI patients.
Cathepsin D is a soluble lysosomal aspartic endopeptidase that synthesizes in the endoplasmic reticulum as pre procathepsin D. It is acknowledged to be linked to non-specific protein degradation in the acidic medium of lysosomes. The role of cathepsin D in the cellular process of atherosclerosis has been proposed3 and recommended as a potential target for therapeutic treatment following the suggestion that it can play a significant role in plaque instability.21 However, a negative correlation has been found between the levels of serum cathepsin D and C-reactive protein, which is an inflammatory marker. A negative correlation was also identified between serum cathepsin D level and SXscore, which indicates that atherosclerosis-induced inflammation or severity of atherosclerosis were not the basis for a correlation between serum cathepsin D levels and SXscore.
In the past decade, a number of studies have demonstrated the regulatory role of cathepsin D in apoptosis.1 From a cardiological point of view, experimental data indicates that ischemic post-conditioning induces the formation of autophagic vacuoles and autophagy-related protein levels (including cathepsin D) in the risk zone of the post-conditioned hearts, which contributes to cardio-protective effects against ischemia/reperfusion injury.4 Kanamori et al. demonstrated how autophagy is activated in surviving cardiomyocytes, as shown by the upregulated expression proteins, including cathepsin D and electron microscopic findings. It has also been suggested that cardiomyocyte autophagy is an innate mechanism that protects against the progression of post-infarction cardiac remodeling.5 Yamac et al. conducted a recent study that explored cathepsin D levels in patients with STEMI.6 They found significantly higher serum cathepsin D activity in patients with STEMI, both after percutaneous coronary intervention and during follow-up, compared to the age-matched controls. Interestingly, in the follow-up process, cathepsin D levels were elevated in patients with new-onset heart failure. Levels initially reduced cardiac function when compared to healthy controls or among patients with preserved and enhanced LVEF after MI.6 In this study, we found a negative correlation between serum cathepsin D and C-reactive protein levels, and SXscore. In addition, we found higher LVEF in patients with higher cathepsin D levels. In light of the results of our study, it may be suggested that lower cathepsin D levels after NSTEMI correlate with a higher SXscore and a poor prognosis, which may reflect the diminished protective role of cathepsin D as it induces less autophagy in those patients.
Our study had several limitations. First, we did not have a control group. Second, coronary angiography was assessed visually, and only major coronary artery lesions can be detected in this way. Third, cathepsin D levels were not compared with other clinical risk scores, such as GRACE and TIMI. Another limitation of this study was the small sample size, and the fact that all participants were admitted to a single centre. Finally, serum cathepsin D levels were not measured during the follow-up period.
CONCLUSIONS
Admission serum cathepsin D levels were significantly and independently lower in NSTEMI patients with high SXscores, high mortality rate, and low LVEF. Therefore, we believe that measuring serum cathepsin D in NSTEMI patients upon their admission to hospital could be beneficial for angiographic and clinic risk assessment, as well as choice of intervention timing. Further studies are required to clarify and identify the role of cathepsin D in patients with NSTEMI.
Acknowledgments
None.
FUNDING
This research was funded by Ankara Numune Education and Research Hospital.
REFERENCES
- 1.Benes P, Vetvicka V, Fusek M. Cathepsin D—many functions of one aspartic protease. Crit Rev Oncol Hematol. 2008;68:12–28. doi: 10.1016/j.critrevonc.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hah YS, Noh HS, Ha JH, et al. Cathepsin D inhibits oxidative stress induced cell death via activation of autophagy in cancer cells. Cancer Lett. 2012;323:208–214. doi: 10.1016/j.canlet.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Ozkayar N, Piskinpasa S, Akyel F, et al. Relation between serum cathepsin D levels and endothelial dysfunction in patients with chronic kidney disease. Nefrologia. 2015;35:72–79. doi: 10.3265/Nefrologia.pre2014.Oct.12609. [DOI] [PubMed] [Google Scholar]
- 4.Wei C, Li H, Han L, et al. Activation of autophagy in ischemic postconditioning contributes to cardioprotective effects against ischemia/reperfusion injury in rat hearts. J Cardiovasc Pharmacol. 2013;61:416–422. doi: 10.1097/FJC.0b013e318287d501. [DOI] [PubMed] [Google Scholar]
- 5.Kanamori H, Takemura G, Goto K, et al. The role of autophagy emerging in postinfarction cardiac remodelling. Cardiovasc Res. 2011;91:330–339. doi: 10.1093/cvr/cvr073. [DOI] [PubMed] [Google Scholar]
- 6.Yamac AH, Sevgili E, Kucukbuzcu S, et al. Role of cathepsin D activation in major adverse cardiovascular events and new-onset heart failure after STEMI. Herz. 2015:1–8. doi: 10.1007/s00059-015-4311-6. [DOI] [PubMed] [Google Scholar]
- 7.Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1:219–227. [PubMed] [Google Scholar]
- 8.Valgimigli M, Serruys PW, Tsuchida K, et al. Cyphering the complexity of coronary artery disease using the syntax score to predict clinical outcome in patients with three-vessel lumen obstruction undergoing percutaneous coronary intervention. Am J Cardiol. 2007;99:1072–1081. doi: 10.1016/j.amjcard.2006.11.062. [DOI] [PubMed] [Google Scholar]
- 9.Capodanno D, Di Salvo ME, Cincotta G, et al. Usefulness of the SYNTAX score for predicting clinical outcome after percutaneous coronary intervention of unprotected left main coronary artery disease. Circ Cardiovasc Interv. 2009;2:302–308. doi: 10.1161/CIRCINTERVENTIONS.108.847137. [DOI] [PubMed] [Google Scholar]
- 10.Garg S, Serruys PW. Coronary stents: current status. J Am Coll Cardiol. 2010;56:1–42. doi: 10.1016/j.jacc.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Caixeta A, Généreux P, Palmerini T, et al. Prognostic utility of the SYNTAX score in patients with single versus multivessel disease undergoing percutaneous coronary intervention (from the acute catheterization and urgent intervention triage strategy [ACUITY] trial). Am J Cardiol. 2014;113:203–210. doi: 10.1016/j.amjcard.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 12.Kundi H, Erel Ö, Balun A, et al. Association of thiol/disulfide ratio with syntax score in patients with NSTEMI. Scand Cardiovasc J. 2015;49:95–100. doi: 10.3109/14017431.2015.1013153. [DOI] [PubMed] [Google Scholar]
- 13.Garg S, Sarno G, Serruys PW, et al. Prediction of 1-year clinical outcomes using the SYNTAX score in patients with acute ST-segment elevation myocardial l infarction undergoing primary percutaneous coronary intervention: a substudy of the STRATEGY (Single High-Dose Bolus Tirofiban and Sirolimus-Eluting Stent Versus Abciximab and Bare-Metal Stentin Acute Myocardial Infarction) and MULTISTRATEGY (Multicenter Evaluation of Single High-Dose Bolus Tirofiban Versus Abciximab With Sirolimus-Eluting Stent or Bare-Metal Stent in Acute Myocardial Infarction Study) trials. JACC Cardiovasc Interv. 2011;4:66–75. doi: 10.1016/j.jcin.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 14.Kundi H, Kiziltunc E, Cetin M, et al. Association of monocyte/HDL-C ratio with SYNTAX scores in patients with stable coronary artery disease. Herz. 2016:1–7. doi: 10.1007/s00059-015-4393-1. [DOI] [PubMed] [Google Scholar]
- 15.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–1598. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Hilfiker-Kleiner D, Kaminski K, Podewski E, et al. A cathepsin D cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell. 2007;128:589–600. doi: 10.1016/j.cell.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 17.Palmerini T, Genereux P, Caixeta A, et al. Prognostic value of the SYNTAX score in patients with acute coronary syndromes undergoing percutaneous coronary intervention: analysis from the ACUITY (Acute Catheterization and Urgent Intervention Triage StrategY) trial. J Am Coll Cardiol. 2011;57:2389–2397. doi: 10.1016/j.jacc.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 18.Scherff F VG, Sürder D, Mantovani A, et al. The SYNTAX score predicts early mortality risk in the elderly with acute coronary syndrome having primary PCI. J Invasive Cardiol. 2011;23:505–510. [PubMed] [Google Scholar]
- 19.Magro M, Nauta ST, Simsek C, et al. Usefulness of the SYNTAX score to predict "no reflow" in patients treated with primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Am J Cardiol. 2012;109:601–606. doi: 10.1016/j.amjcard.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Yadav M, Généreux P, Palmerini T, et al. SYNTAX score and the risk of stent thrombosis after percutaneous coronary intervention in patients with non-ST-segment elevation acute coronary syndromes: an ACUITY trial substudy. Catheter Cardiovasc Interv. 2015;85:1–10. doi: 10.1002/ccd.25396. [DOI] [PubMed] [Google Scholar]
- 21.Durán MC, Martín-Ventura JL, Mohammed S, et al. Atorvastatin modulates the profile of proteins released by human atherosclerotic plaques. Eur J Pharmacol. 2007;562:119–129. doi: 10.1016/j.ejphar.2007.01.077. [DOI] [PubMed] [Google Scholar]