Abstract
Prolyl 4-hydroxylase (EC 1.14.11.2), an alpha 2 beta 2 tetramer, catalyses the formation of 4-hydroxyproline in collagens by the hydroxylation of proline residues in peptide linkages. We report here the isolation of cDNA clones coding for the beta-subunit of prolyl 4-hydroxylase from a human hepatoma lambda gt11 library and a corresponding human placenta library. Five overlapping clones covering all the coding sequences and almost all the non-coding sequences were characterized. The size of the mRNA hybridizing with these clones in Northern blotting is approximately 2.5 kb. The clones encode a polypeptide of 508 amino acid residues, including a signal peptide of 17 amino acids. These human sequences were found to be very similar to those recently reported for rat protein disulphide isomerase (EC 5.3.4.1). The degree of homology between these two proteins was 84% at the level of nucleotide sequences or 94% at the level of amino acid sequences. Southern blot analyses of human genomic DNA with a cDNA probe for the beta-subunit indicated the presence of only one gene containing these sequences. The product of a single gene thus appears to possess two different enzymatic functions depending on whether it is present in cells in monomer form or in the prolyl 4-hydroxylase tetramer.
Full text
PDF
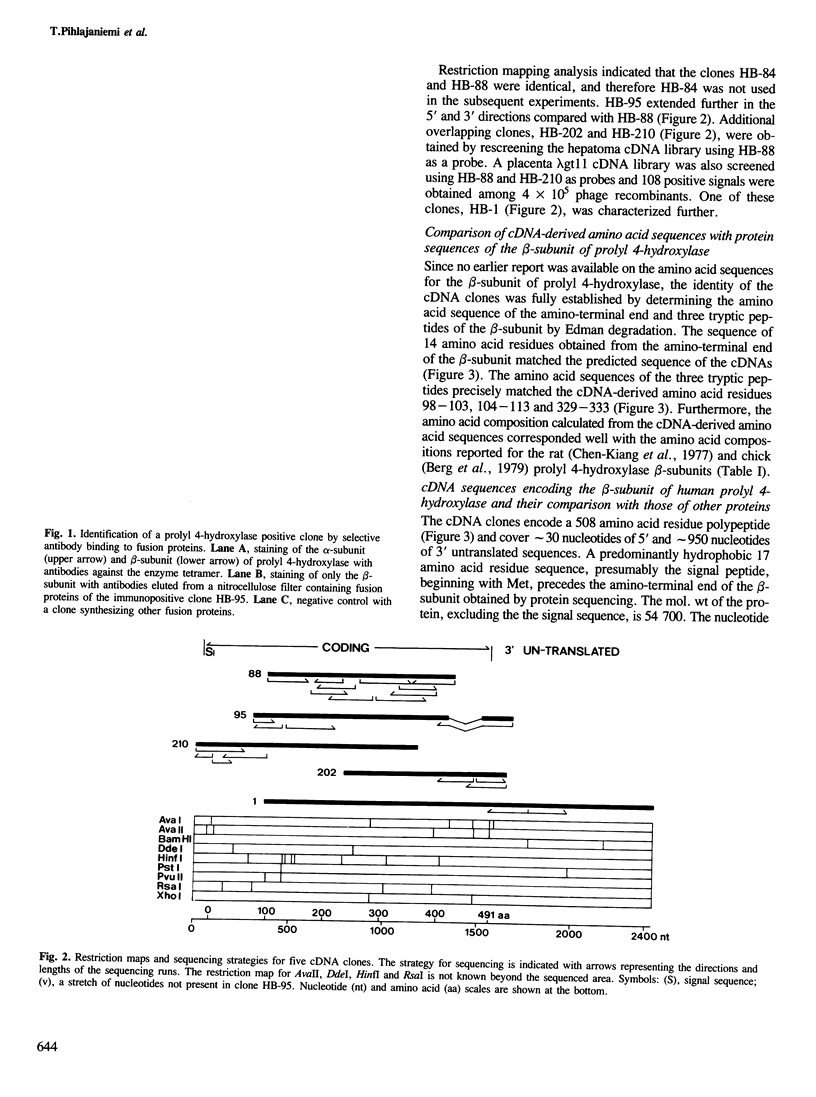

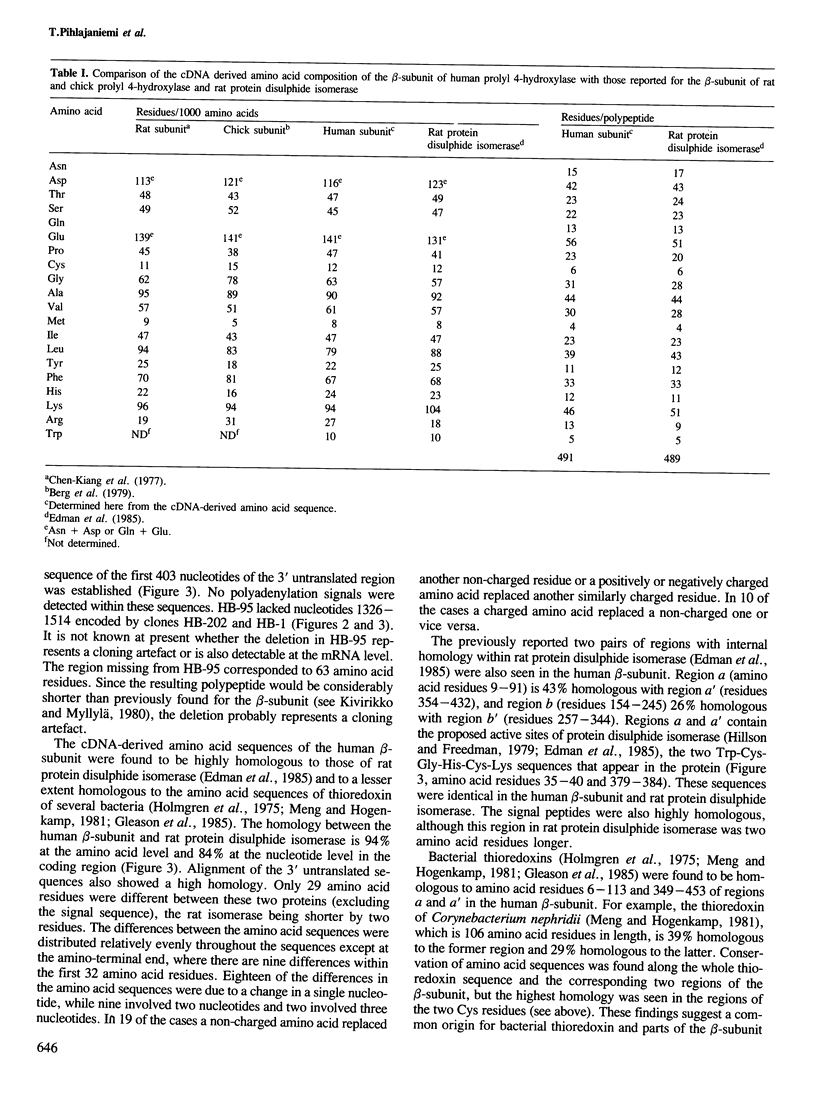



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Teplow D. B., Hood L. E., Kent S. B. Electroblotting onto activated glass. High efficiency preparation of proteins from analytical sodium dodecyl sulfate-polyacrylamide gels for direct sequence analysis. J Biol Chem. 1986 Mar 25;261(9):4229–4238. [PubMed] [Google Scholar]
- Anfinsen C. B., Scheraga H. A. Experimental and theoretical aspects of protein folding. Adv Protein Chem. 1975;29:205–300. doi: 10.1016/s0065-3233(08)60413-1. [DOI] [PubMed] [Google Scholar]
- Berg R. A., Kao W. W., Kedersha N. L. The assembly of tetrameric prolyl hydroxylase in tendon fibroblasts from newly synthesized alpha-subunits and from preformed cross-reacting protein. Biochem J. 1980 Sep 1;189(3):491–499. doi: 10.1042/bj1890491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg R. A., Kedersha N. L., Guzman N. A. Purification and partial characterization of the two nonidentical subunits of prolyl hydroxylase. J Biol Chem. 1979 Apr 25;254(8):3111–3118. [PubMed] [Google Scholar]
- Cardinale G. J., Udenfriend S. Prolyl hydroxylase. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):245–300. doi: 10.1002/9780470122860.ch6. [DOI] [PubMed] [Google Scholar]
- Chen-Kiang S., Cardinale G. J., Udenfriend S. Homology between a prolyl hydroxylase subunit and a tissue protein that crossreacts immunologically with the enzyme. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4420–4424. doi: 10.1073/pnas.74.10.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edman J. C., Ellis L., Blacher R. W., Roth R. A., Rutter W. J. Sequence of protein disulphide isomerase and implications of its relationship to thioredoxin. Nature. 1985 Sep 19;317(6034):267–270. doi: 10.1038/317267a0. [DOI] [PubMed] [Google Scholar]
- Gleason F. K., Whittaker M. M., Holmgren A., Jörnvall H. The primary structure of thioredoxin from the filamentous cyanobacterium Anabaena sp. 7119. J Biol Chem. 1985 Aug 15;260(17):9567–9573. [PubMed] [Google Scholar]
- Goudswaard J., van der Donk J. A., Noordzij A., van Dam R. H., Vaerman J. P. Protein A reactivity of various mammalian immunoglobulins. Scand J Immunol. 1978;8(1):21–28. doi: 10.1111/j.1365-3083.1978.tb00492.x. [DOI] [PubMed] [Google Scholar]
- Günzler V., Hanauske-Abel H. M., Myllylä R., Mohr J., Kivirikko K. I. Time-dependent inactivation of chick-embryo prolyl 4-hydroxylase by coumalic acid. Evidence for a syncatalytic mechanism. Biochem J. 1987 Feb 15;242(1):163–169. doi: 10.1042/bj2420163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hillson D. A., Freedman R. B. Resolution of ox liver thiol-disulphide oxidoreductases by a new application of covalent chromatography. Biochem Soc Trans. 1979 Jun;7(3):573–574. doi: 10.1042/bst0070573. [DOI] [PubMed] [Google Scholar]
- Hillson D. A., Lambert N., Freedman R. B. Formation and isomerization of disulfide bonds in proteins: protein disulfide-isomerase. Methods Enzymol. 1984;107:281–294. doi: 10.1016/0076-6879(84)07018-x. [DOI] [PubMed] [Google Scholar]
- Holmgren A., Söderberg B. O., Eklund H., Brändén C. I. Three-dimensional structure of Escherichia coli thioredoxin-S2 to 2.8 A resolution. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2305–2309. doi: 10.1073/pnas.72.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Hewick R. M., Dreyer W. J., Hood L. E. High-sensitivity sequencing with a gas-phase sequenator. Methods Enzymol. 1983;91:399–413. doi: 10.1016/s0076-6879(83)91038-8. [DOI] [PubMed] [Google Scholar]
- Höyhtyä M., Myllylä R., Piuva J., Kivirikko K. I., Tryggvason K. Monoclonal antibodies to human prolyl 4-hydroxylase. Eur J Biochem. 1984 Jun 15;141(3):472–482. doi: 10.1111/j.1432-1033.1984.tb08217.x. [DOI] [PubMed] [Google Scholar]
- Kao W. W., Berg R. A., Prockop D. J. Ascorbate increases the synthesis of procollagen hydroxyproline by cultured fibroblasts from chick embryo tendons without activation of prolyl hydroxyla. Biochim Biophys Acta. 1975 Dec 5;411(2):202–215. doi: 10.1016/0304-4165(75)90300-1. [DOI] [PubMed] [Google Scholar]
- Kedersha N. L., Tkacz J. S., Berg R. A. Biosynthesis of prolyl hydroxylase: evidence for two separate dolichol-media pathways of glycosylation. Biochemistry. 1985 Oct 8;24(21):5960–5967. doi: 10.1021/bi00342a041. [DOI] [PubMed] [Google Scholar]
- Kivirikko K. I., Myllylä R. Post-translational processing of procollagens. Ann N Y Acad Sci. 1985;460:187–201. doi: 10.1111/j.1749-6632.1985.tb51167.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Litwer S., Danner D. J. Identification of a cDNA clone in lambda gt11 for the transacylase component of branched chain ketoacid dehydrogenase. Biochem Biophys Res Commun. 1985 Sep 16;131(2):961–967. doi: 10.1016/0006-291x(85)91333-6. [DOI] [PubMed] [Google Scholar]
- Majamaa K., Kuutti-Savolainen E. R., Tuderman L., Kivirikko K. I. Turnover of prolyl hydroxylase tetramers and the monomer-size protein in chick-embryo cartilaginous bone and lung in vivo. Biochem J. 1979 Feb 15;178(2):313–322. doi: 10.1042/bj1780313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McGee J. O., Langness U., Udenfriend S. Immunological evidence for an inactive precursor of collagen proline hydroxylase in cultured fibroblasts. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1585–1589. doi: 10.1073/pnas.68.7.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng M., Hogenkamp H. P. Purification, characterization, and amino acid sequence of thioredoxin from Corynebacterium nephridii. J Biol Chem. 1981 Sep 10;256(17):9174–9182. [PubMed] [Google Scholar]
- Pihlajaniemi T., Tryggvason K., Myers J. C., Kurkinen M., Lebo R., Cheung M. C., Prockop D. J., Boyd C. D. cDNA clones coding for the pro-alpha1(IV) chain of human type IV procollagen reveal an unusual homology of amino acid sequences in two halves of the carboxyl-terminal domain. J Biol Chem. 1985 Jun 25;260(12):7681–7687. [PubMed] [Google Scholar]
- Spiker S., Isenberg I. Preparative polyacrylamide gel electrophoresis. Methods Enzymol. 1983;91:214–226. doi: 10.1016/s0076-6879(83)91018-2. [DOI] [PubMed] [Google Scholar]
- Stassen F. L., Cardinale G. J., McGee J. O., Udenfriend S. Prolyl hydroxylase and an immunologically related protein in mammalian tissues. Arch Biochem Biophys. 1974 Jan;160(1):340–345. doi: 10.1016/s0003-9861(74)80042-1. [DOI] [PubMed] [Google Scholar]
- Zimmerman C. L., Appella E., Pisano J. J. Rapid analysis of amino acid phenylthiohydantoins by high-performance liquid chromatography. Anal Biochem. 1977 Feb;77(2):569–573. doi: 10.1016/0003-2697(77)90276-7. [DOI] [PubMed] [Google Scholar]
- de Waal A., de Jong L., Hartog A. F., Kemp A. Photoaffinity labeling of peptide binding sites of prolyl 4-hydroxylase with N-(4-azido-2-nitrophenyl)glycyl-(Pro-Pro-Gly)5. Biochemistry. 1985 Nov 5;24(23):6493–6499. doi: 10.1021/bi00344a028. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Fukushima H., Dewji N. N., Wilcox E., O'Brien J. S., Helinski D. R. Chromogenic immunodetection of human serum albumin and alpha-L-fucosidase clones in a human hepatoma cDNA expression library. DNA. 1984 Dec;3(6):437–447. doi: 10.1089/dna.1.1984.3.437. [DOI] [PubMed] [Google Scholar]





