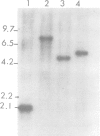
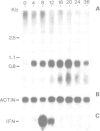
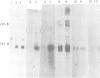
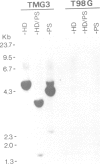
Abstract
A genomic clone encompassing the entire coding region of a murine gene homologous to human erythroid potentiating activity/tissue inhibitor of metalloproteinase (EPA/TIMP) was isolated and sequenced. Based on alignment with human EPA/TIMP cDNAs we deduce a structure comprising five exons and four introns extending over 4.3 kb of DNA. In mouse and hamster cell lines transcription from this gene and interferon genes is induced by Newcastle Disease virus (NDV). Examination of the 5'-flanking sequences of the gene reveals a set of repeated elements with structural similarity to those previously described as inducer-responsive elements in the human IFN-beta 1 gene. The 4.3-kb DNA fragment encompassing the homologous murine EPA/TIMP gene was transfected into human T98G cells and transfectants tested for NDV inducibility. In contrast to the endogenous human gene, the integrated murine EPA/TIMP gene was NDV-inducible and TIMP activity was detectable in the cell culture fluid.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bar-Shavit Z., Teitelbaum S. L., Stricklin G. P., Eisen A. Z., Kahn A. J., Welgus H. G. Differentiation of a human leukemia cell line and expression of collagenase inhibitor. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5380–5384. doi: 10.1073/pnas.82.16.5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bittner M., Kupferer P., Morris C. F. Electrophoretic transfer of proteins and nucleic acids from slab gels to diazobenzyloxymethyl cellulose or nitrocellulose sheets. Anal Biochem. 1980 Mar 1;102(2):459–471. doi: 10.1016/0003-2697(80)90182-7. [DOI] [PubMed] [Google Scholar]
- Cawston T. E., Barrett A. J. A rapid and reproducible assay for collagenase using [1-14C]acetylated collagen. Anal Biochem. 1979 Nov 1;99(2):340–345. doi: 10.1016/s0003-2697(79)80017-2. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Content J., De Wit L., Pierard D., Derynck R., De Clercq E., Fiers W. Secretory proteins induced in human fibroblasts under conditions used for the production of interferon beta. Proc Natl Acad Sci U S A. 1982 May;79(9):2768–2772. doi: 10.1073/pnas.79.9.2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Content J., De Wit L., Poupart P., Opdenakker G., Van Damme J., Billiau A. Induction of a 26-kDa-protein mRNA in human cells treated with an interleukin-1-related, leukocyte-derived factor. Eur J Biochem. 1985 Oct 15;152(2):253–257. doi: 10.1111/j.1432-1033.1985.tb09191.x. [DOI] [PubMed] [Google Scholar]
- Coveney J., Scott G., King R., Burke D. C., Skup D. Changes in the conformation of the interferon beta gene during differentiation and induction. Biochem Biophys Res Commun. 1984 May 31;121(1):290–296. doi: 10.1016/0006-291x(84)90721-6. [DOI] [PubMed] [Google Scholar]
- Docherty A. J., Lyons A., Smith B. J., Wright E. M., Stephens P. E., Harris T. J., Murphy G., Reynolds J. J. Sequence of human tissue inhibitor of metalloproteinases and its identity to erythroid-potentiating activity. Nature. 1985 Nov 7;318(6041):66–69. doi: 10.1038/318066a0. [DOI] [PubMed] [Google Scholar]
- Edwards D. R., Parfett C. L., Denhardt D. T. Transcriptional regulation of two serum-induced RNAs in mouse fibroblasts: equivalence of one species to B2 repetitive elements. Mol Cell Biol. 1985 Nov;5(11):3280–3288. doi: 10.1128/mcb.5.11.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. R., Waterhouse P., Holman M. L., Denhardt D. T. A growth-responsive gene (16C8) in normal mouse fibroblasts homologous to a human collagenase inhibitor with erythroid-potentiating activity: evidence for inducible and constitutive transcripts. Nucleic Acids Res. 1986 Nov 25;14(22):8863–8878. doi: 10.1093/nar/14.22.8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita T., Ohno S., Yasumitsu H., Taniguchi T. Delimitation and properties of DNA sequences required for the regulated expression of human interferon-beta gene. Cell. 1985 Jun;41(2):489–496. doi: 10.1016/s0092-8674(85)80022-2. [DOI] [PubMed] [Google Scholar]
- Galabru J., Robert N., Buffet-Janvresse C., Rivière Y., Hovanessian A. G. Continuous production of interferon in normal mice: effect of anti-interferon globulin, sex, age, strain and environment on the levels of 2-5A synthetase and p67K kinase. J Gen Virol. 1985 Apr;66(Pt 4):711–718. doi: 10.1099/0022-1317-66-4-711. [DOI] [PubMed] [Google Scholar]
- Gasson J. C., Golde D. W., Kaufman S. E., Westbrook C. A., Hewick R. M., Kaufman R. J., Wong G. G., Temple P. A., Leary A. C., Brown E. L. Molecular characterization and expression of the gene encoding human erythroid-potentiating activity. 1985 Jun 27-Jul 3Nature. 315(6022):768–771. doi: 10.1038/315768a0. [DOI] [PubMed] [Google Scholar]
- Goodbourn S., Burstein H., Maniatis T. The human beta-interferon gene enhancer is under negative control. Cell. 1986 May 23;45(4):601–610. doi: 10.1016/0092-8674(86)90292-8. [DOI] [PubMed] [Google Scholar]
- Goodbourn S., Zinn K., Maniatis T. Human beta-interferon gene expression is regulated by an inducible enhancer element. Cell. 1985 Jun;41(2):509–520. doi: 10.1016/s0092-8674(85)80024-6. [DOI] [PubMed] [Google Scholar]
- Higashi Y., Sokawa Y., Watanabe Y., Kawade Y., Ohno S., Takaoka C., Taniguchi T. Structure and expression of a cloned cDNA for mouse interferon-beta. J Biol Chem. 1983 Aug 10;258(15):9522–9529. [PubMed] [Google Scholar]
- Huebner K., Isobe M., Gasson J. C., Golde D. W., Croce C. M. Localization of the gene encoding human erythroid-potentiating activity to chromosome region Xp11.1----Xp11.4. Am J Hum Genet. 1986 Jun;38(6):819–826. [PMC free article] [PubMed] [Google Scholar]
- Kelley K. A., Kozak C. A., Pitha P. M. Localization of the mouse interferon-beta 1 gene to chromosome 4. J Interferon Res. 1985 Summer;5(3):409–413. doi: 10.1089/jir.1985.5.409. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Murphy D., Brickell P. M., Latchman D. S., Willison K., Rigby P. W. Transcripts regulated during normal embryonic development and oncogenic transformation share a repetitive element. Cell. 1983 Dec;35(3 Pt 2):865–871. doi: 10.1016/0092-8674(83)90119-8. [DOI] [PubMed] [Google Scholar]
- Patarca R., Haseltine W. A. A major retroviral core protein related to EPA and TIMP. 1985 Nov 28-Dec 4Nature. 318(6044):390–390. doi: 10.1038/318390a0. [DOI] [PubMed] [Google Scholar]
- Poupart P., De Wit L., Content J. Induction and regulation of the 26-kDa protein in the absence of synthesis of beta-interferon mRNA in human cells. Eur J Biochem. 1984 Aug 15;143(1):15–21. doi: 10.1111/j.1432-1033.1984.tb08332.x. [DOI] [PubMed] [Google Scholar]
- Raj N. B., Fernie B. F., Pitha P. M. Correlation between the induction of mouse interferon and the amount of its mRNA. Eur J Biochem. 1979 Jul;98(1):215–221. doi: 10.1111/j.1432-1033.1979.tb13179.x. [DOI] [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. Analysis of interferon mRNA in human fibroblast cells induced to produce interferon. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7426–7430. doi: 10.1073/pnas.78.12.7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. Synthesis of new proteins associated with the induction of interferon in human fibroblast cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4918–4922. doi: 10.1073/pnas.77.8.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. The messenger RNA sequences in human fibroblast cells induced with poly rI.rC to produce interferon. Nucleic Acids Res. 1980 Aug 11;8(15):3427–3437. doi: 10.1093/nar/8.15.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ryals J., Dierks P., Ragg H., Weissmann C. A 46-nucleotide promoter segment from an IFN-alpha gene renders an unrelated promoter inducible by virus. Cell. 1985 Jun;41(2):497–507. doi: 10.1016/s0092-8674(85)80023-4. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Saunders M. E., Gewert D. R., Tugwell M. E., McMahon M., Williams B. R. Human 2-5A synthetase: characterization of a novel cDNA and corresponding gene structure. EMBO J. 1985 Jul;4(7):1761–1768. doi: 10.1002/j.1460-2075.1985.tb03848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y. M., Hirschhorn R. R., Mercer W. E., Surmacz E., Tsutsui Y., Soprano K. J., Baserga R. Gene transfer: DNA microinjection compared with DNA transfection with a very high efficiency. Mol Cell Biol. 1982 Sep;2(9):1145–1154. doi: 10.1128/mcb.2.9.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skup D., Windass J. D., Sor F., George H., Williams B. R., Fukuhara H., De Maeyer-Guignard J., De Maeyer E. Molecular cloning of partial cDNA copies of two distinct mouse IFN-beta mRNAs. Nucleic Acids Res. 1982 May 25;10(10):3069–3084. doi: 10.1093/nar/10.10.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Hall A., Boll W., Stewart W. E., 2nd, Nagata S., Weissmann C. Target cell specificity of two species of human interferon-alpha produced in Escherichia coli and of hybrid molecules derived from them. Proc Natl Acad Sci U S A. 1981 May;78(5):2848–2852. doi: 10.1073/pnas.78.5.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissenbach J., Chernajovsky Y., Zeevi M., Shulman L., Soreq H., Nir U., Wallach D., Perricaudet M., Tiollais P., Revel M. Two interferon mRNAs in human fibroblasts: in vitro translation and Escherichia coli cloning studies. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7152–7156. doi: 10.1073/pnas.77.12.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn K., Maniatis T. Detection of factors that interact with the human beta-interferon regulatory region in vivo by DNAase I footprinting. Cell. 1986 May 23;45(4):611–618. doi: 10.1016/0092-8674(86)90293-x. [DOI] [PubMed] [Google Scholar]
- Zullo J. N., Cochran B. H., Huang A. S., Stiles C. D. Platelet-derived growth factor and double-stranded ribonucleic acids stimulate expression of the same genes in 3T3 cells. Cell. 1985 Dec;43(3 Pt 2):793–800. doi: 10.1016/0092-8674(85)90252-1. [DOI] [PubMed] [Google Scholar]