This study is the first to assess the reflex control of the thermoregulatory system in individuals living with multiple sclerosis (MS). The novel findings are twofold. First, attenuated increases in sweat rate in subjects with MS compared with healthy controls were observed in response to a moderate increase (0.8°C) in core temperature via passive whole body heat stress. Second, it appears the reflex control of the cutaneous vasculature is preserved in MS.
Keywords: autonomic dysfunction, cutaneous vascular conductance, skin blood flow, sweat rate, thermoregulation
Abstract
Multiple sclerosis (MS) is an autoimmune disease that affects the central nervous system (CNS), disrupting autonomic function. The aim of this study was to test the hypothesis that individuals with MS have blunted control of thermoregulatory reflex increases in sweat rate (SR) and cutaneous vasodilation compared with controls during a passive whole body heat stress (WBH). Eighteen individuals with relapsing-remitting MS and 18 healthy controls (Con) participated in the study. Core temperature (Tcore), skin temperature, heart rate, arterial blood pressure (10-min intervals), skin blood flow (laser-Doppler flux, LDF), and SR were continuously measured during normothermic baseline (34°C water perfusing a tube-lined suit) and WBH (increased Tcore 0.8°C via 48°C water perfusing the suit). Following WBH, local heaters were warmed to 42°C, inducing peak cutaneous vasodilation at the site of LDF collection. Cutaneous vascular conductance (CVC) was calculated as the ratio of LDF to mean arterial pressure and expressed as a percentage of peak achieved during local heating. Individuals with MS had attenuated SR responses to WBH (ΔSR from baseline: Con, 0.65 ± 0.27; MS, 0.42 ± 0.17 mg·cm−2·min−1, P = 0.003), whereas Δ%CVC42C from baseline was similar between groups (Con, 42 ± 16%; MS, 38 ± 12%, P = 0.39). SR responses were blunted as a function of Tcore in MS (interaction: group × Tcore, P = 0.03), of which differences were evident at ΔTcore 0.7°C and 0.8°C (P < 0.05). No interaction was observed in Δ%CVC42C. Taken together, the findings show MS blunts sweating responses, whereas control of the cutaneous vasculature is preserved, in response to WBH.
NEW & NOTEWORTHY This study is the first to assess the reflex control of the thermoregulatory system in individuals living with multiple sclerosis (MS). The novel findings are twofold. First, attenuated increases in sweat rate in subjects with MS compared with healthy controls were observed in response to a moderate increase (0.8°C) in core temperature via passive whole body heat stress. Second, it appears the reflex control of the cutaneous vasculature is preserved in MS.
multiple sclerosis (MS) is an autoimmune disease affecting the central nervous system (CNS). As the most prominent neurological disorder in young adults, the National Multiple Sclerosis Society (NMSS) estimates that 400,000 individuals are currently living with MS in the United States, and 2.3 million worldwide (NMSS 2016). The pathology of MS is marked by chronic autoimmune attacks via activated T cells, leading to the penetration of the blood-brain barrier that ultimately results in demyelination of axons within the CNS, replacing the healthy myelin with lesions (i.e., damaged myelin). Through demyelination of axons within the CNS, there is a loss of conductive properties that increase the risk for reduced conduction velocity (Eisen and Odusote 1980; Schauf and Davis 1974), conduction strength, and, potentially, conduction block (Davis 1970; Smith 1994). Loss of adequate neural conduction has been shown to cause a wide range of MS-related symptomatology, including but not limited to visual dysfunction (Frohman et al. 2011), gait ataxia (Givon et al. 2009; Kalron et al. 2010; Martin et al. 2006), bladder dysfunction (Betts et al. 1993; Miller et al. 1965), and fatigue (Bol et al. 2012; Krupp and Christodoulou 2001; Mollaŏglu and Üstün 2009), as well as autonomic dysfunction (Andersen and Nordenbo 1997; Huang et al. 2015, 2016; Keller et al. 2014; Noronha et al. 1968; Saari et al. 2009).
Although the effects of MS can widely vary among individuals, the majority (60–90%) of MS patients experience a profound heat intolerance known as Uhthoff’s phenomenon (Davis et al. 2010; Flensner et al. 2011; Frohman et al. 2011, 2013), which elicits a temporary worsening of disease-related symptomatology as a result of increased internal temperatures (e.g., warm environments and/or exercise). In some cases, an increase as little as 0.5°C in core temperature (Tcore) is sufficient to elicit Uhthoff’s phenomenon (Rasminsky 1973). Although dangers of heat intolerance (e.g., loss of vision, loss of motor control, extreme fatigue, etc.) in MS are well established (Frohman et al. 2013; Guthrie and Nelson 1995; Romberg et al. 2012; White et al. 2013), little progress has been made in regard to understanding the effect of MS on the control of thermoregulatory reflex responses (i.e., skin blood flow and sweating) that are employed to prevent excessive increases in Tcore.
Autonomic thermoregulatory responses to increases in Tcore require appropriate neural integration within the CNS. Heretofore, little research has been focused on understanding the effect of MS on the reflex control of cutaneous vascular and sudomotor function. Although primarily qualitative, sudomotor dysfunction in MS patients has been reported (Cartlidge 1972; Noronha et al. 1968; Saari et al. 2009; Vas 1969). Using a gross qualitative investigative approach, during a thermoregulatory sweat test (quinzarin powder) investigators have observed alterations or impairments in sweating as a result of MS (Cartlidge 1972; Noronha et al. 1968; Vas 1969). Using a more direct approach, Davis et al. (2005) demonstrated that individuals with MS had a significantly reduced sweat rate and sweat output per gland despite similar total sweat gland recruitment relative to healthy controls when maximal sweat responses from a small area of the dorsal forearm were evoked using iontophoresis of an acetylcholine agonist (pilocarpine). These data are noteworthy because they identify evidence of end-organ impairments, despite MS being primarily a CNS disease, but only provide inferences regarding the maximal capacity rather than a sweat rate (SR) obtained during a patient-relevant heat stress. Additionally, the other primary thermoregulatory mechanism of heat dissipation, skin blood flow, has not been assessed in individuals with MS.
The use of a water perfusion suit to induce a whole body heat stress (WBH) provides a reliable technique to investigate thermoregulatory reflex control of the cutaneous vasculature and sweat glands. By covering ~85% of the body’s surface area with a suit perfusing warm water, investigators can use the suit as a tool to heat the body, ultimately leading to a significant increase in Tcore. Subsequent measurements recorded from an exposed limb (e.g., an uncovered forearm) are thought to be representative of the thermoregulatory reflex (i.e., adequate sensing of increased Tcore, leading to proportional activation of efferent thermoregulatory end organs), because direct local heating from the suit does not influence the unexposed measurement site. Therefore, the use of this technique in assessing the neural control of efferent thermoregulatory responses could provide valuable insight into MS involvement in the neural control and regulation of the cutaneous vasculature and sweat glands.
To date, the effect of MS on the thermoregulatory reflex has yet to be investigated. Therefore, the primary aim of this study was to test the hypothesis that individuals with MS will have impaired reflex control of sweating and skin blood flow to increases in Tcore via passive WBH, leading to diminished sweating and skin blood flow responses compared with matched healthy controls (Con). Collected data, when combined with previous data, also may be able to provide insight into the origin of impairments (central vs. end-organ) in sweat glands and cutaneous blood vessels in individuals with MS.
METHODS
Subjects.
Individuals with definite relapsing-remitting MS (MS; n = 18; expanded disability status scale, EDSS: range 0–6) and age-, sex-, height-, weight-, and body surface area (BSA)-matched healthy controls (Con; n = 18) voluntarily participated in this study. Based on pilot data, an anticipated difference of ~45 mg·cm−2·min−1 in sweating between individuals with MS (n = 4) and healthy controls (n = 4) was expected (effect size = 1.47). A sample size of six subjects per group was derived from a power analysis (α = 0.05, power = 0.80) to find an anticipated difference in SR between groups during WBH. Because of the heterogeneity of symptomatology and disease course in individuals with MS, additional subjects were recruited.
All individuals with MS were otherwise healthy, but clinically diagnosed and currently being treated by neurologists specializing in MS at the MS Clinic at the University of Texas Southwestern Medical Center. This study focused on relapsing-remitting MS because it is the most common disease course: ~85% of individuals with MS are initially diagnosed with this form of the disease (NMSS 2016). This form of MS is characterized by acute attacks (exacerbations) followed by periods of partial or complete recovery (remissions) (NMSS 2016). All individuals with MS were on disease-modifying therapies. According to the 2015 consensus paper by the Multiple Sclerosis Coalition on the use of disease modifying therapies, FDA-approved disease-modifying treatment (DMT) is recommended and should be continued indefinitely (Costello et al. 2015). Individuals with MS on symptom-modifying therapies that are known to affect the CNS and/or thermoregulatory responses (i.e., antidepressants, psychostimulants, anticonvulsants, antispasmatics, and anticholinergics) were excluded from the study. Individuals with MS remained on their DMT as prescribed by their neurologists but were asked to refrain from taking any additional supplements and/or over-the-counter medications before testing. All individuals with MS were tested during a stable phase of their disease and were at least 6 mo removed from their most recent relapse. In an effort to avoid any differences associated with acclimation status, healthy controls were tested during the same time of year and during the same time of day (9:00 AM–12:00 PM) as their MS counterparts. All procedures were approved by the Institutional Review Boards at Southern Methodist University (Dallas, TX) and the University of Texas Southwestern Medical Center (Dallas, TX), and all conformed to the Declaration of Helsinki. All subjects were instructed on the protocol and provided their written informed consent before participation in the study.
Instrumentation.
Internal temperature (Tcore) was assessed using an ingestible telemetry thermometry pill (HQ, Palmetto, FL) taken ~1–2 h before the collection of baseline measurements. Mean skin temperature (Tsk) was measured via the weighted average of six thermocouples attached to the skin (Taylor et al. 1989). Arterial blood pressure was obtained via auscultation of the brachial artery (SunTech Medical Instruments, Raleigh, NC). Heart rate was identified using an electrocardiogram (ECG; Solar 8000i; General Electric, Schenectady, NY) interfaced with a cardiotachometer (CWE, Ardmore, PA). Sweat rate (SR) was measured using capacitance hygrometry (Vaisala, Woburn, MA) via the ventilated capsule method (Bullard 1962; Lang et al. 1993) on a proximal and a distal site on the skin of the exposed dorsal forearm as used previously (Davis et al. 2007). Laser-Doppler flux (LDF; an index of skin blood flow) was measured at a proximal and a distal site on the skin of the exposed dorsal forearm positioned at heart level via integrated laser-Doppler probes (PF413; Perimed, Ardmore, PA) connected to a laser-Doppler flowmeter (PF5010; Perimed). Probes were fitted inside thermostatic probe holders (PF450; Perimed) connected to a local heating device (PF5020; Perimed) capable of controlling local skin temperature at that site. For both SR and LDF, the mean values from the proximal and distal sites were averaged and recorded at each of the corresponding changes in Tcore of interest. To modify Tsk and Tcore, subjects were fitted in a tube-lined perfusion suit (Allen-Vanguard Technologies, Ottawa, ON, Canada), allowing the manipulation of water temperature to provide a thermal stress while leaving the instrumented forearm (as well as the head and feet) exposed for collection of LDF and SR data. All measurements were taken from participants lying in the supine position on a patient bed.
Protocol.
This study consisted of 1 experimental day lasting ~3.5 h. Baseline measurements were taken from the final minute of a 10-min normothermic (NT) baseline period, in which 34°C water perfused the tube-lined suit. After confirmation of adequate baseline measurements, WBH was performed by circulating 48°C water in the suit until an increase in Tcore of 0.8°C was observed. This moderate level of heat stress has been used in similar studies and has been shown to be of sufficient magnitude to induce changes in skin blood flow and sweating (Davis et al. 2007; Low et al. 2011). The following variables were continuously measured throughout the heating protocol: Tcore, Tsk, heart rate, LDF, and SR. Upper arm cuff blood pressures were taken at 10-min intervals. Because the suit was not in contact with the regions of skin where LDF and SR data were assessed, responses from these areas are presumed to be due entirely to thermoregulatory reflex-mediated neural modulation of efferent activity in response to changes in Tcore induced by the suit. Once the desired increase in internal temperature (0.8°C) was achieved, Tcore was stabilized by lowering the temperature of the water perfusing the suit to 42°C. Once a stable elevated Tcore was established, WBH measurements were recorded before subjects were returned toward NT Tcore levels by decreasing the temperature of the water perfusing the suit to 16°C. During this time period, local heating was performed at the sites of LDF collection on the forearm by setting thermostatic probe holders to 42°C. Local heating was terminated after a plateau in LDF values was observed (~30 min). LDF was then normalized relative to peak vasodilation observed at 42°C for each site.
Data analysis.
All data were continuously acquired at a sampling rate of 100 Hz on a 16-channel data acquisition system (Biopac, Santa Barbara, CA). Mean values from all measured variables, with the exception of cuff blood pressure, were obtained from the final minute of NT and WBH. Because of the dramatic increase in heart rate of 40–45% in both groups as a result of WBH, mean arterial pressure (MAP) during both NT and WBH was calculated according to two different equations: 1) the traditional equation MAPtrad = [(systolic blood pressure − diastolic blood pressure)/3 + diastolic blood pressure]; and 2) an adjusted equation (MAPadj), which applies mathematical weighting of systolic blood pressure based on the proportionally less time spent in systole (Moran et al. 1995). Skin blood flow is reported as cutaneous vascular conductance (CVC) and was calculated by dividing mean LDF values (arbitrary units, au) by MAP (both MAPtrad and MAPadj) calculated from brachial artery auscultation. CVC data were also normalized to peak vasodilation obtained during the final minute of local heating at 42°C for 30 min and are expressed as a percentage of peak CVC (%CVC42C). Changes in these variables relative to NT baseline conditions are plotted as means ± SD.
Statistical analysis.
All values are means ± SD. A two-way repeated-measures ANOVA was utilized to compare SR of groups (MS vs. Con) as a function of Tcore (0.1°C increments). Because of the necessity of using estimations of MAP to calculate CVC, it was not possible to appropriately calculate CVC as a function of Tcore. A two-way repeated-measures ANOVA was utilized to compare LDF responses (au) of groups (MS vs. Con) as a function of Tcore (0.1°C increments). Post hoc multiple comparisons were performed utilizing paired t-tests with a Bonferroni adjustment. Paired t-tests were performed on ΔCVC from baseline as a result of WBH (0.8°C increase in Tcore) and local heating responses. For any significant differences between groups, an effect size (Cohen’s d) was calculated to provide practical significance. All analysis was conducted using SPSS version 23 (IBM, Armonk, NY) and plotted via GraphPad Prism 6 (GraphPad Software, La Jolla, CA). Statistical significance was accepted at P < 0.05.
RESULTS
Subject characteristics.
Subject characteristics for both groups are presented in Table 1. The ranges for MS and Con groups were as follows: age (MS: 28–54; Con: 28–53 yr), height (MS: 152–193; Con: 160–196 cm), weight (MS: 48.2–102.9; Con: 48.2–93.1 kg), body surface area (MS: 1.46–2.34; Con: 1.53–2.26 m2), and body mass index (MS: 17.6–35.2; Con: 17.1–30.0 kg/m2). All individuals with MS were diagnosed with relapsing-remitting MS 14 ± 4 yr before participation in the study. Disease-modifying treatments used by individuals with MS included the following: Avonex (interferon β-1a), n = 1; Gilenya (fingolimod), n = 3; Copaxone (glatiramer acetate), n = 3; Tecfidera (dimethyl fumarate), n = 4; Tysabri (natalizumab), n = 5; Plegridy (peginterferon beta-1a), n = 1; and no DMT, n = 1.
Table 1.
Subject characteristics
| MS | Con | P Value | |
|---|---|---|---|
| Age, yr | 41 ± 7 | 39 ± 8 | 0.32 |
| Weight, kg | 72 ± 17 | 70 ± 14 | 0.68 |
| Height, cm | 169 ± 11 | 172 ± 10 | 0.50 |
| BMI, kg/m2 | 24.8 ± 4.3 | 23.6 ± 3.2 | 0.51 |
| BSA, m2 | 1.8 ± 0.3 | 1.8 ± 0.2 | 0.86 |
Values are means ± SD in individuals with multiple sclerosis (MS; n = 18) and healthy control subjects (Con; n = 18). BMI, body mass index; BSA, body surface area.
Normothermic baseline.
Baseline heart rate was significantly greater in the Con group (Con: 72 ± 16; MS: 65 ± 7 beats/min, P = 0.19), whereas baseline systolic (Con: 115 ± 12; MS: 108 ± 13 mmHg, P = 0.13), diastolic (Con: 74 ± 8; MS: 70 ± 10 mmHg, P = 0.26), MAPtrad (Con: 88 ± 9; MS: 83 ± 11 mmHg, P = 0.17), and MAPadj (Con: 88 ± 9; MS: 83 ± 11 mmHg, P = 0.15) were similar between groups. Additionally, there were no group differences in Tcore (Con: 37.1 ± 0.3; MS: 37.2 ± 0.3°C, P = 0.22), Tsk (Con: 35.1 ± 0.4; MS: 34.9 ± 0.5°C, P = 0.12), and SR (Con: 0.11 ± 0.02; MS: 0.10 ± 0.03 mg·cm−2·min−1, P = 0.16). All indexes of skin blood flow were similar during NT baseline: LDF (Con: 18 ± 8; MS: 17 ± 7 au, P = 0.60), mean CVCtrad (Con: 0.20 ± 0.09; MS: 0.20 ± 0.08 au/mmHg, P = 0.98), mean CVCadj (Con: 0.20 ± 0.09; MS: 0.20 ± 0.08 au/mmHg, P = 0.96), %CVC42C:trad (Con: 9.1 ± 5.1; MS: 7.7 ± 3.2, P = 0.32), or %CVC42C:adj (Con: 9.1 ± 5.1; MS: 7.8 ± 3.2, P = 0.38).
Heat stress.
Hemodynamic responses, represented as change from baseline, to WBH are presented in Table 2. During the last minute of WBH, heart rate increased to a similar level (Con: 100 ± 16; MS: 95 ± 14 beats/min, P = 0.44), whereas diastolic blood pressure was similarly maintained (Con: 69 ± 9; MS: 66 ± 5 mmHg, P = 0.14). Interestingly, although systolic blood pressure following heat stress was not statistically different (Con: 125 ± 15; MS: 115 ± 17 mmHg, P = 0.09), MAPtrad (Con: 88 ± 8; MS: 83 ± 8 mmHg, P = 0.08) and MAPadj (Con: 92 ± 8; MS: 86 ± 10 mmHg, P = 0.06) trended higher in the Con group.
Table 2.
Thermal and hemodynamic responses to heat stress
| MS | Con | P Value | |
|---|---|---|---|
| ΔTcore, °C | 0.80 ± 0.01 | 0.80 ± 0.01 | 0.13 |
| ΔHeart rate, beats/min | 30 ± 11 | 28 ± 9 | 0.67 |
| ΔSystolic blood pressure, mmHg | 8 ± 12 | 10 ± 10 | 0.51 |
| ΔDiastolic blood pressure, mmHg | −4 ± 8 | −5 ± 9 | 0.94 |
| ΔMAPtrad, mmHg | 0 ± 8 | 0 ± 17 | 0.78 |
| ΔMAPadj, mmHg | 4 ± 8 | 4 ± 7 | 0.74 |
Values are means ± SD, expressed as changes from baseline following whole body heating, in individuals with multiple sclerosis (MS) and healthy control subjects (Con). Tcore, core temperature; MAPtrad, mean arterial pressure calculated traditionally [(systolic blood pressure − diastolic blood pressure)/3 + diastolic blood pressure]; MAPadj, mean arterial pressure as a function of heart rate.
Thermoregulatory responses, represented as change from baseline, to WBH are presented in Figs. 1, 2, and 3. Of importance, a large effect size (d = 0.81 and d = 1.01) for SR differences between groups was observed at ΔTcore of 0.7°C and 0.8°C, respectively. During the final minute of WBH, the maximal SR achieved by individuals with MS was significantly lower than that of Con (Con: 0.76 ± 0.27; MS: 0.52 ± 0.18 mg·cm−2·min−1, P = 0.003). Tcore (Con: 37.9 ± 0.3; MS 38.0 ± 0.2°C, P = 0.20), Tskin (Con: 38.6 ± 0.5; MS: 38.7 ± 0.5°C, P = 0.50), LDF (Con: 109 ± 38; MS: 106 ± 28, P = 0.92), CVCtrad (Con: 1.3 ± 0.4; MS: 1.3 ± 0.5 au/mmHg, P = 0.55), CVCadj (Con: 1.2 ± 0.4; MS: 1.2 ± 0.5 au/mmHg, P = 0.79), %CVC42C:trad (Con: 53 ± 17; MS: 47 ± 14, P = 0.41), and %CVC42C:adj (Con: 51 ± 16; MS: 45 ± 14, P = 0.30) were similar during the final minute of heating.
Fig. 1.

Mean change in sweat rate (ΔSR) from baseline (±SD) as a function of changes in core temperature (ΔTcore) during passive whole body heating (A) and mean group (bars) and individual summary data (squares) showing ΔSR from baseline following a 0.8°C increase in Tcore (B) in persons with multiple sclerosis (MS) and healthy control subjects (Con). *Differences observed at 0.7°C and 0.8°C (Bonferroni-adjusted P < 0.05).
Fig. 2.
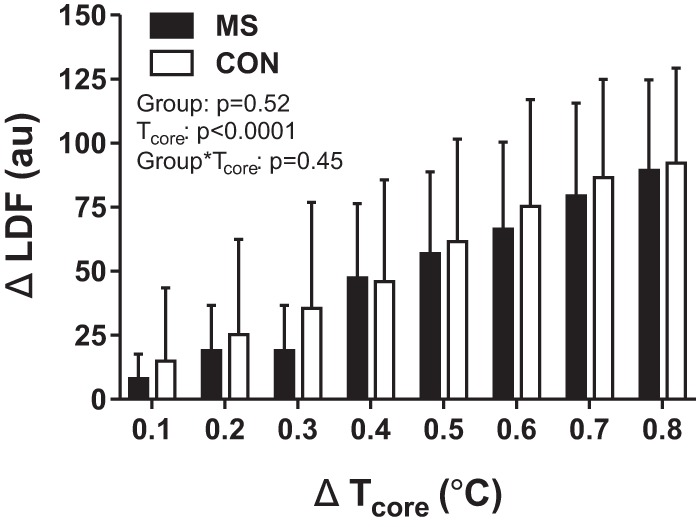
Mean change in laser-Doppler flux (ΔLDF; arbitrary units, au) from baseline (±SD) as a function of changes in core temperature (ΔTcore) during a whole body heat stress in individuals with multiple sclerosis (MS) and healthy control subjects (Con).
Fig. 3.
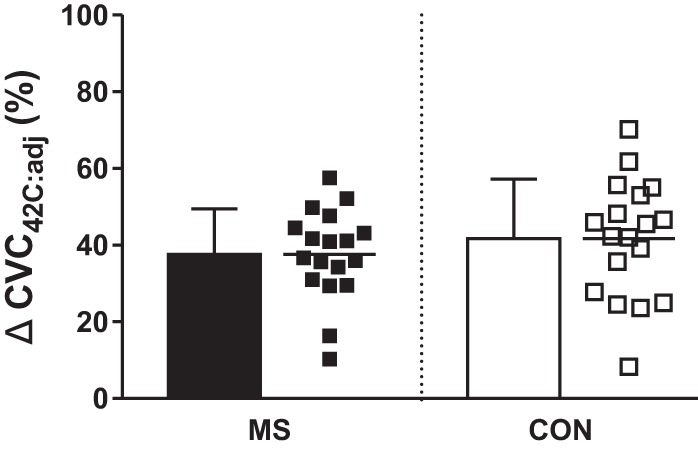
Mean group (bars) and individual (squares) changes in peak cutaneous vascular conductance (ΔCVC42C:adj) from baseline during passive whole body heating in individuals with multiple sclerosis (MS) and healthy control subjects (Con).
Local heating.
Peak LDF responses to 42°C local heating are presented in Fig. 4. In short, there were no significant differences in any peak values for LDF (P = 0.41), CVC42C:trad (P = 0.32), or CVC42C:adj (P = 0.32).
Fig. 4.
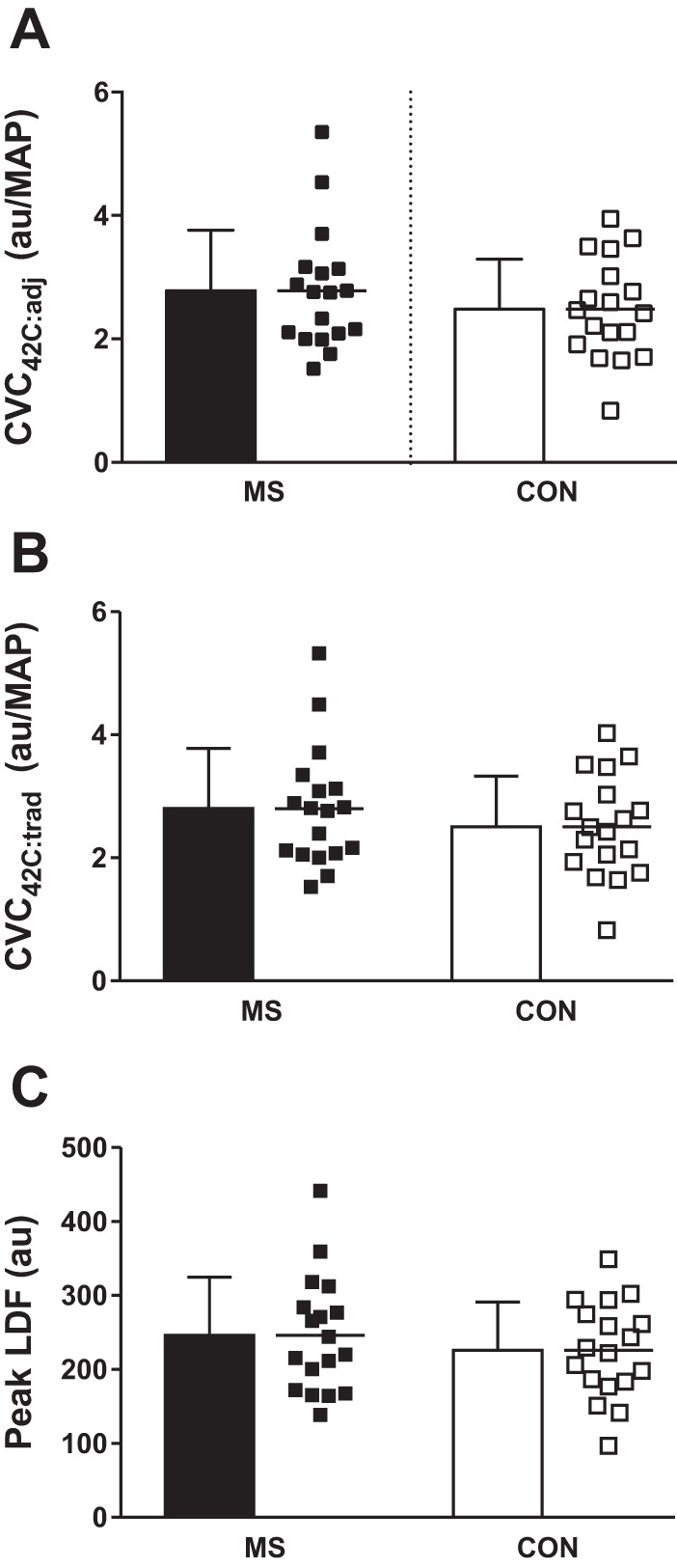
Mean group (bars) and individual (squares) responses in peak heart rate-adjusted cutaneous vascular conductance (CVC42C:adj; A), peak traditionally calculated cutaneous vascular conductance (CVC42C:adj:trad; B), and peak absolute laser-Doppler flux (LDF; C) values during the final minute of local heating to 42°C in individuals with multiple sclerosis (MS) and healthy control subjects (Con).
DISCUSSION
The present study is the first to provide evidence of impairments of thermoregulatory reflex sweating in individuals with MS during passive WBH. Confirming our hypothesis, sweating responses were blunted in individuals with MS during a moderate increase in core temperature compared with healthy controls. Individuals with MS had significantly reduced sweat rates as a function of core temperature and this difference increased as temperature increased. Counter to our hypothesis, the reflex control of the cutaneous vasculature (represented as %CVC42C) appears to be preserved in individuals with MS, because increases in skin blood flow were similar between individuals with MS and healthy controls. Taken together, these findings indicate that MS impairs the reflex control of thermoregulatory responses to passive heat stress.
The thermoregulatory reflex requires complex integration of afferent and efferent signal processing. On exposure to a rising core temperature, thermoreceptors at the skin and the core provide afferent information that is relayed to the preoptic area of the hypothalamus (thermoregulatory center of the brain) (Gordon and Heath 1986; Shibasaki et al. 2006). Once integrated, it is thought this brain center modulates heat dissipation via increasing autonomic signaling to thermoregulatory effector organs (i.e., cutaneous vasculature and sweat glands). The observed reduction in the neural control of sweating in the MS group provides evidence of autonomic impairments as a result of the disease. The mechanisms responsible for this are not readily apparent. With MS being an autoimmune disease affecting the CNS, it is probable that neural conduction within the brain and spinal cord plays a pivotal role in the blunted SR responses observed in this study. As previously mentioned, reduction in myelin (the underlying pathophysiology of MS) has detrimental effects on neural conduction. Reduced conduction velocity, impaired saltatory conduction, and increased incidence of conduction block stems from inadequate insulation properties that are typically provided by an intact myelin sheath (Frohman et al. 2011). Reduced myelin cross sectional area, and thus increased axonal exposure, leads to decreases in the current available relative to the current required for signal propagation (i.e., axonal “safety factor”). Therefore, it remains possible that a dampening of the signal for sweating, impaired integration of the signal for sweating, and/or the efferent signal being relayed from the CNS occurs in MS. Additionally, the increased axonal exposure as a result of decreased myelin cross-sectional area increases the relative reliance on voltage-gated sodium and potassium channels responsible for signal transmission down demyelinated areas of the axon. Over time, this increased voltage-gated channel reliance ultimately leads to increased expression of sodium channels at exposed demyelinated sites (Smith 1994, 2007; Smith et al. 1982), in an effort to ensure action potential propagation. Although this adaptation along the axon may be beneficial for maintaining conduction during normothermic resting conditions, it is thought that these sodium channels are temperature sensitive (Frohman et al. 2011), potentially altering neural signaling/processing within the CNS when internal temperature is increased. On relatively modest increases in Tcore (~0.2–0.5°C), these temperature-sensitive sodium channels close, thus terminating the depolarization phase of subsequent action potentials (Frohman et al. 2011, 2013). Although not directly tested in this study, it is likely this adaptation contributed to the reduction in reflex-mediated sweating responses observed herein.
In the present investigation, the magnitude of increase in SR was blunted during passive WBH. Altered control of sweating in individuals with MS has been identified previously (Cartlidge 1972; Noronha et al. 1968; Saari et al. 2009). Initially, investigators used the technique of staining the skin of participants with quinizarin powder before increasing their body temperature via passive heating (Noronha et al. 1968; Vas 1969). Quinizarin powder, initially yellow in color, stains to a dark shade of purple when in contact with moisture (i.e., perspiration). The use of this technique allows for visual inspection of sweating abnormalities but lacks the capability to quantify sweating responses (qualitative only measurement). More recently, with the use of more quantifiable methods, peripheral sweat gland dysfunction has been identified (Davis et al. 2005). In this study, Davis et al. (2005) demonstrated that postganglionic sympathetic nerves and sweat glands were responsive to maximal peripheral cholinergic stimulation in individuals with MS. However, individuals with MS demonstrated a diminished ability to sweat (decreased output per sweat gland) despite similar sweat gland recruitment compared with healthy counterparts. As a follow-up study, Davis et al. (2005) addressed the potential for decreases in sweat function being attributed to detraining or de-adaptation in this clinical population by exercise training a cohort of MS patients. Expected improvements in sweating responses (e.g., increases in maximal sweating) following aerobic exercise training previously demonstrated in healthy individuals (Crandall et al. 2003; Wilson et al. 2010) were not observed in individuals with MS, suggesting impairments in thermoregulatory control. Given these findings, the current study utilized a passive WBH stress model to investigate sweating responses on an exposed forearm, to target thermoregulatory reflex-mediated modulation of efferent activity in response to changes in Tcore. In agreement with aforementioned studies, the findings of the current study provide further evidence of sweating impairments and suggest these impairments could be attributed to altered neural control of sudomotor function. Taken together, the above findings indicate that MS impairments in sudomotor control within the CNS are complex and could be attributed to interactions of one or more of the following: 1) altered sensory processing, 2) neural integration abnormalities within the CNS, and/or 3) inappropriate end-organ effector processing. Ultimately, these alterations of neural function could lead to end-organ maladaptations in the sweat glands themselves, including a downregulation of cholinergic receptors (number and/or sensitivity) on the eccrine sweat gland, which would ultimately result in lower sweating responses per quanta of acetylcholine released and/or atrophy of sweat glands themselves.
The finding of preserved cutaneous vascular function in individuals with MS in response to an increase in Tcore is an interesting one. The %CVC42C:adj values from the groups in this study, which align well with values reported in a similar study (Davis et al. 2007), showed no apparent differences at any Tcore value. This suggests that not only is the control of the cutaneous vasculature in MS similar to controls in response to heat stress, but sweating and skin blood flow may potentially be under separate neural control. However, it also remains possible that the change in Tcore utilized in this study was not severe enough to reveal significant cutaneous vascular impairment. For healthy participants, in response to light exercise (~30% V̇o2max), Tcore increases nearly 0.8°C, whereas increases in Tcore of ~1.5°C and 2.0°C are seen with moderate (~51% V̇o2max) and moderate-high (~70% V̇o2max) exercise intensities, respectively (Saltin and Hermansen 1966). Additionally, in a study by Low et al. (2011) investigating more severe levels of heat stress in healthy participants (~Δ1.5°C Tcore), the authors demonstrated that CVC, skin sympathetic nerve activity (SSNA), and muscle sympathetic nerve activity (MSNA) increase as a function of Tcore. This increase in SSNA as a result of WBH was likely attributed to activation of sweat glands and increasing dilation of the cutaneous vasculature (i.e., increasing CVC) to facilitate heat dissipation. Although in the current study, differences in %CVC42C:adj responses to a passive heat stress that elicits an 0.8°C change in Tcore were not apparent between groups, potential differences could possibly be revealed at higher levels of heat stress in which more autonomic activity is required. However, a modest change (0.8°C) in Tcore was chosen in the current study in effort to elicit measureable SR and LDF responses while avoiding the onset and/or minimizing symptomatology associated with Uhthoff’s phenomenon. Further research is warranted to investigate the neural control of the cutaneous vasculature at higher Tcore levels in individuals with MS. Given that thermoregulatory reflexes utilize postganglionic nerves to induce both vasodilation and sweating, it is perplexing that SR responses are blunted while the control of skin blood flow is seemingly left intact. Clearly, more research is needed to elucidate these observations and overlying mechanisms.
In this investigation, blunted reflex control of sweating in MS was apparent at relatively moderate increases in Tcore (~0.8°C), whereas control of skin blood flow remained intact. These levels of elevated Tcore are likely encountered on a daily basis among most individuals and lie within the minimum core temperatures necessary to elicit Uhthoff’s phenomenon. Because the evaporation of sweat is the most effective form of heat dissipation among humans, suppressed control of this mechanism is particularly detrimental to individuals with MS. The disease likely renders these individuals unable to adequately prevent heat gain (increased Tcore) via blunting of sweating responses. The combination of heat sensitivity (Uhthoff’s phenomenon) and apparent thermoregulatory dysfunction creates a vicious cycle for individuals vulnerable to excess rises in Tcore, with known heat intolerance.
Limitations and future considerations.
Although our findings are consistent with CNS impairments (Andersen and Nordenbo 1997; Frohman et al. 2011; Noronha et al. 1968; Vas 1969), it remains possible that our results were influenced by DMT prescribed to individuals with MS. Patients were carefully screened for additional medications (i.e., antidepressants, psychostimulants, anticonvulsants, antispasmatics, and anticholinergics), and precautions were taken to remove any clear outliers that might influence group data. With this in mind, immediate prescription of DMT upon diagnosis is the current standard of care (Costello et al. 2015), and thus a critical understanding of individuals with MS currently on these medications is warranted.
CVC data from the current study are referenced to nonnoxious local skin heating to 42°C (Johnson et al. 2014). It may be possible that further vasodilation could have occurred at higher, potentially noxious, local heating temperatures (e.g., 43–46°C). Cutaneous nociceptors robustly express transient receptor potential vanilloid type 1 (TRPV1) channels (Ständer et al. 2004), which are necessary in the heat-pain pathway (Caterina et al. 2000; Davis et al. 2000). These receptors are stimulated at temperatures <42°C (Bevan et al. 2014) even if not consciously processed as pain in normal healthy participants. Given that individuals with MS present with somatosensory abnormalities (e.g., reduction in skin temperature detection thresholds) and central pain (with incidence rates of 50–55%), the use of higher temperatures could become more noxious (Leocani et al. 2003), thus affecting between-group comparisons. Thus, to avoid a potential noxious stimulus and collateral physiological responses, a 42°C local heating protocol was utilized (Johnson et al. 2014). Additionally, as part of the thermoregulatory reflex, elevated local skin temperatures can increase sweat output to aid in heat loss. Because local skin temperature at the SR measurement site was not recorded in the current study, it remains possible that there could be local influences aiding in the responses seen here. That being said, the autonomic control of local skin temperature is part of the thermoregulatory reflex and would only serve to strengthen our findings based on these results.
The use of perfusion suits to induce a tightly controlled passive heat stress limits the ability to assess certain aspects of thermoregulatory function. By covering ~85% of BSA, this heat stress technique effectively reverses skin heat transfer covered by the suit to that of an “outside-in” direction rather than “inside-out.” Although there are circumstances in which humans can face this form of heat stress, the majority of physiological heat stress is encountered from increases in metabolism (i.e., physical activity). To fully understand the impact of impaired sweating in MS on heat dissipation, further research is needed with the use of exercise as an experimental paradigm to increase core temperature. Finally, the experimental design required subjects be positioned in the supine position. Many heat stresses experienced by individuals with MS involve exposures or activity in the upright position. This may be doubly problematic to these patients, because current data suggest that dysautonomia may also manifest itself with the control of arterial blood pressure (Huang et al. 2016; Keller et al. 2014).
Conclusions.
This study utilized a novel approach to examine the reflex control of thermoregulatory responses to a passive heat stress in individuals with MS. These results demonstrate individuals with MS attenuated increases in SR, leading to suppressed SR in response to moderate increases in Tcore. Furthermore, it appears that MS does not affect the reflex control of the cutaneous vasculature in response to moderate elevations in Tcore. These findings are particularly troubling for individuals with MS, because this altered reflex control of thermoregulatory responses could contribute to decreased heat loss capacity. This apparent thermoregulatory dysfunction can compound heat intolerance (Uhthoff’s phenomenon), which may result in increased frequency and susceptibility to this acutely debilitating symptomatology. Further research is warranted to determine whether this attenuated reflex control translates to thermoregulatory dysfunction, especially during physical activity.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grant R15-HL-117224 (to S. L. Davis) and National Multiple Sclerosis Society Grants RG4043A1/1 and RG4696A3/2 (to S. L. Davis).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.R.A., E.M.F., and S.L.D. conceived and designed research; D.R.A., M.H., I.M.P., A.R.D., and S.L.D. performed experiments; D.R.A., M.H., I.M.P., and S.L.D. analyzed data; D.R.A., M.H., E.M.F., and S.L.D. interpreted results of experiments; D.R.A., M.H., and S.L.D. prepared figures; D.R.A. and S.L.D. drafted manuscript; D.R.A., M.H., I.M.P., A.R.D., E.M.F., and S.L.D. edited and revised manuscript; D.R.A., M.H., I.M.P., A.R.D., E.M.F., and S.L.D. approved final version of manuscript.
ACKNOWLEDGMENTS
The considerable time and effort of the participants are greatly appreciated.
REFERENCES
- Andersen EB, Nordenbo AM. Sympathetic vasoconstrictor responses in multiple sclerosis with thermo-regulatory dysfunction. Clin Auton Res 7: 13–16, 1997. doi: 10.1007/BF02267621. [DOI] [PubMed] [Google Scholar]
- Betts CD, D’Mellow MT, Fowler CJ. Urinary symptoms and the neurological features of bladder dysfunction in multiple sclerosis. J Neurol Neurosurg Psychiatry 56: 245–250, 1993. doi: 10.1136/jnnp.56.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S, Quallo T, Andersson DA. TRPV1. Handb Exp Pharmacol 222: 207–245, 2014. doi: 10.1007/978-3-642-54215-2_9. [DOI] [PubMed] [Google Scholar]
- Bol Y, Smolders J, Duits A, Lange IM, Romberg-Camps M, Hupperts R. Fatigue and heat sensitivity in patients with multiple sclerosis. Acta Neurol Scand 126: 384–389, 2012. doi: 10.1111/j.1600-0404.2012.01660.x. [DOI] [PubMed] [Google Scholar]
- Bullard RW. Continuous recording of sweating rate by resistance hygrometry. J Appl Physiol 17: 735–737, 1962. [DOI] [PubMed] [Google Scholar]
- Cartlidge NE. Autonomic function in multiple sclerosis. Brain 95: 661–664, 1972. doi: 10.1093/brain/95.4.661. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288: 306–313, 2000. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Costello K, Halper J, Kalb R, Skutnik L, Rapp R. The Use of Disease-Modifying Therapies in Multiple Sclerosis: Principles and Current Evidence. A Consensus Paper by the Multiple Sclerosis Coalition. Hackensack, NJ: The Multiple Sclerosis Coalition, 2015. [Google Scholar]
- Crandall CG, Shibasaki M, Wilson TE, Cui J, Levine BD. Prolonged head-down tilt exposure reduces maximal cutaneous vasodilator and sweating capacity in humans. J Appl Physiol (1985) 94: 2330–2336, 2003. doi: 10.1152/japplphysiol.00790.2002. [DOI] [PubMed] [Google Scholar]
- Davis FA. Axonal conduction studies based on some considerations of temperature effects in multiple sclerosis. Electroencephalogr Clin Neurophysiol 28: 281–286, 1970. doi: 10.1016/0013-4694(70)90164-1. [DOI] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 405: 183–187, 2000. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- Davis SL, Shibasaki M, Low DA, Cui J, Keller DM, Purdue GF, Hunt JL, Arnoldo TB, Kowalske KJ, Crandall CG. Impaired cutaneous vasodilation and sweating in grafted skin during whole-body heating. J Burn Care Res 28: 427–434, 2007. doi: 10.1097/BCR.0B013E318053D312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SL, Wilson TE, Vener JM, Crandall CG, Petajan JH, White AT. Pilocarpine-induced sweat gland function in individuals with multiple sclerosis. J Appl Physiol (1985) 98: 1740–1744, 2005. doi: 10.1152/japplphysiol.00860.2004. [DOI] [PubMed] [Google Scholar]
- Davis SL, Wilson TE, White AT, Frohman EM. Thermoregulation in multiple sclerosis. J Appl Physiol (1985) 109: 1531–1537, 2010. doi: 10.1152/japplphysiol.00460.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen A, Odusote K. Central and peripheral conduction times in multiple sclerosis. Electroencephalogr Clin Neurophysiol 48: 253–265, 1980. doi: 10.1016/0013-4694(80)90263-1. [DOI] [PubMed] [Google Scholar]
- Flensner G, Ek AC, Söderhamn O, Landtblom AM. Sensitivity to heat in MS patients: a factor strongly influencing symptomology–an explorative survey. BMC Neurol 11: 27, 2011. doi: 10.1186/1471-2377-11-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohman TC, Davis SL, Beh S, Greenberg BM, Remington G, Frohman EM. Uhthoff’s phenomena in MS–clinical features and pathophysiology. Nat Rev Neurol 9: 535–540, 2013. doi: 10.1038/nrneurol.2013.98. [DOI] [PubMed] [Google Scholar]
- Frohman TC, Davis SL, Frohman EM. Modeling the mechanisms of Uhthoff’s phenomenon in MS patients with internuclear ophthalmoparesis. Ann N Y Acad Sci 1233: 313–319, 2011. doi: 10.1111/j.1749-6632.2011.06125.x. [DOI] [PubMed] [Google Scholar]
- Givon U, Zeilig G, Achiron A. Gait analysis in multiple sclerosis: characterization of temporal-spatial parameters using GAITRite functional ambulation system. Gait Posture 29: 138–142, 2009. doi: 10.1016/j.gaitpost.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Gordon CJ, Heath JE. Integration and central processing in temperature regulation. Annu Rev Physiol 48: 595–612, 1986. doi: 10.1146/annurev.ph.48.030186.003115. [DOI] [PubMed] [Google Scholar]
- Guthrie TC, Nelson DA. Influence of temperature changes on multiple sclerosis: critical review of mechanisms and research potential. J Neurol Sci 129: 1–8, 1995. doi: 10.1016/0022-510X(94)00248-M. [DOI] [PubMed] [Google Scholar]
- Huang M, Allen DR, Keller DM, Fadel PJ, Frohman EM, Davis SL. Impaired carotid baroreflex control of arterial blood pressure in multiple sclerosis. J Neurophysiol 116: 81–87, 2016. doi: 10.1152/jn.00003.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M, Jay O, Davis SL. Autonomic dysfunction in multiple sclerosis: implications for exercise. Auton Neurosci 188: 82–85, 2015. doi: 10.1016/j.autneu.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JM, Minson CT, Kellogg DL Jr. Cutaneous vasodilator and vasoconstrictor mechanisms in temperature regulation. Compr Physiol 4: 33–89, 2014. doi: 10.1002/cphy.c130015. [DOI] [PubMed] [Google Scholar]
- Kalron A, Dvir Z, Achiron A. Walking while talking–difficulties incurred during the initial stages of multiple sclerosis disease process. Gait Posture 32: 332–335, 2010. doi: 10.1016/j.gaitpost.2010.06.002. [DOI] [PubMed] [Google Scholar]
- Keller DM, Fadel PJ, Harnsberger MA, Remington GM, Frohman EM, Davis SL. Reduced spontaneous sympathetic nerve activity in multiple sclerosis patients. J Neurol Sci 344: 210–214, 2014. doi: 10.1016/j.jns.2014.06.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp LB, Christodoulou C. Fatigue in multiple sclerosis. Curr Neurol Neurosci Rep 1: 294–298, 2001. doi: 10.1007/s11910-001-0033-7. [DOI] [PubMed] [Google Scholar]
- Lang E, Foerster A, Pfannmüller D, Handwerker HO. Quantitative assessment of sudomotor activity by capacitance hygrometry. Clin Auton Res 3: 107–115, 1993. doi: 10.1007/BF01818995. [DOI] [PubMed] [Google Scholar]
- Leocani L, Martinelli V, Natali-Sora MG, Rovaris M, Comi G. Somatosensory evoked potentials and sensory involvement in multiple sclerosis: comparison with clinical findings and quantitative sensory tests. Mult Scler 9: 275–279, 2003. doi: 10.1191/1352458503ms908oa. [DOI] [PubMed] [Google Scholar]
- Low DA, Keller DM, Wingo JE, Brothers RM, Crandall CG. Sympathetic nerve activity and whole body heat stress in humans. J Appl Physiol (1985) 111: 1329–1334, 2011. doi: 10.1152/japplphysiol.00498.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CL, Phillips BA, Kilpatrick TJ, Butzkueven H, Tubridy N, McDonald E, Galea MP. Gait and balance impairment in early multiple sclerosis in the absence of clinical disability. Mult Scler 12: 620–628, 2006. doi: 10.1177/1352458506070658. [DOI] [PubMed] [Google Scholar]
- Miller H, Simpson CA, Yeates WK. Bladder dysfunction in multiple sclerosis. BMJ 1: 1265–1269, 1965. doi: 10.1136/bmj.1.5445.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollaŏglu M, Ustün E. Fatigue in multiple sclerosis patients. J Clin Nurs 18: 1231–1238, 2009. doi: 10.1111/j.1365-2702.2008.02733.x. [DOI] [PubMed] [Google Scholar]
- Moran D, Epstein Y, Keren G, Laor A, Sherez J, Shapiro Y. Calculation of mean arterial pressure during exercise as a function of heart rate. Appl Human Sci 14: 293–295, 1995. doi: 10.2114/ahs.14.293. [DOI] [PubMed] [Google Scholar]
- NMSS Who Gets MS? (Online). http://www.nationalmssociety.org/What-is-MS/Who-Gets-MS [15 Sept. 2016].
- Noronha MJ, Vas CJ, Aziz H. Autonomic dysfunction (sweating responses) in multiple sclerosis. J Neurol Neurosurg Psychiatry 31: 19–22, 1968. doi: 10.1136/jnnp.31.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasminsky M. The effects of temperature on conduction in demyelinated single nerve fibers. Arch Neurol 28: 287–292, 1973. doi: 10.1001/archneur.1973.00490230023001. [DOI] [PubMed] [Google Scholar]
- Romberg A, Ikonen A, Ruutiainen J, Virtanen A, Hämäläinen P. The effects of heat stress on physical functioning in persons with multiple sclerosis. J Neurol Sci 319: 42–46, 2012. doi: 10.1016/j.jns.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 42.Saari A, Tolonen U, Pääkkö E, Suominen K, Jauhiainen J, Sotaniemi KA, Myllylä VV. Sweating impairment in patients with multiple sclerosis. Acta Neurol Scand 120: 358–363, 2009. doi: 10.1111/j.1600-0404.2009.01164.x. [DOI] [PubMed] [Google Scholar]
- Saltin B, Hermansen L. Esophageal, rectal, and muscle temperature during exercise. J Appl Physiol 21: 1757–1762, 1966. [DOI] [PubMed] [Google Scholar]
- Schauf CL, Davis FA. Impulse conduction in multiple sclerosis: a theoretical basis for modification by temperature and pharmacological agents. J Neurol Neurosurg Psychiatry 37: 152–161, 1974. doi: 10.1136/jnnp.37.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki M, Wilson TE, Crandall CG. Neural control and mechanisms of eccrine sweating during heat stress and exercise. J Appl Physiol (1985) 100: 1692–1701, 2006. doi: 10.1152/japplphysiol.01124.2005. [DOI] [PubMed] [Google Scholar]
- Smith KJ. Conduction properties of central demyelinated and remyelinated axons, and their relation to symptom production in demyelinating disorders. Eye (Lond) 8: 224–237, 1994. doi: 10.1038/eye.1994.51. [DOI] [PubMed] [Google Scholar]
- Smith KJ. Sodium channels and multiple sclerosis: roles in symptom production, damage and therapy. Brain Pathol 17: 230–242, 2007. doi: 10.1111/j.1750-3639.2007.00066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Bostock H, Hall SM. Saltatory conduction precedes remyelination in axons demyelinated with lysophosphatidyl choline. J Neurol Sci 54: 13–31, 1982. doi: 10.1016/0022-510X(82)90215-5. [DOI] [PubMed] [Google Scholar]
- Ständer S, Moormann C, Schumacher M, Buddenkotte J, Artuc M, Shpacovitch V, Brzoska T, Lippert U, Henz BM, Luger TA, Metze D, Steinhoff M. Expression of vanilloid receptor subtype 1 in cutaneous sensory nerve fibers, mast cells, and epithelial cells of appendage structures. Exp Dermatol 13: 129–139, 2004. doi: 10.1111/j.0906-6705.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- Taylor WF, Johnson JM, Kosiba WA, Kwan CM. Cutaneous vascular responses to isometric handgrip exercise. J Appl Physiol (1985) 66: 1586–1592, 1989. [DOI] [PubMed] [Google Scholar]
- Vas CJ. Sexual impotence and some autonomic disturbances in men with multiple sclerosis. Acta Neurol Scand 45: 166–182, 1969. doi: 10.1111/j.1600-0404.1969.tb01230.x. [DOI] [PubMed] [Google Scholar]
- White AT, Vanhaitsma TA, Vener J, Davis SL. Effect of passive whole body heating on central conduction and cortical excitability in multiple sclerosis patients and healthy controls. J Appl Physiol (1985) 114: 1697–1704, 2013. doi: 10.1152/japplphysiol.01119.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson TE, Monahan KD, Fogelman A, Kearney ML, Sauder CL, Ray CA. Aerobic training improves in vivo cholinergic responsiveness but not sensitivity of eccrine sweat glands. J Invest Dermatol 130: 2328–2330, 2010. doi: 10.1038/jid.2010.125. [DOI] [PMC free article] [PubMed] [Google Scholar]


