Abstract
Objectives
Our study analyses the prevalence of ANA, anti-SS-A, anti-SS-B, and ACA and ACPA antibodies in patients with pSS and with dryness symptoms without pSS confirmation, and the association of ACPA and ACA antibodies with specific clinical symptoms.
Materials and methods
113 patients were divided into two groups: I – with diagnosed pSS (N = 75); and II – with dryness without pSS evidence (N = 38). Diagnostics: indirect immunofluorescence (IF; Hep-2 cell line) of antinuclear antibodies (ANA), anti-SS-A anti-SS-B antibodies determined with semi-quantitative method, autoantibody profile (14 antigens, ANA Profil 3 EUROLINE); basic laboratory, ophthalmic examination tests, minor salivary gland biopsy with focus score (FS), joint and lung evaluation, and ESSDAI questionnaire (pSS activity).
Results
88% of group I had ANA antibodies (1 : 320 titre), 5.3% at 1 : 160. Anti-SS-A antibodies were present in 88% of group I, including all ANA 1 : 160. Anti-SS-A antibodies positively correlated with greater and moderate activity of ESSDAI 5 (p = 0.046) and FS. The presence of SS-B antibodies significantly affected disease activity. ACPA present: group I – 13% (associated with higher arthritis incidence; p = 0.003); group II – 8%. ACA antibodies present in 4% of group I, but not in group II. No ACA association with interstitial lung changes (small ACA + group excludes full conclusions).
Conclusions
ANA antibodies should also be considered in a titre of less than 1 : 320, but the presence of anti-SS-A antibodies is still the most important immunological marker for pSS. Anti-SS-A antibodies correlate with higher disease activity (ESSDAI ≥ 5) and higher FS. The presence of the anti-SS-B antibody was significantly affected by higher activity of the disease. The incidence of arthritis was higher in patients with ACPA+ pSS compared to ACPA– (p = 0.003). There was no relationship between ACPA and arthritis in patients with dry-type syndrome without diagnosis of pSS.
Keywords: Sjögren’s syndrome, anticentromere antibodies, anticyclic citrullinated antibodies,
Introduction
As is presently known, B lymphocytes and their activity – the production of immunoglobulins and auto-antibodies, constituting the basic element of the humoral immune response – play a dominant role in the pathogenesis of primary Sjögren’s syndrome (pSS) [1, 2]. Confirmation of the presence of the antinuclear antibodies (ANA) and of the specific type of their immunofluorescence suggest the existence of autoimmunological disease. However, only the presence of the specific antibodies against extractable nuclear antigens (ENA) enables more specific diagnostics. It is known that ANA are present in 80–90% of cases of pSS [3]. These antibodies interact with the elements of the cellular nucleus. They are studied most often by means of indirect immunofluorescence (IF) in Hep-2 (human epithelial cell) cell line. In pSS, ANA are usually present in titres above 1 : 320, but they can also be found in lower titres (1 : 160) and coexist with other types of autoantibodies. Among the antibodies most common in pSS are those against small ribonucleoproteins – SS-A complex (Ro60 and Ro52) and SS-B/La. The antigen for the anti-SS-A antibodies consists of two proteins of different molecular mass: Ro60 and Ro52, coded by separate genes situated in chromosome 19 for Ro60 and in chromosome 11 for Ro52 [4, 5]. Ro52 antigen is a phosphoprotein, production of which is activated through viral infection, type I interferon pathway cascade, or Toll-like receptor stimulation. Ro60 antigen binds to the non-coded RNA, creating the hY-RNA complex, which inhibits immune response [6].
SS-A/Ro (60 D + 52 kD) complex is present in most of the human cells e.g. platelets and red blood cells. It is believed that anti-SS-A antibodies play a role in the pSS pathogenesis, and their presence is associated with more intense symptoms of endocrine glands involvement, lymphadenopathy, larger infiltrates in salivary glands, symptoms of the vasculitis, and with prolonged duration of the disease [6]. Anti-SS-A/Ro52 antibodies are also present in others systemic connective tissue disorders, e.g. in polymyositis, where it is usually accompanied by Jo-1 antibody [7], and in lupus erythematosus (LE), where other antibodies typical for LE, such as anti-dsDNA, anti-Sm, and anti-RNP, are also found. Anti-SS-A antibodies emergence is stimulated by UV radiation, through the intensification of antigen expression on the cell surface. Anti-SS-A presence is associated with aberrations of blood morphology (leukopaenia, anaemia, thrombocytopaenia), lung involvement, and aggravation of the skin lesions. They are found in 5% of pregnant women, creating a risk of their transfer to the foetus through the placenta and causing damage of the foetal cardiac conduction system, including the gravest complication – complete heart block.
Anti-SS-A are also found in scleroderma, rheumatoid arthritis, polymyositis, and dermatomyositis (PM/DM), with those against Ro52 antigen more frequently present [6]. It has been observed that anti-Ro52 antibodies are associated with primary biliary cirrhosis (PBC) and autoimmune hepatitis (AIH) more than antibodies against Ro60 antigen.
Anti-SS-B/La antibodies are less common and usually coexist with anti-SS-A/Ro antibodies. Their presence in systemic diseases other than pSS, in LE in particular (they are found in 25% of LE cases), is associated with skin lesions (erythema, alopecia) and with the serositis, leukopaenia, and dryness symptoms (the latter in the presence of anti-SS-A antibodies in secondary Sjögren’s syndrome) [8].
The occurrence of other autoantibodies, such as anti-centromere antibodies (ACA) and anti-citrullinated protein antibodies (ACPA), has also been described in pSS [9, 10]. ACA are more widely known and analysed. They are comprised of three antibodies associated with proteins (CNEPs) A (19 kD), B (80 kD), and C (140 kD). Gelber et al. [11] demonstrated that CENP-C alone, without the presence of CNEP-A and B, are found in pSS, but coexistence of CEP-B and CENP-C is associated with coincidence of pSS and limited scleroderma. Currently ACA antibodies against numerous antigens, e.g. CENP-D14,15, CENP-E16, and CENP-O17 found in systemic sclerosis (SSc) and CENP-F18, CENP-G19, CENP-H20, and CENP-I21, are being researched [12].
Summarising, for the clinician, knowledge of the patient’s immunological profile gains significance when combined with clinical data such as occurrence of interstitial lesions in lungs or arthritis; it might have some prognostic value. However, it must be taken into consideration, in given clinical circumstances, that various autoantibodies are present in neoplasms and chronic infections, especially granulomatous ones, such as tuberculosis, leprosy, and syphilis.
The presented work aims to analyse the presence of ANA, anti-SS-A, anti-SS-B, ACA and ACPA antibodies in the group of patients with diagnosed pSS compared to patients with dry symptoms, but without confirmation of pSS. The association of ACPA and ACA antibodies with specific clinical symptoms was also studied in those groups.
Material and methods
The research was performed on 113 patients referred to the rheumatology ward with suspected pSS, from which two groups were distinguished.
Group I was with the pSS diagnosis confirmed in the course of the treatment (N = 75); age 22 to 51 years (mean 50.03 ±15.1 years), 65 female [F] (86.7%) and 10 male [M] (10.3%); the female to male ratio was 6.5 : 1. The mean time from the diagnosis of pSS was 2.24 years. The characteristics of that group, concerning the interstitial pulmonary lesions, as well as lesions of the central and peripheral nervous systems, are presented in Table I.
Table I.
Characteristic of group I
| Parameter | N | Number | Percent (%) |
|---|---|---|---|
| Female | 75 | 65 | 87 |
| Male | 75 | 10 | 13 |
| Lung involvement (HRCT) | 75 | 6 | 8 |
| Central nervous system involvement (MRI, neurological examination) | 7 | 11 | 15 |
| Peripheral nervous system involvement (EMG, neurological examination) | 75 | 12 | 16 |
| Arthritis (symptoms, diagnosis confirmation in USG, RTG) | 74 | 15 | 20 |
| Arthralgia (articular pain without confirmation of inflammation) | 75 | 63 | 84 |
Group II was with dryness and no confirmed diagnosis of systemic connective tissue disease (N = 38); age 33 to 58 years (mean 55.11 ±12.4 years), 35 F (92.1%) and 3 M (3.9%); F/M ratio 11.6 : 1.
The pSS diagnosis was based on the examination assessed in accordance with the 2012 ACR criteria current at the time of the research; patients were also evaluated according to the previous AECG (2002) criteria. The 55 patients from group I met both ACR 2012 [13] and AECG [14] criteria, which constituted 80% of the whole group. Solely ACR 2012 criteria were met by only 15 patients (20%) of whom histopathological confirmation was achieved in 11 cases, and in four patients no FS ≥ 1 was found, but simultaneously the anti-SS-A antibodies were detected, and dry-eye symptoms were confirmed with OSS assessment. The characteristics of both groups, including the occurrence of lung lesions and the peripheral and central nervous system changes, are summarised in Tables I and II.
Table II.
Characteristic of group II
| Parameter | N | Number | Percent (%) |
|---|---|---|---|
| Female | 38 | 35 | 92.1 |
| Male | 38 | 3 | 7.9 |
| Lung involvement (HRCT) | 38 | 1 | 2.6 |
| Central nervous system involvement (MRI, neurological examination) | 38 | 5 | 13.2 |
| Peripheral nervous system involvement (EMG, neurological examination) | 38 | 2 | 5.3 |
| Arthritis (symptoms, diagnosis confirmation in USG, RTG) | 37 | 2 | 5.4 |
| Arthralgia (articular pain without confirmation of inflammation) | 37 | 31 | 84 |
All patients were diagnosed using case history, physical examination, and basic laboratory tests. Peripheral blood morphology and white blood count (WBC 4,000–10,000/mm³) were assessed. Electrophoretic separation of serum proteins was performed, and the serum concentrations of gammaglobulins [norm (n) 0.8–1.35 g/dl], C3 (n: 55–120 mg/dl) and C4 (n: 16–47 mg/dl) complement components, rheumatoid factor (RF) of the IgM class (n < 34 IU/ml), creatinine (n: ≤ 1.1 mg/dl), alanine aminotransferase (AlAT n: 13–69 U/l), asparagine aminotransferase (AspAT n: 14–46 U/l), and C-reactive peptide (CRP n: 0–10 mg/l) were assessed. Urinalysis with urine sediment examination was performed. Erythrocyte sedimentation rate (ESR) was assessed, and the serum was examined for the presence of cryoglobulins. Antinuclear antibodies (ANA) were assessed using indirect immunofluorescence (IF) method in HEp-2 (Human Epithelial Cell; HEp-2000) cell line. Anti-SS-A and anti-SS-B antibodies were assessed using semiquantitative Immunoblot (Anti-ENA Profile Plus; Euroimmun, Germany, DL 1590-1601-1 G) method, evaluating antigen-antibody binding strength on a scale from 1 to 3. For the purpose of the statistical analysis, the ANA titres were logarithmised.
The profile of autoantibodies was assessed with a test kit (ANA Profil 3 EUROLINE) for the in vitro qualitative detection of IgG class antibodies against 14 antigens: (anticentromere – ACA, dsRNA, RNP, Scl-70, Jo-1, AMA-M2) with Dot-blot method.
The tests necessary for the confirmation of the symptoms of dryness and diagnosis of pSS were performed. These included ophthalmological assessment of the eye dryness with Schirmer test, lissamine green and fluorescein staining, as well as biopsy of minor salivary glands – the samples were stained with haematoxylin and eosin (HE) and then assessed for the number of foci containing ≥ 50 lymphocytes per 4 mm2 of the affected tissue bordering the healthy gland acini. Each focus constitutes 1 focus score (FS).
In the case of the suspicion of articular involvement (pain, swelling) the X-ray and ultrasound examination (USG) of the joints were performed in order to confirm the inflammation. Classical X-ray of the chest in posterior-anterior projection was also performed; the decision to implement high-resolution computed tomography (HRCT) examination was taken individually in each case by the physician.
Disease activity was assessed with a EULAR Sjögren’s syndrome disease activity index (ESSDAI) questionnaire concerning 12 domains. In each domain, the score was from 0 (no activity) to 3 (high activity). The overall pSS activity is a sum of all points from all domains, with the “strength” of each domain taken into consideration.
The statistical analysis was performed with the Statistica programme version 10.0. The quantitative variables were presented in the form of mean and standard deviations (normal distribution variables) or median and interquartile range (variables with distribution deviating from normal). Qualitative variables are shown in the form of numbers and percentage.
Because the distribution of the studied quantitative variables varied from normal, to compare them the U Mann-Whitney test was employed. For comparison of the qualitative variables the χ2 test, χ2 with Yates correction, or Fisher’s exact test (depending on the multiplicity of the expected values) was used. To establish the relation between the variables Spearman’s correlation coefficient was used. The statistical significance level was set as p < 0.05.
The research was conducted after obtaining the approval of the Bioethics Commission of National Institute of Geriatrics, Rehabilitation, and Rheumatology. The subjects were informed of the aims of the research and the methods employed, signing a form of informed consent for the test.
Results
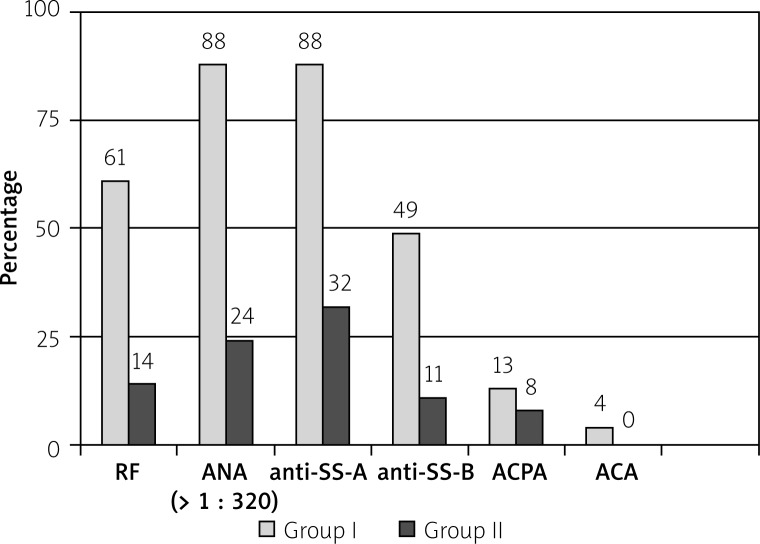
A necessary condition for conducting pSS diagnostics is establishing the presence of the autoantibodies. Therefore, the immunological profile of the patients from group I is shown in Table III. ANA antibodies in titres ≥ 1 : 320 were present in 88% of patients. In four individuals the titre was lower (1 : 160), but in the same patients the presence of anti-SS-A antibodies was revealed. A significant titre of ANA antibodies in group I was found in 93% of individuals. The presence of autoantibodies in both groups are presented in Figure 1.
Table III.
Univariate analysis
| Parameter | Univariate analysis | |
|---|---|---|
| odds ratio (OR)(95% CI) | p | |
| ESR [mm/h] | 1.04 (1.01–1.07) |
0.010 |
| WBC [103/mm³] | 0.97 (0.81–1.17) |
0.780 |
| RF [IU/ml] | 1.02 (1.00–1.03) |
0.011 |
| Gammaglobulins > 1.35 [g/dl] | 1.747 (1.162–2.626) |
0.007 |
| lnANA | 3.681 (2.226–6.087) |
< 0.001 |
| Anti-SS-A | 2.782 (1.905–4.063) |
< 0.001 |
| Anti-SS-B | 2.439 (1.423–4.179) |
0.001 |
| Schirmer test (mean) [mm] | 0.903 (0.858–0.949) |
< 0.001 |
| OSS | 1.621 (1.150–2.285) |
0.006 |
Fig. 1.
Presence (N/%) of the antibodies in both groups.
In univariate analysis (Table III) the distinguishing parameters in pSS patients were: presence of the ANA, anti-SS-A, anti-SS-B antibodies, as well as hypergammaglobulinaemia, RF, elevated ESR, and exacerbated dry eye symptoms assessed with Schirmer test and ocular staining score (OSS) assessment.
ANA antibodies correlated positively with FS (rho = 0.322) and ESSDAI (rho = 0.507).
In the dryness group (II) the presence of autoantibodies, although in a far lower percentage, was revealed as: ANA (N = 9; 24%), anti- SS-A (N = 12; 32%) anti-SS-B (N = 4; 11%) RF (N = 5; 14%), ACPA (N = 3; 8.1%), ACA (N = 0), cryoglobulins (N = 2; 7.7%).
In comparative analysis no statistically significant differences between frequency of ACPA and ACA occurrence in both groups was revealed (Table IV).
Table IV.
ACA and ACPA antibody occurrence in groups I and II
| Sjögren’s syndrome | Symptoms of dryness | p* | |||||
|---|---|---|---|---|---|---|---|
| N | number | percent (%) | N | number | percent (%) | ||
| ACA+ | 71 | 3 | 4 | 33 | 0 | 0 | 0.314 |
| ACPA+ | 75 | 10 | 13 | 37 | 3 | 8 | 0.318 |
Fisher’s test
There was a statistically significant positive correlation between the presence of ACPA antibodies in pSS patients with arthritis occurrence (Table V). There was no correlation between the presence of ACPA antibodies and disease activity measured with ESSDAI (p = 0.749).
Table V.
Correlation between the presence of ACPA antibodies in pSS patients with the arthritis occurrence
| pSS | ACPA+ | ACPA– | p | ||||
|---|---|---|---|---|---|---|---|
| N | number | percent (%) | N | number | percent (%) | ||
| Arthritis | 10 | 6 | 60 | 64 | 9 | 14 | 0.003 |
In the patients from group II with ACPA positive arthritis was not revealed (p = 0.838).
In group I anti-SS-A and anti-SS-B antibodies, RF, and FS value had statistically significant positive correlation; respectively, p = 0.046; p = 0.015; p = 0.71; p = 0.036. There was no such correlation for ANA presence or for gammaglobulin concentration.
Discussion
Analysis of the group with diagnosed pSS shows that in 88% of the ANA group were present in titre 1 : 320 and in 5% in the lower titre – 1 : 160, such results being consistent with the previous reports of other researchers [15, 16].
In all individuals with ANA titre 1 : 160, anti-SS-A antibodies were also present. This suggests that further diagnostics of the immune profile should be employed in the case of lower ANA titres. Positive correlation of ANA titres with FS, hypergammaglobulinaemia, decreased C4 complement component (data not shown), and elevated ESSDAI > 5 suggests the role of ANA in pSS, local inflammation, and disease activity.
The role of anti-SS-A in pSS has been widely described, but their incidence in pSS has been differently reported: from around 40% [17] up to 70% [18].
In the present study, anti-SS-A antibodies were found in 88% of patients from the group with diagnosed pSS. Among these patients, anti-SS-A antibodies revealed high binding strength in 56% (as assessed using semiquantitative method). In group II anti-SS-A antibodies were observed in 12% of individuals only. This indicates that anti-SS-A antibodies are a valuable marker for pSS diagnosis (p = 0.004). Such a conclusion was anticipated in the current EULAR/ACR criteria [19], in which anti-SS-A antibodies were left as the sole immunologic marker for pSS. A positive correlation between ESSDAI ≥ 5 and the presence of anti-SS-A antibodies (p = 0.046) and anti-SS-B antibodies (p = 0.015) was confirmed in the study group of patients with pSS.
Thus, it was confirmed that the presence of these autoantibodies was associated with more severe disease and multi-organ involvement [20, 21].
Antibodies to SS-B antigen were present in 49% of patients with pSS, but they did not appear in the pSS alone, without the presence of anti-SS-A antibodies. This indicates a strong correlation between anti-SS-B antibodies and anti-SS-A antibodies in pSS. The above described relationship was also presented in previous studies, e.g. Theander et al. [22].
These investigators also noted the very rare separate occurrence of anti-SS-B antibodies [23].
The occurrence of other autoantibodies in pSS, CENP-B in particular, as well as ACA, has been reported in the literature. In group I ACA were present in approximately 4%, a frequency corresponding to those reported by other researchers; they were not present in group II. Tests were performed for the presence of CENP-A and CENP-B only because the tests for anti-CENP-C antibodies were not available for the research, this being a limitation of the presented work.
The relationship between lung lesions (such as active interstitial changes or fibrosis) and ACA presence has not been demonstrated, but the ACA+ group (three persons) was too small to perform a complete, reliable statistical analysis. Research performed by other authors on larger patient groups showed a link between the presence of ACA antibodies and the occlusion of exocrine glands, and also showed that in the ACA+ group anti-SS-A and anti-SS-B autoantibodies were less common [20]. In the present work, in three individuals with ACA, anti-SS-A antibodies were also found.
It is known that one of the clinical signs of pSS may be non-destructive arthritis. In this work, most of the subjects had arthralgia (84%) and 13% of group I (N = 10) had ACPA antibodies. From the ACPA-positive pSS patients, 60% (N = 6) showed non-destructive arthritis.
ACPA antibodies were found in low concentrations, and patients with ACPA+ met certain criteria for the diagnosis of pSS. It appears, however, from the observation of other researchers, that patients with pSS and the presence of ACPA antibodies require further follow-up concerning possible development of rheumatoid arthritis [24].
In the He et al. [25] observation pSS preceded the development of rheumatoid arthritis by from 9 to even 29 years.
According to some research, the presence of anticentromere antibodies in patients with pSS may be associated with increased interstitial lung disease (ILD), Raynaud’s syndrome, and dysphagia [9]. It is therefore suggested that this group may be a separate subgroup of patients with pSS [26]. In the presented analysis, however, we did not show similar relationships.
Conclusions
The significance of the presence of various autoantibodies in pSS, specific for other connective tissue disorders, should be a subject for further studies. The presented work also confirms that the presence of ACPA antibodies in patients with pSS may be associated with increased incidence of arthritis. These patients should be monitored for the increased risk of developing RA (SS/RA) and for the risk of developing active disease with higher disease activity (ESSDAI). The indication of the presence of other specific autoantibodies in pSS may suggest that another systemic disease coexists with pSS (overlap syndrome) or that the Sjögren’s syndrome is in fact secondary to another systemic disease.
The present work leads to the following conclusions:
ANA antibodies should be considered in pSS diagnosis also in titres below 1 : 320, but the most important immunological marker for pSS is the presence of anti-SS-A antibodies,
presence of anti-SS-A antibodies correlates with higher intensity of the disease (ESSDAI) and higher FS,
it was demonstrated that the incidence of arthritis in ACPA+ pSS patients is higher than in ACPA– patients (p = 0.003) [3, 4],
the correlation between the presence of the ACPA antibodies and occurrence of arthritis in patients with sicca syndrome, but no diagnosis of the pSS, was not observed in the studied group.
Footnotes
The authors declare no conflict of interest.
Grant National Science Centre 2012/05/N/NZ5/02838.
References
- 1.Youinou P, Pers JO. Disturbance of cytokine networks in Sjögren’s syndrome. Arthritis Res Ther. 2011;13:227. doi: 10.1186/ar3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maślińska M, Przygodzka M, Kwiatkowska B, Sikorska-Siudek K. Sjögren’s syndrome: still not fully understood disease. Rheumatol Int. 2015;35:233–241. doi: 10.1007/s00296-014-3072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kroese FG, Abdulahad WH, Haacke E, et al. B-cell hyperactivity in primary Sjögren’s syndrome. Exp Rev Clin Immunol. 2014;10:483–499. doi: 10.1586/1744666X.2014.891439. [DOI] [PubMed] [Google Scholar]
- 4.Retamozo S, Brito-Zerón P, Gandía, et al. In: Immunological Tests in Primary Sjögren Syndrome. Ramos-Casals M, Stone JH, Moutsopoulos HM, editors. Springer, London: Sjögren’s Syndrome.; 2012. pp. 402–403. [Google Scholar]
- 5.Huang YF, Cheng Q, Jiang CM, et al. The immune factors involved in the pathogenesis, diagnosis, and treatment of Sjogren’s syndrome. Clin Dev Immunol. 2013;2013:160491. doi: 10.1155/2013/160491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dugar M, Cox S, Limaye V, et al. Diagnostic utility of anti-Ro52 detection in systemic autoimmunity. Postgrad Med J. 2010;86:79–82. doi: 10.1136/pgmj.2009.089656. [DOI] [PubMed] [Google Scholar]
- 7.Yamagata H, Akizuki M, Tojo T, Homma M. Anti-Ro/SSA and -La/SSB antibodies in patients with connective tissue diseases. Scand J Rheumatol Suppl. 1986;61:98–101. [PubMed] [Google Scholar]
- 8.Rao L, Liu G, Li Ch, et al. Specificity of anti-SSB as a diagnostic marker for the classification of systemic lupus erythematosus. Exp Ther Med. 2013;5:1710–1714. doi: 10.3892/etm.2013.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bournia VK, Diamanti KD, Vlachoyiannopoulos PG, Moutsopoulos HM. Anticentromere antibody positive Sjögren’s Syndrome: a retrospective descriptive analysis. Arthritis Res Ther. 2010;12:R47. doi: 10.1186/ar2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Payet J, Goulvestre C, Bialé L, et al. Anticyclic citrullinated peptide antibodies in rheumatoid and nonrheumatoid rheumatic disorders: experience with 1162 patients. J Rheumatol. 2014;41:2395–2402. doi: 10.3899/jrheum.131375. [DOI] [PubMed] [Google Scholar]
- 11.Gelber AC, Pillemer SR, Baum BJ, et al. Distinct recognition of antibodies to centromere proteins in primary Sjogren’s syndrome compared with limited scleroderma. Ann Rheum Dis. 2006;65:1028–1032. doi: 10.1136/ard.2005.046003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song G, Hu C, Zhu H, et al. New centromere autoantigens identified in systemic sclerosis using centromere protein microarrays. Rheumatology. 2013;40:461–468. doi: 10.3899/jrheum.120264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiboski SC, Shiboski CH, Criswell L, et al. American College of Rheumatology classification criteria for Sjögren’s syndrome: a data-driven, expert consensus approach in the Sjögren’s International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken) 2012;64:475–487. doi: 10.1002/acr.21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61:554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kyriakidis NC, Kapsogeorgou EK, Tzioufas AG. A comprehensive review of autoantibodies in primary Sjogren’s syndrome: clinical phenotypes and regulatory mechanisms. J Autoimmun. 2003;51 C:67–74. doi: 10.1016/j.jaut.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Nardi N, Brito-Zerón P, Ramos-Casals M, et al. Circulating auto-antibodies against nuclear and non-nuclear antigens in primary Sjögren’s syndrome: prevalence and clinical significance in 335 patients. Clin Rheum. 2006;25:341–346. doi: 10.1007/s10067-005-0059-3. [DOI] [PubMed] [Google Scholar]
- 17.Routsias JG, Tzioufas AG. Sjögren’s syndrome – study of autoantigens and autoantibodies. Clin Rev Allergy Immunol. 2007;32:238–251. doi: 10.1007/s12016-007-8003-8. [DOI] [PubMed] [Google Scholar]
- 18.Abrol E, González-Pulido C, Praena-Fernández JM, Isenberg DA. A retrospective study of long-term outcomes in 152 patients with primary Sjogren’s syndrome: 25-year experience. Clin Med (Lond) 2014;14:157–164. doi: 10.7861/clinmedicine.14-2-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shiboski CH, Shiboski SC, Seror R, et al. 2016 American College of Rheumatology/European League Against Rheumatism Classification Criteria for Primary Sjögren’s Syndrome. A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheum. 2017;69:35–45. doi: 10.1002/art.39859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baer AN, Medrano L, McAdams-DeMarco M, Gniadek TJ. Anti-centromere antibodies are associated with more severe exocrine glandular dysfunction in Sjögren’s syndrome: Analysis of the Sjögren’s International Collaborative Clinical Alliance cohort. Arthritis Care Res (Hoboken) 2016;68:1554–1559. doi: 10.1002/acr.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang YF, Cheng Q, Jiang CM, et al. The immune factors involved in the pathogenesis, diagnosis, and treatment of Sjogren’s syndrome. Clin Dev Immunol. 2013;2013:160491. doi: 10.1155/2013/160491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Theander E, Jonsson R, Sjöström B, et al. Prediction of Sjogren’s syndrome years before diagnosis and identification of patients with early onset and severe disease course by auto-antibody profiling. Arthritis Rheum. 2015;67:2427–2436. doi: 10.1002/art.39214. [DOI] [PubMed] [Google Scholar]
- 23.Salliot C, Gottenberg JE, Bengoufa D, et al. Anticentromere antibodies identify patients with Sjögren’s syndrome and autoimmune overlap syndrome. J Rheumatol. 2007;34:2253–2258. [PubMed] [Google Scholar]
- 24.Atzeni F, Sarzi-Puttini P, Lama N, et al. Anti-cyclic citrullinated peptide antibodies in primary Sjögren syndrome may be associated with non-erosive synovitis. Arthritis Res Ther. 2008;10:R51. doi: 10.1186/ar2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.He J, Ding Y, Feng M, et al. Characteristics of Sjögren’s syndrome in rheumatoid arthritis. Rheumatology (Oxford) 2013;52:1084–1089. doi: 10.1093/rheumatology/kes374. [DOI] [PubMed] [Google Scholar]
- 26.Nakamura H, Kawakami A, Hayashi T. Anti-centromere antibody-seropositive Sjögren’s syndrome differs from conventional subgroup in clinical and pathological study. BMC Musculoskelet Disord. 2010;11:140. doi: 10.1186/1471-2474-11-140. [DOI] [PMC free article] [PubMed] [Google Scholar]