Abstract
Cerebral microbleeds are increasingly recognised as biomarkers of small vessel disease. Several preclinical and clinical studies have suggested that chronic disruption of the blood–brain barrier is one of the mechanisms for the development of cerebral microbleeds.
A 51-year-old man experienced two left parieto-occipital lobar intracerebral haemorrhages (ICHs) in the timespan of 2 years. Multiple microbleeds surrounding the two haemorrhages were found on MRI, but not at location distant from the haemorrhages. Ten months after the last haemorrhage, an MRI demonstrated a right occipital focus of contrast enhancement. Twenty months after the last ICH, a new cerebral microbleed had developed exactly at the location of the earlier contrast enhancement.
This case demonstrates that blood–brain barrier disruption may be an important factor preceding the development of cerebral microbleeds.
Keywords: Stroke, Radiology
Background
Cerebral microbleeds (CMBs) are small areas of blood product deposition in the brain, seen as signal voids on MR sequences susceptible to the paramagnetic effects of bloods products, such as T2*-weighted gradient echo. Since the initial report by Fazekas et al,1 they have received increasing attention as biomarkers of cerebral small vessel disease. They are most prevalent in patients with recurrent intracerebral haemorrhage (ICH)2 but also observed in patients with ischaemic stroke,2 cognitive decline3 and normal ageing.4 Two of the main underlying disease processes leading to the development of CMBs are cerebral amyloid angiopathy (CAA) and hypertensive vasculopathy.5
Disturbance of the blood–brain barrier has previously been implicated as one of the mechanisms involved in the development of CMBs.6 7 Indeed, CMBs have been demonstrated in other conditions with blood–brain barrier disruption apart from small vessel disease, including infective endocarditis8 and radiation therapy.9 A recent study showed that after acute lacunar stroke, the presence of biomarkers for blood–brain barrier disruption (S100B and RAGE) in plasma was associated with an increasing number of CMBs.10
Case presentation
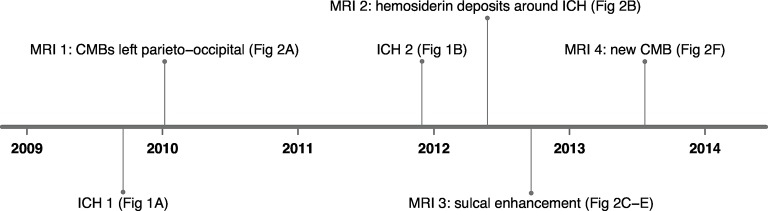
A 51-year-old Caucasian right-handed male with a medical history of diabetes mellitus type 2 and hypercholesterolaemia and a documented normal blood pressure presented with acute headache and a right homonymous quadrantanopia. He never smoked, had no history of alcohol or drug abuse and the family history was unremarkable. CT imaging revealed a haemorrhage in the left parieto-occipital region (figure 1A). On MRI 1 (Philips Healthcare), no underlying cause was identified other than some indication of small vessel disease consisting of mild white matter lesions, most prominent adjacent to the posterior horn of the left ventricle (Fazekas 1). Two years later, the patient reported fatigue, abnormal coordination of his right arm, and difficulties with calculations and reading. A CT scan showed a recurrent ICH in the same region, cranial to the tissue defect from the first haematoma (figure 1B). CT angiography and a digital subtraction angiography performed at that time revealed no underlying macrovascular cause. Eight months after the second ICH, the patient was referred to our centre. At neurological examination, the patient displayed difficulties performing arithmetic tasks. Further examination was unremarkable.
Figure 1.
(A) First ICH and (B) second ICH on CT. ICH, intracerebral haemorrhage.
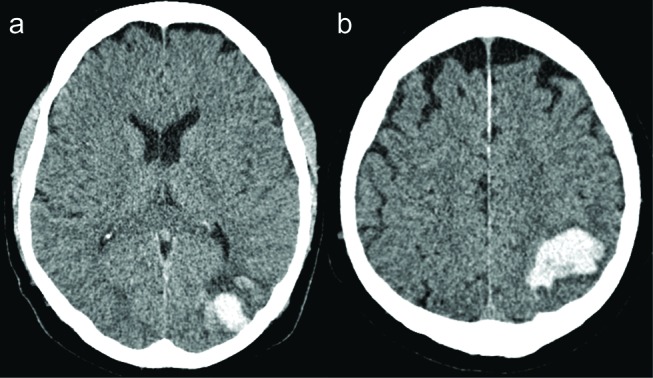
After the first ICH, MRI (MRI 1, figure 2A) with a T2* sequence demonstrated numerous round signal voids in the area of the haematoma, consistent with CMBs and no signs indicative of a tumour or cavernous malformation. A subsequent MRI 6 months after the second ICH (MRI 2, figure 2B, Siemens) showed an increase in the number of signal voids in the area of the two haematomas, consistent with the deposition of new blood products. No additional microbleeds were seen at locations distant from the two haemorrhages.
Figure 2.
MRI 1: 1T T2* gradient echo 4 months after the first ICH (A), MRI 2: 1T T2* gradient echo 6 months after the second ICH (B). MRI 3: 7T three-dimensional fluid-attenuated inversion recovery (10 month after second ICH) before contrast (C) and after contrast (D): small focus of enhancement (inset). MRI 3: 7T T2* gradient echo: no microbleed in the right occipital lobe (E). MRI 4: 3T susceptibility-weighted imaging 10 months later showing new microbleed in the same location as previous enhancement (inset) (F). ICH, intracerebral haemorrhage.
Ten months after the second ICH, we performed a high-field 7 Tesla MR scan (MRI 3, Philips Healthcare). As we had not been able to establish a cause for the ICHs in this patient, we used high-field MRI to detect CMBs or superficial siderosis that would support a diagnosis of CAA with maximum sensitivity. This MRI included a dual echo T2*-weighted sequence. We reconstructed minimum intensity projections of the first and second echo. In addition, we included precontrast and postcontrast 3D fluid-attenuated inversion recovery (FLAIR) images to investigate signs of blood–brain barrier leakage as part of our scientific interest in ICH. We found no additional CMBs (figure 2E) or superficial siderosis. On the 3D FLAIR acquired 10 min after administration of gadolinium contrast, we found one sulcal focus of contrast enhancement in the right occipital region (figure 2C,D). In this region, there were no signs of a parenchymal lesion at that time. In addition, several foci of enhancement were observed in the left parieto-occipital region, adjacent to the haemorrhage. We performed a 3 Tesla MR scan (MRI 4, Philips Healthcare) 20 months after the second ICH to determine whether new CMBs had developed over time that would lend support to the diagnosis of CAA. On this examination, a CMB had developed in the right occipital region, exactly at the location where we had previously observed the contrast enhancement (figure 2F). No gadolinium was administered during this examination.
Figure 3 summarises the timeline of clinical symptoms and CT and MR scans.
Figure 3.
A timeline illustrating the timing of the 2 ICHs and the 4 MRIs. CMB, cerebral microbleed; ICH, intracerebral haemorrhage.
Differential diagnosis
At the time of referral, a macrovascular cause and a tumour had already been excluded. The clinical symptoms and course had not been suggestive of vasculitis or endocarditis, and the family history was negative for genetic causes of ICH or its associated symptoms (HCHWA-D, CADASIL, COL4A1). Hypertension-related vasculopathy was unlikely because of the lobar location of the ICHs and the documented absence of high blood pressure. An overview of the differential diagnosis of ICH is listed in box.
Box. Differential diagnosis of non-traumatic intracerebral haemorrhage.
-
Spontaneous intracerebral haemorrhage
Hypertension-related small vessel disease
Cerebral amyloid angiopathy
-
Secondary intracerebral haemorrhage
Arteriovenous malformation
Aneurysm
Cerebral venous sinus thrombosis
Cerebral cavernous malformation
Dural arteriovenous fistula
Vasculitis
Moyamoya disease
Intracranial dissection
Coagulopathy
Haemorrhagic transformation of ischaemic stroke
Neoplasm
Drug abuse
Genetic: HCHWA-D, CADASIL, COL4A1
Despite the relatively young age of the patient at the time of his first haemorrhage, we concluded that the most likely cause of his disease was CAA, based on the absence of hypertension, the lobar parieto-occipital location of the haemorrhages, the occurrence of a new occipital CMB in the contralateral hemisphere over time and the absence of macrovascular causes. No other causes of microangiopathy were identified. As he was below 55 years of age, he did not fulfil the modified Boston criteria for probable CAA.11 Because of the lack of therapeutic consequences, we did not perform a biopsy to confirm the diagnosis.
Outcome and follow-up
The patient remained clinically stable at follow-up 33 months after the last ICH.
Discussion
We observed the emergence of a new cerebral microbleed in the exact same area where contrast enhancement was found 10 months earlier in a patient with a history of two lobar ICHs in the contralateral hemisphere.
Gadolinium contrast enhancement is a known biomarker of blood–brain barrier disruption and has previously been used to demonstrate blood–brain barrier disruption in patients with ICH in the acute phase,12 Alzheimer’s disease,13 lacunar stroke and white matter lesions.14 Studies of spontaneously hypertensive stroke-prone rats have suggested that blood–brain barrier hyperpermeability precedes haemorrhage and occurs early in the pathological cascade of cerebral small vessel disease.6 15 Stasis of erythrocytes in the microvasculature may be the first histologically observable sign of the pathological cascade that involves blood–brain barrier damage and subsequently microthrombosis, microbleeds, macrobleeds and infarcts.6 16
In a study of 19 patients with CAA, two patients showed blood–brain barrier leakage of gadolinium.17 In both patients, there were severe white matter changes and one of the patients had presented with a posterior reversible encephalopathic syndrome 10 months earlier.17 In the same study, the authors demonstrated that in isolated rat brain microvessels amyloid-β40 decreased the expression of tight junction proteins and increased expression of matrix metalloproteinase 2 and 9, both indicating increased blood–brain barrier permeability.17 Moreover, permeability was increased in brain microvessels from transgenic human amyloid precursor protein-overexpressing mice compared with microvessels from wild-type controls.17
Our case is the first to demonstrate in vivo that asymptomatic focal gadolinium leakage is followed by emergence of a microbleed at exactly the location of the gadolinium leakage several months later. It also illustrates that patients with a likely diagnosis of CAA may experience their first ICH before the age of 55 years—the minimum age to fulfil the modified Boston criteria for CAA.
Our case observation lends further support to the hypothesis that the blood–brain barrier disruption is associated with small vessel diseases that cause ICH. Treatment aimed at prevention or restoring of blood–brain leakage may be a potential treatment target to prevent haemorrhage in patients with ICH due to small vessel disease such as CAA and hypertensive vasculopathy.
Learning points.
New microbleeds in patients with small vessel disease-related intracerebral haemorrhage may be preceded by blood–brain barrier disruption.
Restoration of the blood–brain barrier should be evaluated as a potential novel treatment target to prevent haemorrhage in patients at risk of intracerebral haemorrhage caused by small vessel disease, including cerebral amyloid angiopathy.
Relatively young age does not preclude the clinical phenotype of cerebral amyloid angiopathy.
Footnotes
Contributors: CJMK conceived the case report. All authors contributed to the analysis and interpretation of the case. KMvN wrote the first draft of the manuscript. CJMK and JH critically revised the manuscripts intellectual content. All authors read and approved the final version for publication.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Fazekas F, Kleinert R, Roob G, et al. . Histopathologic analysis of foci of signal loss on gradient-echo T2*-weighted MR images in patients with spontaneous intracerebral hemorrhage: evidence of microangiopathy-related microbleeds. AJNR Am J Neuroradiol 1999;20:637–42. [PMC free article] [PubMed] [Google Scholar]
- 2.Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain 2007;130:1988–2003. 10.1093/brain/awl387 [DOI] [PubMed] [Google Scholar]
- 3.Poels MMF, Ikram MA, van der Lugt A, et al. . Cerebral microbleeds are associated with worse cognitive function: the Rotterdam scan study. Neurology 2012;78:326–33. 10.1212/WNL.0b013e3182452928 [DOI] [PubMed] [Google Scholar]
- 4.Poels MM, Vernooij MW, Ikram MA, et al. . Prevalence and risk factors of cerebral microbleeds: an update of the Rotterdam scan study. Stroke 2010;41:S103–S106. 10.1161/STROKEAHA.110.595181 [DOI] [PubMed] [Google Scholar]
- 5.Greenberg SM, Vernooij MW, Cordonnier C, et al. . Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol 2009;8:165–74. 10.1016/S1474-4422(09)70013-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schreiber S, Bueche CZ, Garz C, et al. . Blood brain barrier breakdown as the starting point of cerebral small vessel disease? - New insights from a rat model. Exp Transl Stroke Med 2013;5:4 10.1186/2040-7378-5-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher M, French S, Ji P, et al. . Cerebral microbleeds in the elderly: a pathological analysis. Stroke 2010;41:2782–5. 10.1161/STROKEAHA.110.593657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein I, Iung B, Labreuche J, et al. . Cerebral microbleeds are frequent in infective endocarditis: a case-control study. Stroke 2009;40:3461–5. 10.1161/STROKEAHA.109.562546 [DOI] [PubMed] [Google Scholar]
- 9.Lupo JM, Chuang CF, Chang SM, et al. . 7-Tesla susceptibility-weighted imaging to assess the effects of radiotherapy on normal-appearing brain in patients with glioma. Int J Radiat Oncol Biol Phys 2012;82:e493–e500. 10.1016/j.ijrobp.2011.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao L, Sun W, Lan W, et al. . Correlation between cerebral microbleeds and S100B/RAGE in acute lacunar stroke patients. J Neurol Sci 2014;340:208–12. 10.1016/j.jns.2014.03.006 [DOI] [PubMed] [Google Scholar]
- 11.Linn J, Halpin A, Demaerel P, et al. . Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 2010;74:1346–50. 10.1212/WNL.0b013e3181dad605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kidwell CS, Burgess R, Menon R, et al. . Hyperacute injury marker (HARM) in primary hemorrhage: a distinct form of CNS barrier disruption. Neurology 2011;77:1725–8. 10.1212/WNL.0b013e318236ef46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Starr JM, Farrall AJ, Armitage P, et al. . Blood–brain barrier permeability in Alzheimer's disease: a case–control MRI study. Psychiatry Res 2009;17141:232–41. [DOI] [PubMed] [Google Scholar]
- 14.Topakian R, Barrick TR, Howe FA, et al. . Blood-brain barrier permeability is increased in normal-appearing white matter in patients with lacunar stroke and leucoaraiosis. J Neurol Neurosurg Psychiatry 2010;81:192–7. 10.1136/jnnp.2009.172072 [DOI] [PubMed] [Google Scholar]
- 15.Lee J-M, Zhai G, Liu Q, et al. . Vascular permeability precedes spontaneous intracerebral hemorrhage in Stroke-Prone spontaneously hypertensive rats. Stroke 2007;38:3289–91. 10.1161/STROKEAHA.107.491621 [DOI] [PubMed] [Google Scholar]
- 16.Schreiber S, Bueche CZ, Garz C, et al. . The pathologic cascade of cerebrovascular lesions in SHRSP: is erythrocyte accumulation an early phase? J Cereb Blood Flow Metab 2012;32:278–90. 10.1038/jcbfm.2011.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartz AM, Bauer B, Soldner EL, et al. . Amyloid-β contributes to blood-brain barrier leakage in transgenic human amyloid precursor protein mice and in humans with cerebral amyloid angiopathy. Stroke 2012;43:514–23. 10.1161/STROKEAHA.111.627562 [DOI] [PMC free article] [PubMed] [Google Scholar]