Abstract
A 67-year-old man was referred with a history of a right-sided neck lump and dysphonia, secondary to a lesion in the thyroid gland. After undergoing a total thyroidectomy, he was found to have an exceedingly rare combination of follicular carcinoma, insular carcinoma, thyrolipomatosis and an amyloid goitre in his thyroid gland. He subsequently underwent further radioactive iodine ablation and has been in remission. He was also later incidentally diagnosed with systemic amyloidosis, which explained the amyloid deposition in his thyroid gland.
Keywords: Thyroid disease, Endocrine cancer, Head and neck cancer, Pathology, Head and neck surgery
Background
Diffuse thyrolipomatosis is an extremely rare condition characterised by diffuse infiltration of the thyroid stroma by mature adipose tissue.1 2 Fewer than 15 cases have been reported in the literature since its first description by Dhayagude in 1942.3 It has been associated with systemic amyloidosis, where the fat deposition produces an ‘amyloid goitre.’4 To date, only two cases have been associated with carcinomas, both being the papillary subtype.1 5
Insular carcinoma (also known as poorly differentiated thyroid carcinoma, PDTC) is an uncommon subtype of thyroid cancer that lies both morphologically and behaviourally between well-differentiated and undifferentiated/anaplastic carcinomas.6 7 They make up about 1.8%–15% of all thyroid carcinomas, and are generally poorly understood due to their rarity.8 9
Here, we describe the first known case of a PDTC arising completely within a follicular carcinoma, on a background of diffuse thyrolipomatosis with amyloid deposition, likely secondary to Amyloid A (AA) amyloidosis. Neither of these tumours has been associated with diffuse thyrolipomatosis.
Case presentation
A 67-year-old man was referred to the otolaryngology service with a 2-week history of dysphonia and right-sided neck lump. This was discovered incidentally while the patient was admitted to hospital for dyspnoea secondary to bronchiectasis. The man was otherwise asymptomatic and had no signs of thoracic inlet obstruction. The patient suffered from chronic renal impairment, which was thought to be secondary to hypertension.
Investigations
An ultrasound of the neck showed a 42×42×37 mm homogeneous nodule in the right thyroid lobe. The thyroid gland was enlarged, with an estimated volume of 186 mL. There were no microcalcifications on the ultrasound scan. A CT scan of the neck again demonstrated this lesion, which was hyperdense with central hypodensity. There was diffuse fatty conversion of the thyroid parenchyma (figure 1). There was no cervical, mediastinal or hilar lymphadenopathy on the CT scan. A fine needle aspiration biopsy of the nodule was non-diagnostic. The thyroid function tests were within normal limits.
Figure 1.
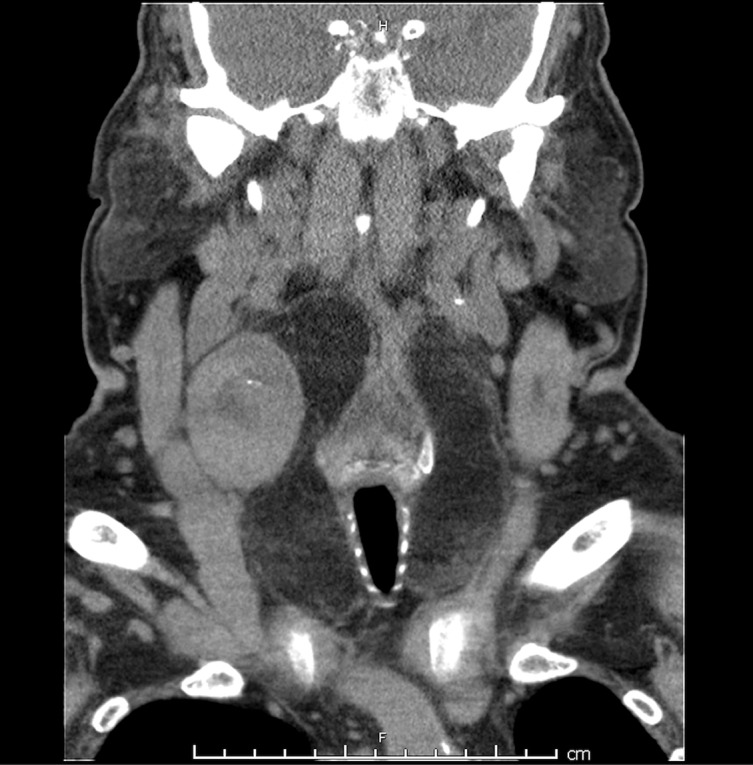
Coronal section of the patient’s neck CT scan, demonstrating the large fatty goitre and a solid lesion in the right lobe of the thyroid.
Due to the suspicious radiological appearance of the nodule on CT and ultrasound, a right hemithyroidectomy was performed to obtain a histological diagnosis, with a view of proceeding to a staged completion thyroidectomy pending histological confirmation of malignancy. Intraoperatively, the right thyroid lesion was found to have central necrosis. The lesion was not invading the recurrent laryngeal nerve or trachea.
The histological examination of the right hemithyroid showed a 45×40×35 mm nodule. The lesion was consistent with a follicular carcinoma, showing signs of lymphovascular invasion. Within the follicular neoplasm, there was also a patchy distribution of solid nests, islands and trabeculae punctuated by variable numbers of small abortive follicles (figures 2–4). The tumour cells within these areas stained positive for HBME-1 and thyroid transcription factor-1 (TTF-1). Chromogranin, synaptophysin and carcinoembryonic antigen (CEA) staining were all negative. There were no signs of extrathyroidal extension. Based on the AJCC guidelines (seventh edition), this was in keeping with a pT2 N0 insular/PDTC arising within the follicular carcinoma.10
Figure 2.
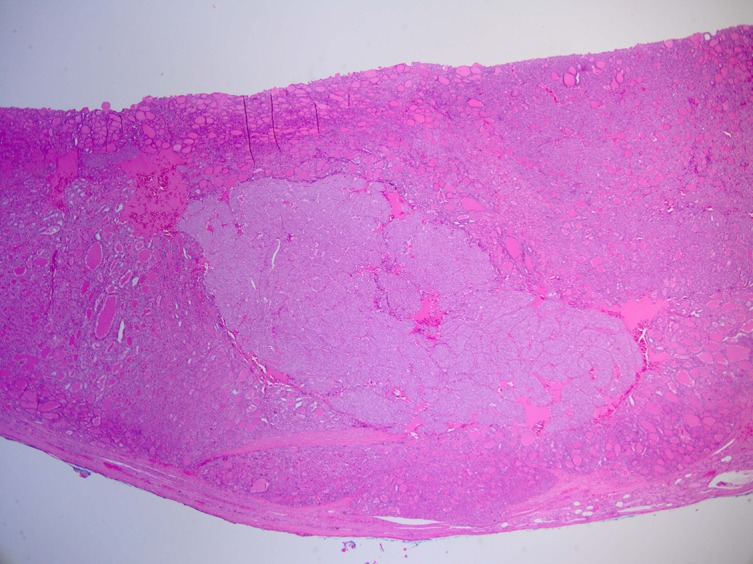
Insular carcinoma completely surrounded by follicular carcinoma.
Figure 3.
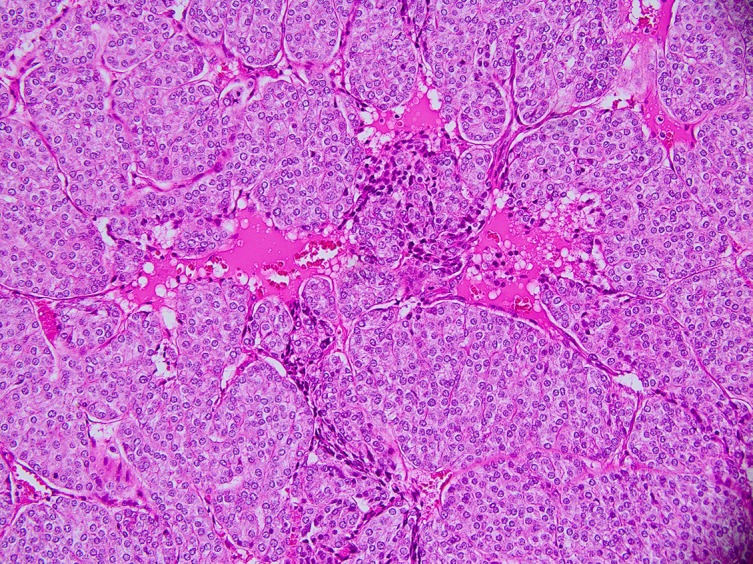
Close-up of insular carcinoma, showing high mitotic activity and an insular growth pattern.
Figure 4.
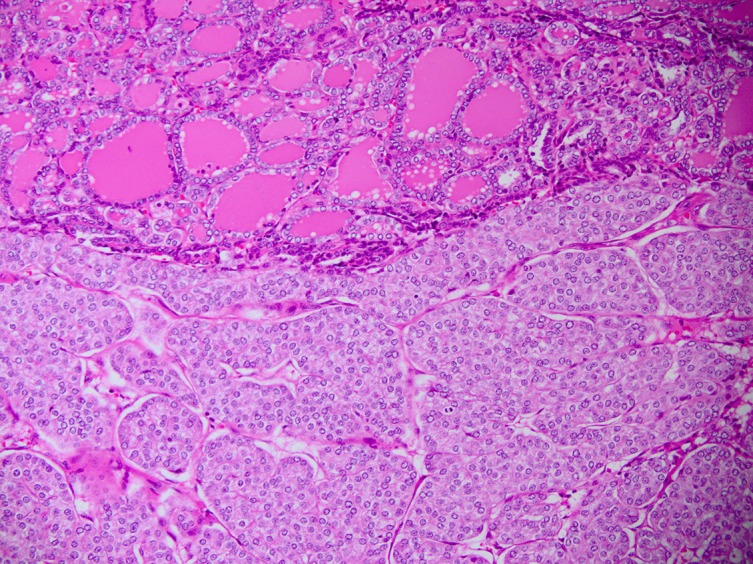
Close-up of both insular (bottom) and follicular (top) carcinomas.
Outside of the thyroid nodule, the thyroid gland showed follicles within a stroma that had diffuse adipose tissue infiltration, that is, thyrolipomatosis. Moreover, there was also abnormal amyloid deposition within the perifollicular areas (figure 5). Further staining of this favoured AA-type amyloidosis over the amyloid light-chain (AL) type. Investigations for chronic inflammatory conditions were negative.
Figure 5.
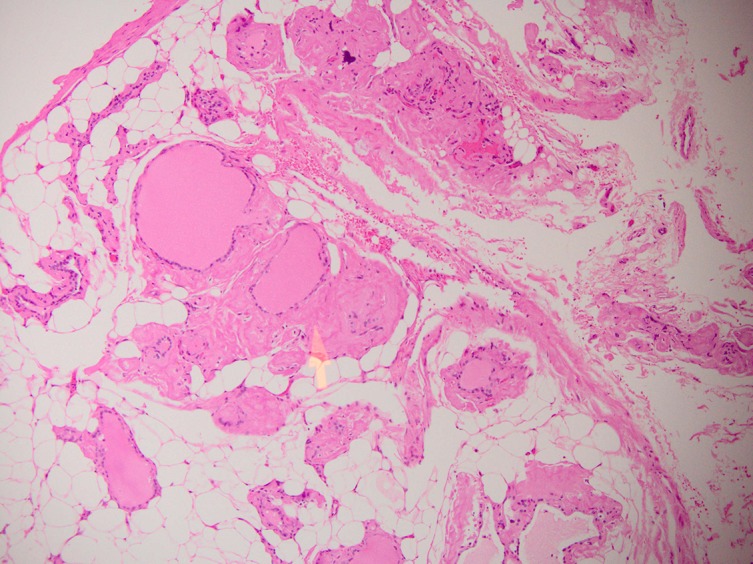
H+E stained section of the thyroid parenchyma not involved by cancer. Diffuse adipose infiltration of the parenchyma is seen. The yellow arrow shows amyloid protein deposition.
Treatment
A subsequent completion left hemithyroidectomy was performed after the histological diagnosis of malignancy, largely due to the high-risk histological features identified on the initial hemithyroidectomy. There were also diffuse thyrolipomatosis and amyloid deposition but no signs of malignancy in the left thyroid lobe. The immediate postoperative recovery was complicated by post-thyroidectomy hypocalcaemia, which stabilised with calcium supplementation. Due to the intermediate-high risk for recurrence in PDTC, the patient then completed a course of adjuvant I-131 radioablative therapy (3623 mBq) 1 month after the completion thyroidectomy. He was also commenced on thyroid stimulating hormone (TSH) suppression therapy—thyroxine doses were titrated against thyroid-stimulating hormone levels, aiming for a TSH level of less than 0.1 for 10 years.11
Outcome and follow-up
After the therapy, surveillance was performed clinically and also with thyroglobulin monitoring. There have been no signs of recurrence at 1 year from the initial surgery. Incidentally, he had a renal biopsy 3 months after the thyroidectomy due to a deterioration of his renal function. The biopsy was consistent with AA amyloid nephropathy. No chronic inflammatory disease has been found on screening investigations to date.
Discussion
According to the Turin criteria, PDTC is recognised by the presence of (1) a solid/trabecular/insular growth pattern, (2) absence of conventional nuclear features of papillary carcinoma, and (3) presence of convoluted nuclei, mitotic activity or necrosis.12 PDTC has generally been associated with poorer outcomes than well-differentiated carcinomas, with a 5-year disease-specific mortality rate ranging from 17% to 34% in the literature.8 12–14 PDTCs that do not produce thyroglobulin or take up radioiodine have a worse prognosis.15 These tumours are known to arise de novo or from pre-existing follicular and papillary carcinomas, although little is known about the specific boundaries and relationships between well differentiated and PDTC.12 In this case, the complete nesting of the PDTC within the follicular carcinoma would suggest dedifferentiation of the follicular neoplasm into PDTC. Unfortunately, we were unable to obtain further immunohistochemical analysis to further substantiate this.
Amyloid deposition in the thyroid gland is a known phenomenon, commonly seen in medullary thyroid carcinoma.16 This usually occurs when the excess calcitonin produced by the neoplastic C cells aggregates into insoluble fibrils. Rarely, systemic calcitonin amyloidosis has been reported in medullary thyroid carcinoma.17 Systemic amyloidosis is more commonly seen in excess production of immunoglobulin light chains (AL amyloid) in plasma cell neoplasms, or less frequently, an excess of acute-phase proteins (serum amyloid A associated, or AA amyloid). Rarely, the excess of acute-phase proteins that causes AA amyloidosis is associated with malignancies, typically renal cell carcinoma and Hodgkin’s lymphoma. However, AA amyloidosis has been described in a wide variety of other carcinomas, such as that of the bladder, uterus and the oesophagus.18 In this patient’s case, it is possible that the AA amyloidosis could have arisen as a result of the chronic inflammation precipitated by the thyroid malignancy.
The mechanism of fatty infiltration of the thyroid gland in systemic amyloidosis is unclear. Based on observations in other solid organs, Schröder et al had suggested that ischaemia due to capillary destruction by amyloid deposition triggers metaplasia of stromal fibroblasts, causing a fatty change.19 In this case, the presence of an invasive neoplasm may have further contributed to this via vascular invasion. Apart from the thyroid carcinoma, there were no other inflammatory or infective conditions found on investigation. It is uncertain whether the thyroid cancer may have precipitated the AA amyloidosis and thyrolipomatosis. Interestingly, despite the multiple pathologies in this case, the oncological outcome was good.
Learning points.
Suspicious thyroid nodules should be investigated promptly with a combination of clinical examination, imaging and, when indicated, biopsies.
Poorly differentiated thyroid carcinoma is an aggressive type of thyroid cancer that can arise from follicular carcinoma.
Thyrolipomatosis is a rare condition that can occur in amyloidosis and can present as a goitre—its mechanism of pathogenesis is unknown but may be related to hypoxia, and in this case, possibly malignancy.
The presence of amyloid in the thyroid gland, on a background of undiagnosed chronic renal impairment, should raise suspicion for systemic amyloidosis
Footnotes
Contributors: TLL: drafting and primary author of manuscript. HP, SL, RBA: revision and editing of manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Ge Y, Luna MA, Cowan DF, et al. Thyrolipoma and thyrolipomatosis: 5 case reports and historical review of the literature. Ann Diagn Pathol 2009;13:384–9. 10.1016/j.anndiagpath.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 2.Gnepp DR, Ogorzalek JM, Heffess CS. Fat-containing lesions of the thyroid gland. Am J Surg Pathol 1989;13:605–12. 10.1097/00000478-198907000-00009 [DOI] [PubMed] [Google Scholar]
- 3.Dhayagude RG. Massive fatty infiltration in a colloid goiter. Arch Pathol 1942;33:357–60. [Google Scholar]
- 4.James PD. Amyloid goitre. J Clin Pathol 1972;25:683–8. 10.1136/jcp.25.8.683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nandyala HS, Madapuram S, Yadav M, et al. Diffuse lipomatosis of the thyroid gland with papillary microcarcinoma: report of a rare entity. Indian J Pathol Microbiol 2015;58:348–50. 10.4103/0377-4929.162890 [DOI] [PubMed] [Google Scholar]
- 6.Carcangiu ML, Zampi G, Rosai J. Poorly differentiated ("insular") thyroid carcinoma. A reinterpretation of Langhans' "wuchernde Struma". Am J Surg Pathol 1984;8:655–68. [DOI] [PubMed] [Google Scholar]
- 7.Sakamoto A, Kasai N, Sugano H. Poorly differentiated carcinoma of the thyroid. A clinicopathologic entity for a high-risk group of papillary and follicular carcinomas. Cancer 1983;52:1849–55. [DOI] [PubMed] [Google Scholar]
- 8.Papotti M, Bussolati G, Ashfaq F, Vuitch F, Delgado R, Albores‐Saavedra J. Papillary and follicular thyroid carcinomas with an insular component. Cancer 1994;74:2599–600. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez JM, Parrilla P, Moreno A, et al. Insular carcinoma: an infrequent subtype of thyroid Cancer. J Am Coll Surg 1998;187:503–8. 10.1016/S1072-7515(98)00233-6 [DOI] [PubMed] [Google Scholar]
- 10.Edge S, Byrd D, Compton C, et al. ; AJCC Cancer Staging Manual. 7th ed New York: Springer-Verlag, 2010. [Google Scholar]
- 11.Haugen BR, Alexander EK, Bible KC, et al. 2015 american thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid Cancer: the american thyroid Association guidelines Task Force on thyroid nodules and differentiated thyroid Cancer. Thyroid 2016;26:1–133. 10.1089/thy.2015.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Volante M, Collini P, Nikiforov YE, et al. Poorly differentiated thyroid carcinoma: the Turin proposal for the use of uniform diagnostic criteria and an algorithmic diagnostic approach. Am J Surg Pathol 2007;31:1256–64. 10.1097/PAS.0b013e3180309e6a [DOI] [PubMed] [Google Scholar]
- 13.Jung TS, Kim TY, Kim KW, et al. Clinical features and prognostic factors for survival in patients with poorly differentiated thyroid carcinoma and comparison to the patients with the aggressive variants of papillary thyroid carcinoma. Endocr J 2007;54:265–74. 10.1507/endocrj.K06-166 [DOI] [PubMed] [Google Scholar]
- 14.Mizukami Y, Nonomura A, Michigishi T, et al. Poorly differentiated ('insular') carcinoma of the thyroid. Pathol Int 1995;45:663–8. 10.1111/j.1440-1827.1995.tb03519.x [DOI] [PubMed] [Google Scholar]
- 15.Lam KY, Lo CY, Chan KW, et al. Insular and anaplastic carcinoma of the thyroid: a 45-year comparative study at a single institution and a review of the significance of p53 and p21. Ann Surg 2000;231:329–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sletten K, Westermark P, Natvig JB. Characterization of amyloid fibril proteins from medullary carcinoma of the thyroid. J Exp Med 1976;143:993–8. 10.1084/jem.143.4.993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Azzopardi JG, Lehner T. Systemic amyloidosis and malignant disease. J Clin Pathol 1966;19:539–48. 10.1136/jcp.19.6.539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koopman T, Niedlich-den Herder C, Stegeman CA, et al. Kidney involvement in systemic calcitonin Amyloidosis Associated with medullary thyroid carcinoma. Am J Kidney Dis 2017;69:546–9. 10.1053/j.ajkd.2016.09.027 [DOI] [PubMed] [Google Scholar]
- 19.Schröder S, Böcker W, Hüsselmann H, Adenolipoma DH, et al. Adenolipoma (thyrolipoma) of the thyroid gland report of two cases and review of literature. Virchows Arch A Pathol Anat Histopathol 1984;404:99–103. 10.1007/BF00704254 [DOI] [PubMed] [Google Scholar]


