Abstract
We describe the case of a 30-year-old man with pathological myopia with a phakic intraocular lens (IOL) (Visian ICL V4c model; STAAR, Monrovia, California, USA) in situ having complaints of metamorphopsia in the left eye with documented myopic foveoschisis on swept-source optical coherence tomography (DRI OCT Triton; Topcon, Tokyo, Japan). The patient underwent pars plana vitrectomy with internal limiting membrane peeling. This report discusses the intraoperative challenges occurring as a result of increased optical aberrations in the presence of a phakic IOL.
Keywords: Ophthalmology, Retina, Macula
Background
Phakic intraocular lens (pIOL) implantation in patients with myopia is a frequently performed refractive procedure. Pathological myopia is associated with a spectrum of degenerative fundus changes, one of which is myopic traction maculopathy (MTM). Intraocular manipulation, temporary hypotony during the pIOL surgery, superimposed on the changes in vitreous structures in myopes may accelerate the development of posterior segment abnormalities in these patients.1–4 These patients may require retinal surgery to treat the posterior segment pathology.
The presence of a pIOL within the posterior chamber in our patient posed difficulties in visualising the fundus intraoperatively due to aberrations noticed at the edges of the optical zone of the pIOL. We report a case of myopic foveoschisis with pIOL in situ and discuss the intraoperative challenges faced. To the best of our knowledge, there are no reports discussing challenges in vitrectomy for MTM with pIOL in situ.
Case presentation
A 30-year-old male patient with pathological myopia presented to our clinic with complaints of gradually progressive metamorphopsia in the left eye for the past 15 months. He gave history of both eye pIOL implantation (Visian ICL V4c model) (ICL; STAAR, Monrovia, California, USA) 18 months prior to presentation, with good uncorrected visual acuity postoperatively. The complains started 3 months after surgery and was gradually progressive, resulting in difficulty in routine activities for the patient.
The patient also gave a history of retinal detachment OD 14 months after pIOL implantation, which was managed with a scleral buckling procedure at another centre 3 months back.
At presentation, the patient had a distance Best Corrected Visual Acuity (BCVA) of OD 6/9 and OS 6/9 and a near vision of N6 in both eyes.
A pIOL with a central aperture was present in the posterior chamber and was well centred in both the eyes (figure 1C). He had clear crystalline lenses in both eyes and the intraocular pressure was 16 mm Hg in OD and 16 mm Hg in OS.
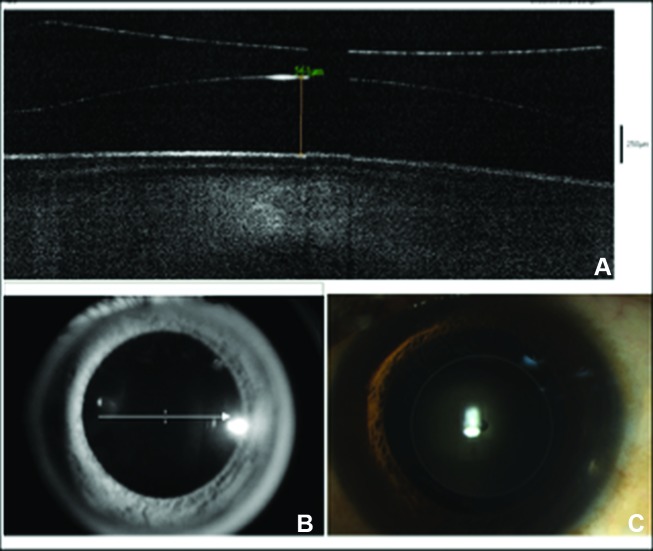
Figure 1.
(A,B) Anterior segment optical coherence tomography demonstrating the phakic intraocular lens (pIOL) in situ and the preoperative vaulting of 561 μm in the left eye. (C) Anterior segment photograph showing pIOL (V4c model) in situ.
Posterior segment OD revealed a tessellated fundus with a tilted disc, a temporal crescent and a posterior staphyloma. An encirclage indent with cryotherapy marks around the retinal breaks on the indent was seen. The retina was well attached. OS showed complete posterior vitreous detachment (PVD) with Weiss ring and a tessellated fundus with a tilted disc, a temporal crescent and a posterior staphyloma. A trilobed shaped outer lamellar hole was present at the fovea.
There was no history of any systemic illness. There was no significant family history.
The fundus findings prior to the pIOL implantation and details of prerefractive surgery work-up were not available.
Investigations
The axial length measurement using optical biometry (IOLMaster 500; Carl Zeiss Meditec, Jena, Germany) was OD 28.98 mm and OS 27.88 mm. Specular microscopy revealed an endothelial cell density of OD 2546 cells/ mm2 and OS 2566 cells/mm2. The anterior segment optical coherence tomography (OCT) (RTVue FD-OCT, Optovue, Fremont, California, USA) revealed a pIOL vaulting of 590 μm and 561 μm OD and OS, respectively (figure 1A,B).
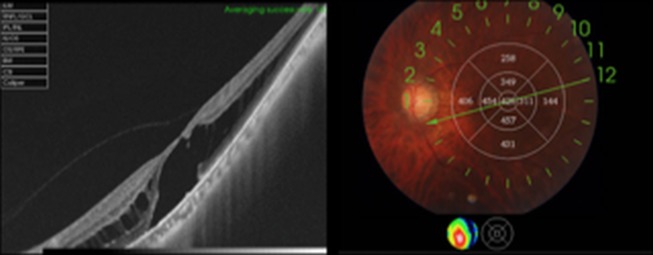
Swept-source optical coherence tomography (SS-OCT) of the left eye demonstrated retinoschisis at the outer plexiform layer with an outer lamellar hole (figure 2).
Figure 2.
Preoperative swept-source optical coherence tomography of the left eye demonstrating myopic foveoschisis with an outer lamellar hole.
Treatment
In view of gradually progressive symptoms hampering routine activities, the patient underwent surgical intervention instead of conservative management. The patient underwent 23-gauge three-port pars plana vitrectomy with triamcinolone-assisted PVD induction. ILM-Blue (brilliant blue G 0.025% in combination with 4% polyethylene glycol; DORC, Zuidland, The Netherlands) assisted fovea-sparing internal limiting membrane (ILM) peeling was done using intraoperative OCT guidance (OCT system attached to the Zeiss Opmi Lumera700/Rescan microscope; Carl Zeiss Meditec). This was followed by 25% SF6 (Sulfur Hexafluoride) injection. Postoperatively the patient was advised prone positioning for 1 day and a short course of topical antibiotics, topical steroids and topical cycloplegics.
Outcome and follow-up
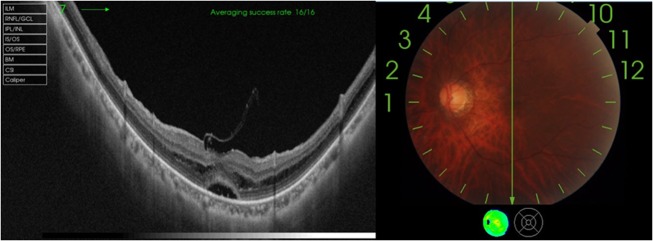
The patient had an uneventful intraoperative and postoperative period. The patient's last follow-up visit was 2 months postsurgery, with best corrected visual acuity of 6/9 and with significant subjective improvement of metamorphopsia. OCT with fundus image shows resolution of retinoschisis, and restoration of foveal contour with remnants of spared ILM seen over fovea (fovea-sparing ILM peeling) and with minimal residual neurosensory detachment (NSD) (figure 3A–C).
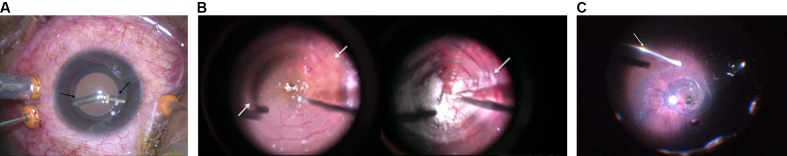
Figure 3.
(A) Arrows marking the blurring of the image of the vitrectomy instruments at the edge of optical zone of the phakic intraocular lens. (B) Ghost images viewed through the Chalam SSV lens (plano-concave) during tricot-assisted removal of vitreoschisis and ILM peeling (fovea-sparing) over the macula. (C) Aberrations noted at the edges while viewing through the MiniQuad XL lens.
Discussion
pIOL implantation is a frequently performed refractive procedure in highly myopic individuals. Rarely retinal breaks leading to retinal detachment have been reported as complications after this procedure.1–4 The posterior segment pathology in such eyes can be pre-existing, unrelated to the pIOL surgery or occur secondary to the pIOL implantation. Pathological myopia can be associated with MTM, which may manifest as foveoschisis and associated NSD. With the use of OCT, the prevalence of MTM in such eyes ranges from 9% to 34%.5–8
The STAAR Visian implantable collamer lens (ICL) is placed behind the iris and anterior to the crystalline lens in the sulcus through a clear corneal incision. The V4c model −14.5 Diopter Sphere (DS) ICL placed in our patient is a plano-concave plate. This has a central 100 μm thin optical zone diameter of 4.9 mm. The centre of the optical zone has a 0.36 mm KS AquaPORT aperture. Surrounding this is haptic zone of about 500 μm in thickness. The refractive index is uniform throughout the lens and measures about 1.452.
The intraoperative difficulties that we faced during Pars Plana Vitrectomy (PPV) in this patient included problems in visualisation due to aberrations produced at the edges of the optical zone of the pIOL. The aberrations were present with both the contact flat lens (Chalam Direct SSV lens; Volk Optical) and wide-angle viewing system (MiniQuad XL contact wide-angle viewing lens, Volk Optical). The aberrations were more marked while using the Chalam Direct SSV lens probably due to a smaller field of view, a higher magnification and an increased depth of focus.
The presence of ghost images at the edges of the optical zone caused difficulties in intraoperative visualisation especially during critical steps such as ILM peeling and peripheral vitrectomy.
The presence of such aberrations while manipulating in the macular region can also increase the risk of complications. Aberrations produced at the edges of the optic are demonstrated in figure 4.
Figure 4.
Postoperative swept-source optical coherence tomography of the left eye demonstrating significant resolution of retinoschisis, restoration of foveal contour with remnants of spared ILM seen over fovea (fovea-sparing ILM peeling) and with minimal residual neurosensory detachment. ILM, internal limiting membrane.
The STAAR ICL pIOL has shown to induce higher order aberrations (HOAs) in patients after refractive surgery. The innate optical properties of lenses result in increased negative spherical aberration and trefoil aberrations with the increasing myopic pIOL power. This may be explained by the reduction in the optical zone diameter with increasing myopic power.9 10
Experimental studies comparing the optical quality and HOAs between the two ICL models V4b and V4c without and with the central aperture (KS AquaPORT), respectively, and those comparing different myopic powers of the different models revealed no significant differences.11–13
No study has evaluated the effect of optical aberrations induced by pIOL during retinal surgery. Studies on multifocal (in the bag) intraocular lenses have shown that refractive and diffractive multifocal IOLs reduce the observation of the retinal image through a flat contact lens, but the observations are affected less when viewed through the wide-field viewing system. Reduction in contrast of the retinal image, defocusing of image and centrifugally oriented ghost images have been described.14 Similar observations were noted in our case with pIOL in situ wherein aberrations were more significant with the flat contact Chalam Direct SSV lens as compared with the MiniQuad XL wide-field viewing system. Because the light rays with the wide-field viewing system pass through only the centre of the IOLs, the aberrations noticed with these are less as compared with the flat contact lens.
Thus, the presence of a pIOL along with the natural crystalline lens and the varying optic zone diameter depending on the power of pIOL can produce intraoperative optical aberrations during retinal surgery.
In order to decrease the difficulties due to aberration, most of the surgical steps may be carried out using wide-angle viewing lens as the aberrations encountered are less (figure 3C). However the use of high-magnification macular lenses like Chalam SSV lens is inevitable in critical steps like ILM peeling leading to aberrations and difficulties in intraoperative visualisation. Note that the aberrations are more in the periphery of the magnified image (figure 3B). Thus a wider area of peeling may be achieved by multiple pinch and grasp technique, keeping the area of interest in the centre of the field after each pinch, rather than attempting a wider peel in a single attempt. This might help reduce intraoperative complications due to blurring of image.
There is no established causal association between pIOL implantation and development or progression of MTM. In our patient, there is a temporal correlation between pIOL implantation and the posterior segment manifestations. The patient developed complaints of metamorphopsia in his left eye 3 months postoperatively, and the SS-OCT exam revealed the presence of myopic foveoschisis, associated macular detachment and an outer lamellar hole. However, the review of preoperative records do not document OCT prior to the pIOL implantation and a causal association could not be established. No previous case studies have highlighted the presence of MTM after pIOL implantation. However, when a patient with pIOL in situ presents with MTM, the additional surgical challenges posed may necessitate routine screening for MTM prior to refractive surgery in the eyes with myopia.
Protocols for preoperative assessment before the refractive surgery may vary between institutions. Besides the routine biometry and refractive check-up, investigations usually include a complete posterior segment evaluation including macula examination with a dilated fundus examination.15 Early stages of MTM and subtle foveal changes may be missed on routine biomicroscopy and fundus examination. The advent of OCT in the past few years has allowed us to detect and diagnose MTM more frequently.
We recommend a routine screening with a posterior segment OCT in such highly myopic individuals to look for macular pathologies prior to recommending a refractive surgery procedure.
Learning points.
Myopic traction maculopathy (MTM) can occur following phakic intraocular lens (pIOL) implantation.
The presence of pIOL can lead to difficulties in visualisation during retinal surgery, especially while using flat contact lenses for fundus viewing.
Posterior segment optical coherence tomography should be included as part of the prerefractive work-up in all patients with myopia undergoing refractive procedures.
The causal association of pIOL implantation surgery and development of posterior segment complication including MTM needs to be evaluated in larger patient groups.
Footnotes
Contributors: AK: managed the patient and was involved in planning, conception and design, interpretation of data, and critical revision of the manuscript.
RDR: conception and planning, monitoring data acquisition, and drafting and revision of manuscript.
AM: data acquisition and drafting of manuscript.
PK: monitoring data acquisition, drafting and revision of manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Jun JH, Kim YC, Kim KS. Macular hole after phakic intraocular lens implantation: two cases with divergent manifestations. Semin Ophthalmol 2014;29:213–7. 10.3109/08820538.2013.835839 [DOI] [PubMed] [Google Scholar]
- 2. Alió JL, Ruiz-Moreno JM, Artola A. Retinal detachment as a potential hazard in surgical correction of severe myopia with phakic anterior chamber lenses. Am J Ophthalmol 1993;115:145–8. 10.1016/S0002-9394(14)73916-9 [DOI] [PubMed] [Google Scholar]
- 3. Rizzo S, Belting C, Genovesi-Ebert F. Two cases of giant retinal tear after implantation of a phakic intraocular lens. Retina 2003;23:411–3. 10.1097/00006982-200306000-00024 [DOI] [PubMed] [Google Scholar]
- 4. Ruiz-Moreno J, Montero J, de la Vega C, et al. . Retinal detachment in myopic eyes after phakic intraocular Lens implantation. Am J Ophthalmol 2006;22:247–52. 10.1016/j.ajo.2006.05.020 [DOI] [PubMed] [Google Scholar]
- 5. Panozzo G, Mercanti A. Optical coherence tomography findings in myopic traction maculopathy. Arch Ophthalmol 2004;122:1455–60. 10.1001/archopht.122.10.1455 [DOI] [PubMed] [Google Scholar]
- 6. Baba T, Ohno-Matsui K, Futagami S, et al. . Prevalence and characteristics of foveal retinal detachment without macular hole in high myopia. Am J Ophthalmol 2003;135:338–42. 10.1016/S0002-9394(02)01937-2 [DOI] [PubMed] [Google Scholar]
- 7. Benhamou N, Massin P, Haouchine B, et al. . Macular retinoschisis in highly myopic eyes. Am J Ophthalmol 2002;133:794–800. 10.1016/S0002-9394(02)01394-6 [DOI] [PubMed] [Google Scholar]
- 8. Akiba J, Konno S, Sato E, et al. . Retinal detachment and retinoschisis detected by optical coherence tomography in a myopic eye with a macular hole. Ophthalmic Surg Lasers 2000;31:240–2. [PubMed] [Google Scholar]
- 9. Pérez-Vives C, Domínguez-Vicent A, Ferrer-Blasco T, et al. . Optical quality of hyperopic and myopic phakic intraocular lenses. Indian J Ophthalmol 2014;62:437–41. 10.4103/0301-4738.119423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim SW, Yang H, Yoon G, et al. . Higher-order aberration changes after implantable collamer lens implantation for myopia. Am J Ophthalmol 2011;151:653–62. 10.1016/j.ajo.2010.10.031 [DOI] [PubMed] [Google Scholar]
- 11. Pérez-Vives C, Ferrer-Blasco T, Madrid-Costa D, et al. . Optical quality comparison of conventional and hole-visian implantable collamer lens at different degrees of decentering. Am J Ophthalmol 2013;156:69–76. 10.1016/j.ajo.2013.01.030 [DOI] [PubMed] [Google Scholar]
- 12. Bhandari V, Karandikar S, Reddy JK, et al. . Implantable collamer lens V4b and V4c for correction of high myopia. J Curr Ophthalmol 2016;27:76–81. 10.1016/j.joco.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Domínguez-Vicent A, Ferrer-Blasco T, Pérez-Vives C, et al. . Optical quality comparison between 2 collagen copolymer posterior chamber phakic intraocular lens designs. J Cataract Refract Surg 2015;41:1268–78. 10.1016/j.jcrs.2014.09.050 [DOI] [PubMed] [Google Scholar]
- 14. Inoue M, Noda T, Ohnuma K, et al. . Quality of image of grating target placed in vitreous of isolated pig eyes photographed through different implanted multifocal intraocular lenses. Acta Ophthalmol 2011;89:e561–6. 10.1111/j.1755-3768.2011.02173.x [DOI] [PubMed] [Google Scholar]
- 15. American Academy of Ophthalmology Refractive Management/Intervention Panel. Preferred Practice Pattern® Guidelines Refractive errors & refractive surgery. San Francisco, CA: American Academy of Ophthalmology, 2013. www.aao.org/ppp. [Google Scholar]