Abstract
We described a rare case of the syndrome of inappropriate antidiuretic hormone secretion (SIADH) and severe unconsciousness accompanied by bilateral hypothalamic and anterior thalamic lesions with positive serum antiaquaporin 4 (AQP4) antibody. A 29-year-old man was admitted to our hospital due to the subacute progression of an unconscious state. He was observed to be hyponatraemic secondary to SIADH. Brain MRI showed bilateral hypothalamic and anterior thalamic lesions. Anti-AQP4 antibody was detected in his serum. After the administration of intravenous methylprednisolone pulse therapy, his symptoms improved with complete recovery from SIADH and regression of the hypothalamic and anterior thalamic lesions. The patient was transferred to another hospital for rehabilitation with 20 mg/day of oral prednisolone 127 days after admission. This case highlights the importance of testing for anti-AQP4 antibody in patients with unexplainable SIADH, subacute progressive unconsciousness and bilateral hypothalamic and anterior thalamic lesions.
Keywords: Endocrinology, Neurology, Adult intensive care, Immunology, Fluid electrolyte and acid–base disturbances
Background
The syndrome of inappropriate antidiuretic hormone secretion (SIADH) is a well-known cause of hyponatraemia.1 It is important to determine the cause of SIADH because it can be secondary to a number of other pathological states, such as malignant diseases, pulmonary disorders, disorders of the central nervous system and drugs.1
The presence of antiaquaporin 4 (AQP4) antibody is specific for neuromyelitis optica (NMO) and NMO spectrum disease (NMOSD).2 Patients with anti-AQP4 antibody sometimes have hypothalamic abnormalities.3 Moreover, a previous cohort study suggested that SIADH was observed in some patients with anti-AQP4 antibody.4 In this context, we report a young man presenting with SIADH and subacute progressive unconsciousness accompanied by bilateral hypothalamic and anterior thalamic lesions and serum anti-AQP4 antibody. It is important to test for anti-AQP4 antibody when a patient presents with unexplainable hypothalamic lesions and SIADH, although few such cases have been reported.
Case presentation
A 29-year-old man was admitted to our hospital for uncontrollable hyponatraemia and an unconscious state. Two weeks prior to admission, he had a mild fever of 38°C and presented to a general physician. He was diagnosed with upper respiratory inflammation and the doctor prescribed non-steroidal anti-inflammatory drugs (NSAIDs) and antibiotics. However, his symptoms did not improve, and he began to experience nausea, headache and poor appetite 1 week prior to admission. Two days before hospital admission his serum sodium level was measured to be 112 mEq/L. He gradually progressed to an unconscious state, despite infusion with a litre of 0.9% saline, and his mother brought him to the emergency department of our hospital. His medical history was unremarkable. His mother had a history of pharyngeal cancer. Other family members did not have any medical history, including endocrinopathy. He had not taken any medications or supplements before this episode. On admission, his body temperature (BT) was 36.2°C, blood pressure was 127/83 mm Hg and pulse rate was 60 beats/min. His Glasgow Coma Scale (GCS) was E4V4M6, and extraocular movements were full and smooth. He had bilateral sluggish papillary responses to light, and poor tendon reflexes bilaterally at the biceps brachii, triceps brachii, brachioradialis, patella and achilles. His Babinski and Chaddock signs showed planter responses. His physical examination was otherwise normal.
Investigations
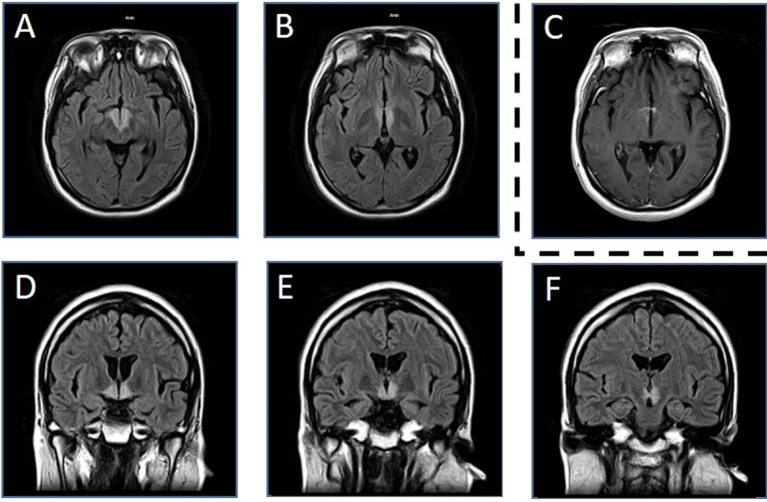
Table 1 shows the results of laboratory testing. The patient was hyponatraemic, had hypo-osmolar serum and a serum antidiuretic hormone (ADH) level of 3.6 pg/mL (reference range: 0–4 pg/mL). He did not have any signs of dehydration, heart failure or liver cirrhosis. His adrenal, thyroid and renal functions were all normal. He had low levels of luteinising hormone, follicle-stimulating hormone and testosterone. The test results for the following autoimmune antibodies were negative: antinuclear, antineutrophil cytoplasmic, antimitochondrial, anti-double-strand DNA and anti-SS-A/SS-B (Sjögren Syndrome type A and B) antibodies. PCR investigation for tuberculosis and Mycobacterium avium complex was negative. Examination of the cerebrospinal fluid (CSF) revealed mild pleocytosis (10/µL), normal protein levels (37 mg/dL), negative oligoclonal bands and elevated myelin basic protein (291 pg/mL, reference range: ≤102 pg/mL). The CSF IgG index was 0.68. PCR investigation for herpes simplex virus and varicella zoster virus was also negative. Blood and CSF cultures were negative. Electroencephalography on the third day after hospital admission showed poor 8–9 Hz alpha wave activity with dominant presence in the occipital lobe, mixed with bilateral frontal pole-dominant or parieto-occipital dominant delta wave activity. His brain MRI showed high-intensity lesions of the bilateral hypothalamus including the anterior thalamus in the T2-weighted image and fluid-attenuated inversion recovery images (figure 1). We also observed high signal intensity of the same lesions by contrast-enhanced MRI using a gadolinium-based contrast agent (figure 1). There were no abnormal findings in an MRI of the entire spinal cord. On the 50th day after admission, anti-AQP4 antibody was detected in a blood sample that was collected on the sixth day after admission.
Table 1.
Laboratory findings
| Blood samples | Urine samples | |||||||
| WBC | 6.8×109 | /L | Cr | 0.47 | mg/dL | Cr | 46.89 | mg/dL |
| Hb | 15.8 | g/dL | Na | 121 | mEq/L | Na | 92 | mEq/L |
| Plt | 185×109 | /L | K | 3.5 | mEq/L | K | 15 | mEq/L |
| Alb | 3.8 | g/dL | Cl | 87 | mEq/L | Cl | 104 | mEq/L |
| AST | 28 | IU/L | UA | 1.3 | mg/dL | UA | 29.9 | mg/dL |
| ALT | 77 | IU/L | CRP | 0.99 | mg/dL | UN | 213.4 | mg/dL |
| BUN | 4.2 | mg/dL | OSM | 247 | mOsm/kg | OSM | 372 | mOsm/kg |
| Endocrine function (taken at early morning) | ||||||||
| TSH | 1.67 | µIU/mL | LH | 0.58 | mIU/mL | ADH | 3.6 | pg/mL |
| Free T4 | 1.3 | ng/dL | FSH | 0.70 | mIU/mL | GH | 2.57 | ng/mL |
| Free T3 | 2.2 | pg/mL | PRL | 35.1 | ng/mL | IGF-1 | 267 | ng/mL |
| PRA | 4.3 | ng/mL/h | ACTH | 21.4 | pg/mL | Testosterone | 0.2 | ng/mL |
| Aldosterone | 187 | pg/mL | Cortisol | 7.1 | µg/dL | DHEA-S | 146 | µg/dL |
ACTH, adrenocorticotropic hormone; ADH, antidiuretic hormone; Alb, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; CRP, C reactive protein; DHEA-S, dehydroepiandrosterone sulfate; FSH, follicle-stimulating hormone; GH, growth hormone; Hb, haemoglobin; IGF-1, insulin-like growth factor-1; LH, luteinising hormone; Plt, platelet; PRA, plasma renin activity; PRL, prolactin; OSM, osmolality; TSH, thyroid-stimulating hormone; UA, uric acid; UN, urea nitrogen; WBC, white blood cell; T3, triiodothyronine; T4, thyroxine.
Figure 1.
MRI on the fifth day after admission. We found bilateral hypothalamic and anterior thalamic high-intensity lesions in fluid-attenuated inversion recovery images (3.0 T; TR 6200 ms, TE 105 ms) on the fifth day after admission (A,B: axial; D–F: coronal). We also observed high signal intensity of the same lesions by contrast-enhanced MRI using a gadolinium-based contrast agent (C) (T1-weighted image, 3.0 T; TR 557 ms, TE 12 ms). T, Tesla; TE, echo time; TR, repetition time.
Differential diagnosis
We diagnosed this patient with SIADH from the relatively high ADH secretion (3.6 pg/mL), low serum sodium levels (121 mEq/L) and lack of clinical features of dehydration. Low levels of uric acid (1.3 mg/dL), unsuppressed plasma renin activity (4.3 ng/mL/h) and the gap of osmolality between plasma and urine samples (plasma, 247 mOsm/kg, and urine, 372 mOsm/kg) were consistent with this diagnosis. He was not observed to have an ectopic ADH producing tumour by chest and abdominal CT. He did not have an episode of excessive water intake, and he had never taken any diuretics. NSAIDs, which were prescribed before admission, might increase the action of ADH1 and worsen his hyponatraemia.
In regard to the brain abnormalities, acute disseminated encephalomyelitis was considered less likely, because of his subacute deterioration, the bilateral symmetrical lesions in his brain, the subtle elevation of C reactive protein level and the mild pleocytosis and normal protein levels in CSF.5 Central pontine and extrapontine myelinolysis (CPM/EPM) were also considered. However, he had no history of a rapid increase of serum sodium levels, and the bilateral hypothalamic lesions are not typical sites of CPM/EPM.6 Neuropsychiatric systemic lupus erythematosus was ruled out because of the negative results for antineutrophil cytoplasmic antibodies and anti-double-strand DNA antibodies.7
Treatment
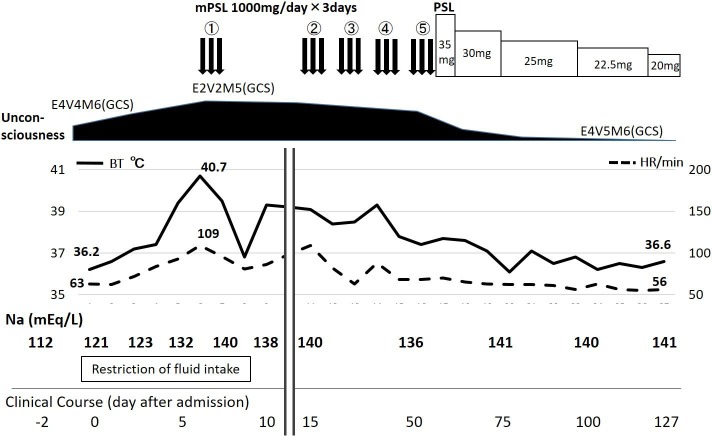
His clinical course is shown in figure 2. On the sixth day after admission, he had a fever of 40.7°C and progressed to unconsciousness. He consistently showed bradycardia relative to his high temperature (figure 2). His GCS was E2V2M5, although hyponatraemia was corrected (to 132 mEq/L) by fluid intake restriction. We considered that he might have an autoimmune disorder because he had a clinical history of upper respiratory inflammation 2 weeks prior to admission, and some cases of NMO had been reported to be accompanied by bilateral hypothalamic lesions.4 We introduced intravenous methylprednisolone pulse therapy (IVMP; methylprednisolone 1000 mg/day) 3 days/week, beginning on the seventh day after admission.
Figure 2.
Clinical course. His hyponatraemia was corrected by the restriction of fluid intake, but he had elevated BT and decreased consciousness on the fifth day after admission. He consistently showed bradycardia, relative to his high temperature. His condition gradually improved after four courses of the intravenous mPSL pulse therapy (IVMP). After an additional course of IVMP, he returned to being afebrile and his consciousness dramatically recovered. He was finally transferred to another hospital for rehabilitation with 20 mg/day of oral PSL 127 days after admission. Antiaquaporin 4 antibody was revealed to be positive on the 50th day after admission, using the blood sample taken before the introduction of IVMP. BT, body temperature; HR, heart rate; GCS, Glasgow Coma Scale; mPSL, methylprednisolone; PSL, prednisolone.
Outcome and follow-up
His condition gradually improved after four courses of IVMP, and a follow-up MRI on the 47th day after admission showed a significant reduction of signal intensity in the bilateral hypothalamus and anterior thalamus (figure 3). He recovered completely from SIADH with the regression of the hypothalamic lesion. His consciousness gradually recovered after another course of IVMP, and subsequent oral prednisolone (PSL) (35 mg/day) administration was continued from the 53rd day after admission. We tapered the PSL dose (about 5 mg every 3 weeks) according to a review of the treatment for patients with NMO8 (figure 2). A follow-up brain MRI on the 110th day after admission showed improvement of the hypothalamic lesions (figure 3). The patient was transferred to another hospital for rehabilitation on the 127th day after admission with 20 mg/day of oral PSL (figure 2).
Figure 3.
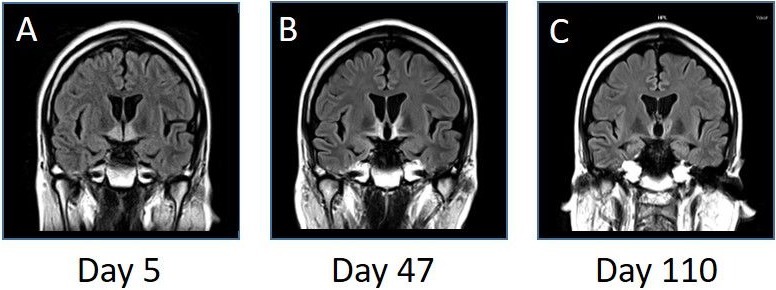
MRI (fluid-attenuated inversion recovery images, coronal, 3.0 T; TR 10 000 ms, TE 104 ms) on the fifth (A), 47th (B) and 110th (C) day after admission. The bilateral hypothalamic and anterior thalamic high-intensity lesions gradually improved on the following MRI. T, Tesla; TE, echo time; TR, repetition time.
Discussion
To the best of our knowledge, this is the first case of a young man with initial SIADH and subacute progressive unconsciousness, accompanied by bilateral hypothalamic and anterior thalamic lesions with positive serum anti-AQP4 antibody. We assumed that his SIADH was caused by the bilateral hypothalamic and anterior thalamic lesions, which could in turn be due to anti-AQP4 antibody development as a consequence of his clinical history of upper respiratory inflammation 2 weeks prior to admission.9
Anti-AQP4 antibody is one of the most important antibodies in patients with NMO/NMOSD,10 and our case highlights the importance of investigating for anti-AQP4 antibody in cases with lesions in areas surrounding the hypothalamus. It has been reported that high levels of AQP4 expression were observed with hypothalamic, brainstem and periventricular lesions.11 A pathological study reported that astrocyte cytotoxicity of anti-AQP4 antibody might cause demyelination,12 which would damage the regulation of the secretion of ADH in the hypothalamus.13 A Chinese cohort study using patients with anti-AQP4 antibody showed that 14.6% (6/41) of the patients met criteria for SIADH, and 26.8% (11/41) of the patients had hypothalamic lesions.4 Some previous reports also described hypothalamic lesions in patients with anti-AQP4 antibody,14–17 regardless of whether or not they met the criteria of NMO/NMOSD.2 Table 2 shows the review of cases with SIADH as an initial manifestation, abnormal hypothalamic lesions observed by MRI and positive anti-AQP4 antibody. In these patients, bilateral hypothalamic abnormalities had been reported in at least two female patients. Two cases had thalamic abnormalities as well as hypothalamic abnormalities, and their clinical course included unconsciousness. Their symptoms deteriorated over the course of a few days, as in our present case, although the symptoms developed over a longer duration in the other cases reported. Serum sodium levels varied in each individual, and they were not related to the severity of the disease. Our case did not meet criteria for NMO/NMOSD because the patient was not a recurrent case, and there was no evidence of optic neuritis or myelitis confirmed by MRI images.2 18 We need to follow up carefully to determine whether he has these abnormalities in the future.
Table 2.
Review of SIADH as an initial manifestation of abnormal hypothalamic lesions with a positive anti-AQP4 antibody
| References | Year | Age | Sex | Unconsciousness | Duration* | Laterality† | Na (mEq/L) | Therapy |
| Nakajima et al 14 | 2011 | 63 | M | – | NA‡ | Unilateral (left) | 114 | IVMP |
| Suzuki et al 16 | 2012 | 21 | F | + | 3 days | Bilateral | 127 | IVMP |
| Lotze et al 15 | 2012 | 15 | F | – | 21 days | Bilateral | 111 | IVMP IVIG |
| Sakai et al 13 | 2014 | 36 | M | +§ | 5 days | Unilateral (right) | 121 | IVMP PE |
| Pu et al 4 | 2015 | 24 | F | NA¶ | NA | NA** | 114 | IVMP |
| Present case | 2016 | 29 | M | + | 7 days | Bilateral | 112 | IVMP |
*Duration from the emergence of symptoms to admission.
Laterality of the hypothalamic lesions in the MRI.
They described that it took 3 months from the first episode of SIADH to the emergence of neurological disabilities, although there was no information about admission.
This case was accompanied with central and extrapontine myelinolysis.
Laterality was not described in this report.
They did not describe whether the patient had unconsciousness or not, although they stated that four out of the six patients in their report experienced confusion and decreased consciousness.
AQP4, aquaporin 4; F, female; IVIG, intravenous immunoglobulin; IVMP, intravenous methylprednisolone pulse; M, male; NA, not applicable; PE, plasma exchange; SIADH, syndrome of inappropriate antidiuretic hormone secretion.
Moreover, we reasoned that the unconsciousness in this patient might have been due to the anterior thalamic lesions in addition to his hyponatraemia, because his condition continued to get worse despite improvement of the hyponatraemia. The report of patients who had a stroke with bilateral anterior thalamic lesions supports the clinical course of this patient, whose unconsciousness improved in parallel with the improvement of the thalamic lesions.19 Nozaki et al 20 showed the correlation between the orexin level of the patient and the cause of unconsciousness in patients with NMOSD. Although we did not measure the orexin levels in his CSF, we excluded narcolepsy as a possible reason for the improvement of his unconsciousness by treatment without methamphetamine or other medicines for narcolepsy.
We need comprehensive management strategies for secondary dysfunctions induced by hypothalamic disruption. The present case showed hypogonadotropic hypogonadism, and uncontrollable hyperthermia for more than 2 months during his clinical course. The hypothalamus plays an important role in homeostasis, such as controlling BT, appetite and sleep through the secretion of oxytocin, vasopressin and other hypothalamic-releasing hormones.21 Therefore, in addition to causing SIADH, bilateral hypothalamic lesions have the potential to trigger secondary diseases, such as uncontrollable hyperthermia, malfunction due to low appetite and hypopituitarism.
In conclusion, our report indicates the importance of searching for bilateral hypothalamic and anterior thalamic lesions in patients with unexplainable SIADH and unconsciousness. Moreover, measurement of anti-AQP4 antibody is preferred in such cases, because the result can provide important information to understand the patients’ physiological status and to prescribe the best treatment for them.
Patient’s perspective.
We thank all of the doctors and nurses who engaged in providing care to my son. We are so impressed by his great recovery. We would be happy if this case report of my son helps someone with similar diseases in the world. (Comment from mother of the patient)
Learning points.
Young patients with unexplainable syndrome of inappropriate antidiuretic hormone secretion (SIADH) and prolonged unconsciousness might have bilateral hypothalamic and anterior thalamic lesions.
Measurement of antiaquaporin 4 antibody is recommended for patients with SIADH and bilateral hypothalamic lesions.
Bilateral hypothalamic lesions might cause endocrinopathy and thermoregulation disturbances which would require comprehensive management.
Footnotes
Contributors: KI mainly treated the patient and wrote this manuscript. TN and AK edited the manuscript from the perspective of neurology. JS gave a helpful advice for the treatment of the patient and finally reviewed the manuscript.
Competing interests: None declared.
Patient consent: Obtained from guardian.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Ellison DH, Berl T. The syndrome of inappropriate antidiuresis. N Engl J Med 2007;356:2064–72. 10.1056/NEJMcp066837 [DOI] [PubMed] [Google Scholar]
- 2. Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis optica. Lancet Neurol 2007;6:805–15. 10.1016/S1474-4422(07)70216-8 [DOI] [PubMed] [Google Scholar]
- 3. Pittock SJ, Lennon VA, Krecke K, et al. Brain abnormalities in neuromyelitis optica. Arch Neurol 2006;63:390–6. 10.1001/archneur.63.3.390 [DOI] [PubMed] [Google Scholar]
- 4. Pu S, Long Y, Yang N, et al. Syndrome of inappropriate antidiuretic hormone secretion in patients with aquaporin-4 antibody. J Neurol 2015;262:101–7. 10.1007/s00415-014-7537-y [DOI] [PubMed] [Google Scholar]
- 5. Pohl D, Alper G, Van Haren K, et al. Acute disseminated encephalomyelitis: updates on an inflammatory CNS syndrome. Neurology 2016;87(9 Suppl 2):S38–45. 10.1212/WNL.0000000000002825 [DOI] [PubMed] [Google Scholar]
- 6. Kleinschmidt-Demasters BK, Rojiani AM, Filley CM. Central and extrapontine myelinolysis: then… and now. J Neuropathol Exp Neurol 2006;65:1–11. 10.1097/01.jnen.0000196131.72302.68 [DOI] [PubMed] [Google Scholar]
- 7. Tay SH, Mak A. Diagnosing and attributing neuropsychiatric events to systemic lupus erythematosus: time to untie the Gordian knot? Rheumatology 2016:kew338 10.1093/rheumatology/kew338 [DOI] [PubMed] [Google Scholar]
- 8. Kimbrough DJ, Fujihara K, Jacob A, et al. Treatment of neuromyelitis optica: review and recommendations. Mult Scler Relat Disord 2012;1:180–7. 10.1016/j.msard.2012.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koga M, Takahashi T, Kawai M, et al. A serological analysis of viral and bacterial infections associated with neuromyelitis optica. J Neurol Sci 2011;300:19–22. 10.1016/j.jns.2010.10.013 [DOI] [PubMed] [Google Scholar]
- 10. Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 2004;364:2106–12. 10.1016/S0140-6736(04)17551-X [DOI] [PubMed] [Google Scholar]
- 11. Pittock SJ, Weinshenker BG, Lucchinetti CF, et al. Neuromyelitis optica brain lesions localized at sites of high aquaporin 4 expression. Arch Neurol 2006;63:964–8. 10.1001/archneur.63.7.964 [DOI] [PubMed] [Google Scholar]
- 12. Misu T, Fujihara K, Kakita A, et al. Loss of aquaporin 4 in lesions of neuromyelitis optica: distinction from multiple sclerosis. Brain 2007;130:1224–34. 10.1093/brain/awm047 [DOI] [PubMed] [Google Scholar]
- 13. Sakai W, Matsui N, Fujita K, et al. [Case of neuromyelitis optica spectrum disorder associated with central pontine and extrapontine myelinolysis preceded by syndrome of inappropriate antidiuretic hormone secretion]. Rinsho Shinkeigaku 2014;54:556–60. 10.5692/clinicalneurol.54.556 [DOI] [PubMed] [Google Scholar]
- 14. Nakajima H, Fujiki Y, Ito T, et al. Anti-aquaporin-4 antibody-positive neuromyelitis optica presenting with syndrome of inappropriate antidiuretic hormone secretion as an initial manifestation. Case Rep Neurol 2011;3:263–7. 10.1159/000334129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lotze TE, Northrop JL, Hutton GJ, et al. Spectrum of pediatric neuromyelitis optica. Pediatrics 2008;122:e1039–47. 10.1542/peds.2007-2758 [DOI] [PubMed] [Google Scholar]
- 16. Suzuki K, Nakamura T, Hashimoto K, et al. Hypothermia, hypotension, hypersomnia, and obesity associated with hypothalamic lesions in a patient positive for the anti-aquaporin 4 antibody: a case report and literature review. Arch Neurol 2012;69:1355–9. 10.1001/archneurol.2012.300 [DOI] [PubMed] [Google Scholar]
- 17. Nakano T, Fujimoto T, Fukuda Y, et al. [Neuromyelitis optica with syndrome of inappropriate secretion of antidiuretic hormone and hypersomnia associated with bilateral hypothalamic lesions: a case report]. Rinsho Shinkeigaku 2011;51:599–602. 10.5692/clinicalneurol.51.599 [DOI] [PubMed] [Google Scholar]
- 18. Wingerchuk DM, Lennon VA, Pittock SJ, et al. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485–9. 10.1212/01.wnl.0000216139.44259.74 [DOI] [PubMed] [Google Scholar]
- 19. Schmahmann JD. Vascular syndromes of the thalamus. Stroke 2003;34:2264–78. 10.1161/01.STR.0000087786.38997.9E [DOI] [PubMed] [Google Scholar]
- 20. Nozaki H, Shimohata T, Kanbayashi T, et al. A patient with anti-aquaporin 4 antibody who presented with recurrent hypersomnia, reduced orexin (hypocretin) level, and symmetrical hypothalamic lesions. Sleep Med 2009;10:253–5. 10.1016/j.sleep.2007.11.022 [DOI] [PubMed] [Google Scholar]
- 21. Hall JE. Guyton and hall textbook of medical physiology Philadelphia: Saunders/Elsevier, 2015. [Google Scholar]