Abstract
Fibrosing mediastinitis (FM) is a rare disorder resulting from abnormal immunological-mediated fibro-proliferative reaction in the mediastinum. Here, we describe a case of a 46-year-old female with an incidentally found 11×9 cm posterior mediastinal mass. Multiple biopsies of this unresectable, 18-fluorodeoxyglucose avid mass revealed marked fibrosis without any evidence of malignancy, suggesting idiopathic fibrosing mediastinitis as our initial diagnosis. Multiple interventions including a trial of steroids, fluconazole, and azathioprine to target fibrosing mediastinitis were not successful. Repeat biopsy was consistent with primary mediastinal follicular dendritic cell sarcoma. The manuscript highlights the heightened need for suspecting occult malignancies in cases of FM presenting with an indeterminate cause.
Keywords: Pathology, Cardiothoracic surgery, Surgical oncology
Background
Follicular dendritic sarcoma (FDCS) is a rare neoplasm originating from follicular dendritic cells (FDC) and can occur at both nodal and extra nodal sites. The chances of misdiagnosing this relatively less known tumour are high, especially when it occurs in extranodal or uncommon sites. Fibrosing mediastinitis (FM) is also a rare entity that occurs due to an abnormal immunological-mediated fibroproliferative reaction in the mediastinum, usually triggered by histoplasmosis and rarely by tuberculosis, autoimmune disease, radiation therapy, viral infections or trauma. FM is often confused with other malignancies that occur in the mediastinum such as lung carcinoma, lymphoma especially nodular sclerosing Hodgkin’s lymphoma, low-grade sarcoma and mesothelioma. This manuscript highlights the heightened need for suspecting occult and rare malignancies in cases of FM presenting with an indeterminate cause.
Case presentation
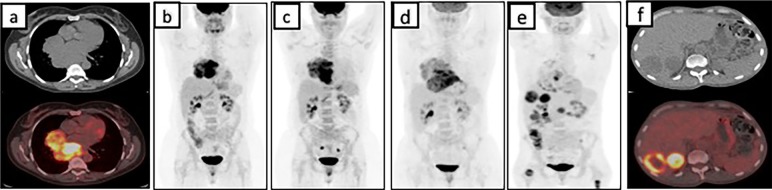
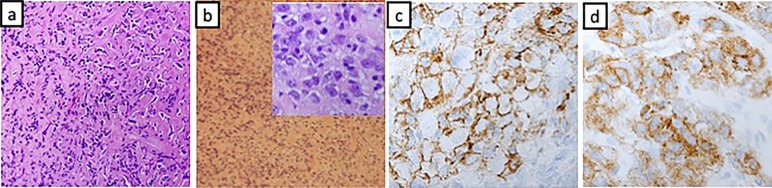
A 46-year-old African-American female was brought to the emergency department after she was involved in a motor vehicle accident in January 2014. A routine CT scan of the chest revealed a posterior mediastinal mass measuring 11×9 cm without any evidence of mediastinal lymphadenopathy. Preceding the accident, the patient denied any constitutional symptoms, cough, haemoptysis, odynophagia, dysphagia, chest pain or chronic heartburn. Pertinent medical, familial and surgical history were negative. Physical examination was grossly normal. A CT-guided percutaneous core needle biopsy of the mediastinal mass revealed a mixed inflammatory infiltrate including small mature lymphocytes, plasma cells and scattered larger atypical lymphocytes in a dense fibrous background. No Reed-Sternberg cells were identified by CD30 or CD15 staining. CD20 and Pax-5 showed abundant B-lymphocytic aggregates between the fibrotic bands. CD3-stained small T lymphocytes. MUM-1 highlighted scattered plasma cells. CD15 stained the granulocytes. No fungal organisms were seen in the Gomori methenamine silver (GMS) stain. IgG stain identified numerous non-clonal plasma cells, of which only a small minority were IgG4 positive. Cytokeratin AE1/AE3 stains were negative. Occasional lymphocytes are positive for BCL-6. CD10 and synaptophysin were unremarkable. S-100 stain was diffusely positive. Acid fast bacilli stain and GMS showed no evidence of micro-organisms. Epstein-Barr virus (EBV)-encoded RNA (EBER) was negative for Epstein-Barr virus. While the scattered large cells of the fibrohistocytic background were worrisome for classical Hodgkin’s lymphoma (HL), the cells did not express CD30 and CD20 markers. Flow cytometry did not reveal any abnormal clonal cell population. 18-fluorodeoxyglucose (FDG) positron emission tomography (PET) and CT scan demonstrated a large heterogeneous mediastinal mass with intense FDG uptake on PET scan (figure 1A and B). Due to a high degree of suspicion for malignancy, video-assisted thoracoscopic surgery was performed, which demonstrated a large globular mass in the mediastinum extending towards the pericardium, grossly adherent to the pleura and parenchyma of the lung. Intraoperatively, this was converted to right thoracotomy to minimise injury to the major vascular structures, pericardium and phrenic nerve. Biopsy revealed dense fibrotic tissue and fragments of lymphoid tissue consistent with lymph node (figure 2A). The immunohistochemistry staining was exactly the same from before (see above). The differential diagnoses included sclerosing mediastinitis and/or HL. Since immunohistochemical and flow cytometry studies did not reveal any positive stains or clonality suggesting HL, fibrosing mediastinitis was our working diagnosis. Infectious work-up involving HIV I/II, hepatitis panel, histoplasma and tuberculosis were negative. Rheumatological work up including antinuclear antibody, rheumatoid factor, cyclic citrullin antibody was also negative. ACE levels were also within normal limits. As the diagnosis still remained obscure, an empirical trial of prednisone and fluconazole was initiated without any sign of radiological or clinical improvement (figure 1C). As patient was asymptomatic, she declined repeat biopsies or trial of immunosuppressant therapy and preferred observation. In April of 2015, cardiac echocardiogram done for evaluation of continued mild chest pain after her thoracotomy revealed large pericardial effusion. Pericardiocentesis and pericardial stripping was performed but once again fluid analysis, pathology and microbial cultures were all negative. Lack of other definite diagnosis and worsening of patient’s condition led to initiation of azathioprine for presumptive diagnosis of FM. Patient’s chest pain progressed and was now associated with symptoms of severe dysphagia, weight loss, nausea and vomiting. Diagnostic upper endoscopy revealed an area of ulceration with extrinsic compression of the midoesophagus, and biopsy of this suspicious area revealed granulation tissue only. PET/CT done in October of 2015 demonstrated progression of the disease with development of new FDG avid subcentimetre lung lesions (figure 1D). A repeat thoracotomy and mediastinal biopsy of the most FDG avid part of the mediastinal mass demonstrated dense stromal sclerosis with focal aggregates of mature lymphocytes interspersed by oval elongated spindle cells with vesicular nuclei and vacuolated reticular cytoplasm (figure 2B), and these cells were strongly positive for CD21, CD35, CD68 and weakly positive for CD31 (figure 2C and D). MPO, CD1a, S100, CD15, CD30, CD10, WT-1, ALK-1, CD34, AE1/AE3, PLAP, D2-40 and TdT were negative. CD3 and CD20 stained T-lymphocytes and B-lymphocytes, respectively, but were negative in the tumour cells. This led to the diagnosis of primary mediastinal follicular dendritic cell sarcoma. Reanalysis of initial core biopsy samples was positive for CD21, CD35 and CD68 immunohistochemical (IHC) stains.
Figure 1.
PET/CT images at various stages during the presentation. In A and B, PET/CT demonstrates a FDG avid mediastinal mass; C and D show an MIP image on PET/CT demonstrating an increase in the size of the mediastinal mass; E and F showing a decreased FDG uptake in the mediastinum and appearance of new FDG avid metastasis in the liver on PET/CT. FDG, 18-fluorodeoxyglucose; MIP, maximal intensity projection; PET, positron emission tomography.
Figure 2.
(A) Marked fibrosis with lymphocytic infiltrate and spindle cells. (B) Stromal sclerosis and mature lymphocytes interspersed by round to oval and elongated spindle cells with inset showing 100× magnified image of the same. (C and D) Positive CD21 and CD 35 (respectively) staining of malignant cells.
Treatment
Patient was started on six cycles of intravenous cyclophosphamide (750 mg/m2), doxorubicin (50 mg/m2), vincristine (1 mg/m2) and prednisone (1 mg/kg) given every 3 weeks. After an initial improvement in her constitutional symptoms and evidence of partial response on PET/CT in February of 2016, patient’s tolerance to chemotherapy continued to decline. After chemotherapy, palliative mediastinal radiation was performed for local symptom control. Although local control was achieved in the mediastinum, the tumour continued to rapidly progress in the liver(figure 1E and F). A trial of salvage nivolumab (3 mg/kg every 21 days) was attempted without any success.
Outcome and follow-up
Three months after disease progression with liver metastasis, the patient elected for hospice and expired soon after.
Discussion
FDCS is a very rare neoplasm and it was first described by Monda et al in 1986 in their account of four cases of painless unilateral lymphadenopathy with histology and immunohistochemistry consistent with non-lymphomatous primary lymph node malignancy.1 Over the next three decades, about 500 cases of FDCS were reported.2
FDCS is a tumour of the follicular dendritic cells which are mesenchymal in origin and are mainly distributed in primary and secondary lymphoid follicles. WHO grouped FDCS in histiocytic and dendritic cell neoplasms along with histiocytic sarcoma, tumours derived from Langerhans cells, interdigitating dendritic cell sarcoma, other rare dendritic cell tumours and disseminated juvenile xanthogranuloma.3 Histological examination of the FDCS reveals a neoplastic proliferation of spindle cells which forms fascicles, storiform patterns, whorls, trabeculae, diffuse sheets or a mixture of these patterns. The tumour is infiltrated with small lymphocytes that may be predominantly B or T or mix of both B and T cells. The individual tumour cells are ovoid to elongated with a moderate amount of eosinophilic cytoplasm and indistinct cellular borders. The nuclei are oval or elongated with vesicular or finely granular dispersed chromatin. The nuclear membranes are delicate with common nuclear pseudoinclusions. The mitotic rate is usually low with 0 to 10 mitoses per 10 high-power fields, although some cases with significant cytological atypia may show much higher mitotic rates, atypical bizarre-looking mitoses and necrosis.4 Cases with focal to diffuse background of dense fibrosis have also been described.5
IHC studies are indispensable in the diagnosis of FDCS and usually demonstrate the immunophenotypic profile of non-neoplastic follicular dendritic cells from which they seem to originate like CD21, CD23 and/or CD35. Cells are almost always positive for clusterin that is usually negative or weakly positive in other dendritic tumours.5 Podoplanin, desmoplakin, vimentin, fascin, human leucocyte antigen-DR and epithelial membrane antigen are usually positive. The variable positive stains include S-100, epidermal growth factor receptor (EGFR) and CD68. Staining for CD1a, lysosomes, myeloperoxidase, CD34, CD3, CD79a, CD30 and HMB45 are negative.4 No specific genetic abnormalities have been attributed to FDCS. BRAF mutation was detected in 18.5% cases in a study that looked at BRAF mutations in histiocytic and dendritic cell neoplasms.6 Our patient was negative for this mutation.
The mean age at diagnosis is 49 years with even sex distribution except for the inflammatory pseudotumour variant which has a slight female predilection.2 7 Most of the cases are idiopathic. The inflammatory pseudotumour variant is linked with EBV.8 In a review of 481 cases of FDCS by Facchetti et al, 25% were nodal origin mainly cervical, axillary, abdominal and mediastinal, 65% were extranodal including mediastinum, skin, tonsil, gastrointestinal tract, lung, pleura and soft tissue and about 10% were characterised under both nodal and extranodal. Of the 442 cases in the study, 10.4% were mediastinal (both nodal and extranodal) in origin.2 FDCS can be often missed as these are rare tumours and the tumour-specific immunohistochemistry studies for CD 21 and CD 35 are less likely to be done especially when they occur in extranodal or uncommon sites. It may resemble a wide variety of tumours based on the location of the primary tumour. Although the pathology and immunohistochemistry of the tumour are well defined, the biology, risk factors, clinical course and effective treatment are incompletely understood. On CT scans, these tumours demonstrate variable vascularity with large tumours demonstrating central necrosis. These tumours also demonstrate moderate to extremely high-metabolic activity on PET imaging.9–11 Specific diagnosis by imaging modalities is not possible due to the non-specific nature of the imaging findings. Local recurrences occur in 28% of the cases, and distant metastasis occurs in about 27% cases. The common sites of recurrences include liver, lung, lymph nodes and bones.12 Large tumour size (greater than 6 cm), intra-abdominal tumours, the presence of coagulative necrosis, mitotic count greater than 5 mitoses per 10 high-power fields and significant cellular atypia have been described as unfavourable factors for prognosis.12
FDCS usually presents as a painless swelling although systemic symptoms do occur in some patients with inflammatory pseudotumour-like variant.6 Other symptoms depend on the site of the tumour. FDCS is known to be associated with paraneoplastic pemphigus in rare cases.13 Most of the data on treatment of FDCS is from small case series or case reports. There are no prospective studies. Complete surgical resection, wherever possible, appears to be the treatment of choice for both primary and recurrent lesions. Adjuvant radiation has not shown to prolong survival in early disease when compared with surgery alone.12 However, its utility is not well defined in late/metastatic disease. Chemotherapy regimens primarily designed to target lymphoma including cyclophosphamide, vincristine, doxorubicin, prednisolone (CHOP), ifosfamide, carboplatin, etoposide, and adriamycin, bleomycin, vincristine, dacarbazine were employed in the treatment of FDCS with variable success.12 In a report of 14 cases by Soriano et al, CHOP was used as primary modality of treatment in four cases with complete remission in only one case. They also used multimodal therapy with surgery followed with CHOP and radiation and achieved complete remission in six of seven cases. In one case, surgery alone followed by intraoperative salvage external beam radiation treatment resulted in complete remission. In the same series, it was noted gemcitabine plus taxotere when used as a third-line treatment in cases of relapse resulted in complete remission in two of three cases.14 Newer potential targeted therapies for FDCSs include a BRAF enzyme inhibitor (vemurafenib) and EGFR inhibitors.
Fibrosing mediastinitis is characterised by an ill-defined soft-tissue mass involving the mediastinum which results from an abnormal immunological mediated fibroproliferative reaction in the mediastinum. It is usually seen in histoplasmosis and rarely with tuberculosis, aspergillosis, mucormycosis, blastomycosis and cryptococcosis. Behçet disease, rheumatic fever, radiation therapy trauma, Hodgkin disease and drug therapy with methysergide maleate have also been implicated in some reports.15 The presence of extensive paucicellular fibrous tissue infiltrating into the adipose tissue with or without patchy infiltrate of mononuclear cells in the absence of malignancy should raise suspicion for fibrosing mediastinitis.16 Cases have been reported where it masked underlying malignancies such as lung carcinoma,17 lymphoma especially nodular sclerosing Hodgkin’s lymphoma,18 mesothelioma19 and proved to be a diagnostic challenge. In one of these cases, the patient underwent two thoracotomies and the biopsies were consistent with fibrosing mediastinitis, only to reveal desmoplastic malignant mesothelioma on autopsy.19 Simple fibrosis, fibromatosis and low-grade sarcoma should also be considered in the differential diagnosis.15 In our case, IHC stains for CD21, CD35 and CD68 stains were not done due to a low suspicion FDCS, which might have potentially delayed the diagnosis. It has been proposed to perform an open thoracotomy with extensive sampling to diagnose underlying malignancy.17 In localised disease, surgical resection may be attempted. Although various treatments including systemic steroids, antifungals, immunomodulators such as azathioprine have been proposed, none of them have been consistently effective.15
Learning points.
Fibrosing mediastinitis is a diagnosis of exclusion and any infiltrating and fibrous lesion must be sampled extensively to exclude occult malignancy underlying the fibrosis and low-grade sarcoma should be considered in the differential diagnosis.
The chances of misdiagnosing FDCS are very high when it occurs in extranodal sites or in uncommon sites. Thus, it is important to be aware of this entity and to perform appropriate inmunohistochemical studies, specifically with CD21 and CD35 markers on tumours that exhibit a histological pattern of a sarcoma or histiocytic or dendritic cell tumour, which will aid in the recognition of this entity.
As definite guidelines assisting FDCS management are lacking, surgery when feasible, followed by multimodal approach involving chemotherapy and radiation continue to be an optimal strategy.
Incorporation of next-generation sequencing to identify possible driver mutations will hopefully lead to targeted approaches in this rare disease.
Footnotes
Contributors: SRC, MAS, AT contributed to data collection. SRC, PP and MAS are responsible for drafting the article. PP and SRC are responsible for critical revision of the article. All authors have approved the final version.
Competing interests: None declared.
Patient consent: Consent obtained from guardian.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Monda L, Warnke R, Rosai J. A primary lymph node malignancy with features suggestive of dendritic reticulum cell differentiation. A report of 4 cases. Am J Pathol 1986;122:562. [PMC free article] [PubMed] [Google Scholar]
- 2.Facchetti F, Lorenzi L. Follicular dendritic cells and related sarcoma. Semin Diagn Pathol 2016;33:262–76. 10.1053/j.semdp.2016.05.002 [DOI] [PubMed] [Google Scholar]
- 3.Grogg KL, Lae ME, Kurtin PJ, et al. Clusterin expression distinguishes follicular dendritic cell tumors from other dendritic cell neoplasms: report of a novel follicular dendritic cell marker and clinicopathologic data on 12 additional follicular dendritic cell tumors and 6 additional interdigitating dendritic cell tumors. Am J Surg Pathol 2004;28:988–98. [DOI] [PubMed] [Google Scholar]
- 4.Wu A, Pullarkat S. Follicular dendritic cell sarcoma. Arch Pathol Lab Med 2016;140:186–90. 10.5858/arpa.2014-0374-RS [DOI] [PubMed] [Google Scholar]
- 5.Swerdllow SH, Campo E, Harris NL. WHO classification of tumours of haematopoietic and lymphoid tissues. 2008 France: IARC Press, 2008. [Google Scholar]
- 6.Go H, Jeon YK, Huh J, et al. Frequent detection of BRAF(V600E) mutations in histiocytic and dendritic cell neoplasms. Histopathology 2014;65:261–72. 10.1111/his.12416 [DOI] [PubMed] [Google Scholar]
- 7.Ge R, Liu C, Yin X, et al. Clinicopathologic characteristics of inflammatory pseudotumor-like follicular dendritic cell sarcoma. Int J Clin Exp Pathol 2014;7:2421. [PMC free article] [PubMed] [Google Scholar]
- 8.Cheuk W, Chan JK, Shek TW, et al. Inflammatory pseudotumor-like follicular dendritic cell tumor: a distinctive low-grade malignant intra-abdominal neoplasm with consistent Epstein-Barr virus association. Am J Surg Pathol 2001;25:721–31. [DOI] [PubMed] [Google Scholar]
- 9.Dong A, Wang Y, Zuo C. FDG PET/CT in follicular dendritic cell sarcoma with extensive peritoneal involvement. Clin Nucl Med 2014;39:534–6. 10.1097/RLU.0b013e318292aa9e [DOI] [PubMed] [Google Scholar]
- 10.Subesinghe M, Smith JT, Chowdhury FU. F-18 FDG PET/CT imaging of follicular dendritic cell sarcoma of the mediastinum. Clin Nucl Med 2012;37:204–5. 10.1097/RLU.0b013e31823ea1a5 [DOI] [PubMed] [Google Scholar]
- 11.Kim H, Park CM, Jeon YK, et al. Follicular dendritic cell sarcoma of the mediastinum: CT and 18 F-fluoro-2-deoxyglucose PET findings. Thorac Cancer 2013;4:203–6. 10.1111/j.1759-7714.2012.00143.x [DOI] [PubMed] [Google Scholar]
- 12.Saygin C, Uzunaslan D, Ozguroglu M, et al. Dendritic cell sarcoma: a pooled analysis including 462 cases with presentation of our case series. Crit Rev Oncol Hematol 2013;88:253–71. 10.1016/j.critrevonc.2013.05.006 [DOI] [PubMed] [Google Scholar]
- 13.Su Z, Liu G, Liu J, et al. Paraneoplastic pemphigus associated with follicular dendritic cell sarcoma: report of a case and review of literature. Int J Clin Exp Pathol 2015;8:11983. [PMC free article] [PubMed] [Google Scholar]
- 14.Soriano AO, Thompson MA, Admirand JH, et al. Follicular dendritic cell sarcoma: a report of 14 cases and a review of the literature. Am J Hematol 2007;82:725–8. 10.1002/ajh.20852 [DOI] [PubMed] [Google Scholar]
- 15.Rossi SE, McAdams HP, Rosado-de-Christenson ML, et al. Fibrosing mediastinitis. Radiographics 2001;21:737–57. 10.1148/radiographics.21.3.g01ma17737 [DOI] [PubMed] [Google Scholar]
- 16.Peikert T, Colby TV, Midthun DE, et al. Fibrosing mediastinitis: clinical presentation, therapeutic outcomes, and adaptive immune response. Medicine 2011;90:412–23. 10.1097/MD.0b013e318237c8e6 [DOI] [PubMed] [Google Scholar]
- 17.Mole TM, Glover J, Sheppard MN. Sclerosing mediastinitis: a report on 18 cases. Thorax 1995;50:280–3. 10.1136/thx.50.3.280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flannery MT, Espino M, Altus P, et al. Hodgkin's disease masquerading as sclerosing mediastinitis. South Med J 1994;87:921–3. 10.1097/00007611-199409000-00012 [DOI] [PubMed] [Google Scholar]
- 19.Crotty TB, Colby TV, Gay PC, et al. Desmoplastic malignant mesothelioma masquerading as sclerosing mediastinitis: a diagnostic dilemma. Hum Pathol 1992;23:79–82. 10.1016/0046-8177(92)90017-W [DOI] [PubMed] [Google Scholar]