Abstract
Isolated amyloidomas derived from insulin are extremely rare, and there is only one reported case to date of insulin-derived amyloidoma in the breast.
We present the case of a 36-year-old woman reporting a lump in the right breast. It was clinically assessed as a probable fibroadenoma but was removed surgically given the size of the lesion. On histological analysis, the lesion had features consistent with amyloid. Further investigations showed the amyloid to be derived from insulin. The lump was removed in its entirety, and the patient made a full recovery.
Keywords: Breast surgery, Pathology
Background
Amyloidosis is a rare condition characterised by the aggregation of misfolded proteins. This occurs when certain ‘precursor proteins’ that are normally soluble become misfolded and stick together to form an insoluble deposit—amyloid. The amyloid can be further categorised by the particular precursor protein involved, and more than 30 different types of precursor protein have been identified.1 The most common forms of systemic amyloid deposition are AL amyloidosis (immunoglobulin light chain), followed by AA amyloidosis (serum amyloid A).
More commonly, amyloid can be deposited in a single organ or body part. This ‘localised amyloidosis’ is overall more common than systemic amyloidosis, though the process often occurs as part of the pathogenesis of another clinical entity. Two well-known examples of this include the extracellular deposition of beta amyloid-Aβ in the brain as part of Alzheimer’s disease, and amyloid formation from islet amyloid polypeptide (IAPP) in the pancreas, which is implicated in type 2 diabetes mellitus. Less commonly, amyloid can be found deposited in subcutaneous tissues as a solitary lesion, which is often termed an ‘amyloidoma’.
Amyloidomas have been reported in a wide variety of tissues, including the breast. Though most breast amyloid deposits are as part of systemic amyloidosis, isolated amyloidomas are sometimes identified. The largest case series to date was over 18 years at a specialist amyloid centre in the USA; only 0.5% of cases referred (seven cases) had a localised amyloid deposit in the breast.2 The majority of cases were in older women (median age 63 years), and none were associated with systemic disease. All of the cases in this series presented with microcalcified lesions on screening mammography. This case was very different, involving a young patient and quite reassuring appearances on imaging.
The precursor protein could not be identified in the cases reported in this series, however. Other case reports exist with successful identification of the precursor protein, most of which are AL or AA amyloid.3
Insulin-related amyloid deposits (Ains) are an increasingly common subtype of localised amyloid. A case series in 2009 documented four new cases in addition to five historical cases where an amyloid deposit was identified and subsequently confirmed to be derived from insulin.4 The cases were highly variable, reported from various sites including thigh, abdominal wall, shoulder, as well as other injection sites. The pathogenesis of insulin-derived amyloidomas is poorly understood, and it has been conjectured that the trauma of injection can provide a focus for amyloid fibril formation.5 An excellent review of insulin-derived amyloid cases revealed that over time the number of cases reported has increased dramatically from <5 cases per year up to 2007, to 20 cases in 2008–2011, and 75 cases in 2012–2015.6 This is likely, at least in part, to improvements in access to diagnostic services, and the technological capabilities of those services.
This case brings together these two rare entities, giving a unique opportunity to revisit the rarer causes of breast lumps, as well as explore the emerging phenomenon of insulin-derived amyloidomas.
Case presentation
A 36-year-old woman was referred by her general practitioner (GP) to the triple assessment breast clinic having reported the sensation of a lump in the right breast. She had first noticed the lump about 10 weeks prior to visiting the GP. She reported no other symptoms and denied experiencing any pain, nipple discharge, other lumps or skin changes on either breast.
Her medical history included diabetes mellitus type 1 and mild asthma. She had no personal or family history of breast cancer and had never been pregnant. She used a basal bolus regimen for her insulin delivery; 36 units of long-acting insulin in the morning and 1 unit per 5 g of carbohydrates short-acting insulin bolus after meals. She had evidence of background diabetic retinopathy, and her most recent glycated haemoglobin was 66 mmol/mol. She used e-cigarettes but not tobacco and drank alcohol occasionally.
Otherwise, she was clinically very well, and examination of the right breast revealed a lump in the upper inner quadrant, between the one and two o’clock positions. The lump was approximately 2×2 cm in size, non-tender, with no suspicious features. Examination of the left breast was normal, and there was no palpable lymphadenopathy in either axilla.
Investigations
Ultrasound assessment of the lesion revealed a 4×1 cm well-defined lesion in the right breast. It was assessed as having no malignant features, and was labelled as a U2 lesion (likely benign).
A core biopsy of the lesion was taken under ultrasound guidance, yielding two cores of tissue. On microscopy, they showed fragments of amorphous eosinophilic material and minimal fibrous stroma, without evidence of a definitive lesion. It was therefore reported as an inadequate sample.
Differential diagnosis
At this point, the most likely diagnosis given her clinical findings and investigation results was a benign fibroadenoma.
Treatment
In light of the size of the lesion, she was offered a lumpectomy. At the time of operation, it was noticed by the operating surgeon that the lesion was unusually friable and did not have a surrounding capsule. It was difficult to recover whole, and so was excised in pieces. The procedure was otherwise uneventful, with no intraoperative complications.
Outcome and follow-up
The patient made an excellent recovery from the procedure. The excised tissue was sent for histological analysis.
The excision specimen was received as multiple pieces of fibrofatty tissue aggregating 50×50×20 mm. Histologically, there was extensive extracellular deposition of amorphous eosinophilic material focally arranged as bands and bundles (figure 1). The amyloid deposits substantially replaced the normal structure and so no identifiable breast glandular tissue was seen anywhere in the submitted material. Admixed chronic inflammatory cells and multinucleated giant cells were seen which are thought to be due to a foreign-body-like reaction to the amyloid. Areas of fine dystrophic calcification were identified, but osseous metaplasia has also been reported in some amyloid deposits.
Figure 1.
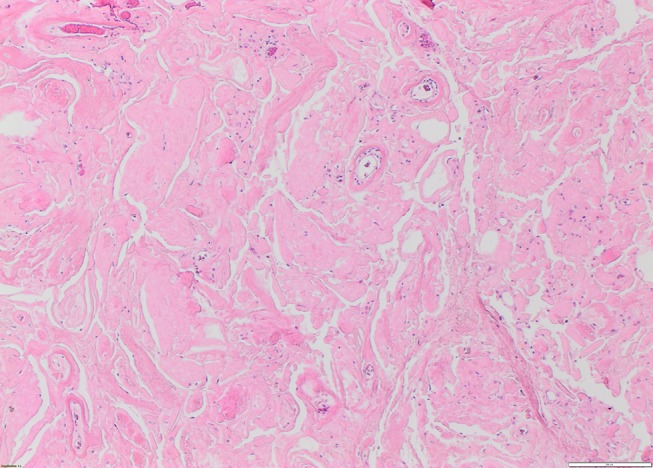
Extensive deposition of amorphous eosinophilic material (H&E ×10 magnification).
A reticulin stain highlighted the fibrillar structure of the protein (figure 2). The amorphous amyloid material stained red orange with Congo Red stain and apple green birefringence was exhibited when the Congo Red stain was examined under high-intensity cross-polarised light (figures 3 and 4). When electron microscopy was performed, it revealed the presence of straight, non-branching, haphazardly arranged amyloid fibrils 5–12 nm enmeshed with bands of collagen fibres.
Figure 2.
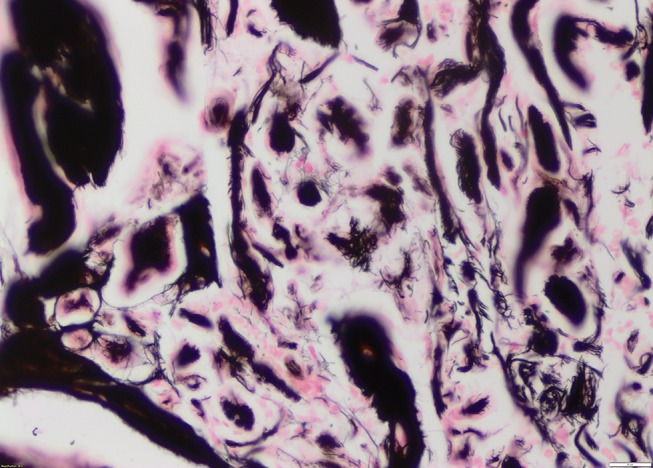
Reticulin special stain showing fibrillar nature of amyloid deposits (H&E ×20 magnification).
Figure 3.
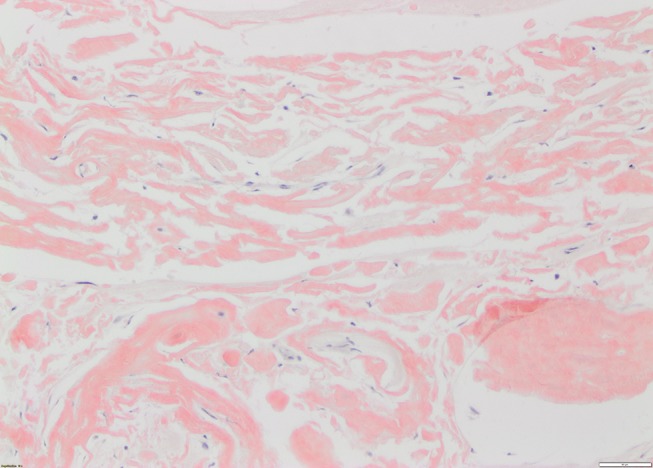
Congo Red special stain showing orange red colouration to band-like amyloid deposits (×20 magnification).
Figure 4.
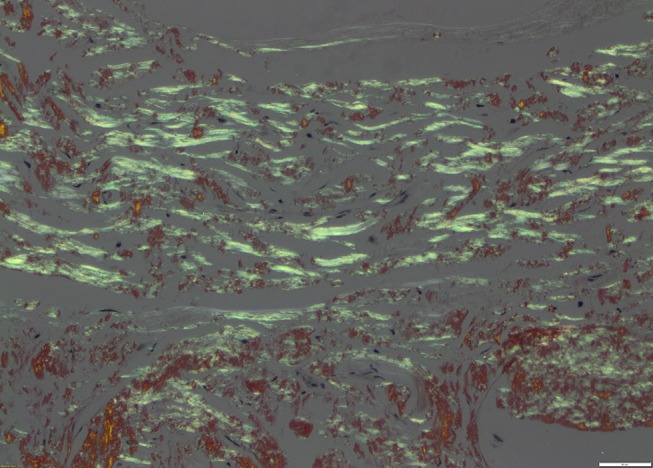
Same area as figure 3 under cross-polarised light exhibiting apple green birefringence within band-like amyloid deposits (×20 magnification).
After local diagnosis as an amyloidoma, the case was sent for specific immunohistochemical typing at the National Amyloidosis Centre which proved elusive as there was no staining for any of the monospecific antibodies reactive with serum amyloid A protein (SAA), transthyretin (TTR) and with Kappa and Lambda immunoglobulin light chains. Proteomic analysis of the amyloid indicated insulin to be the amyloid fibril protein with a high degree of certainty.
The patient was reassured that no further treatment was required and discharged.
Discussion
Only one similar case has been reported in the literature.7 The patient was middle- aged (41 years) with type 1 diabetes and was initially investigated for a self-reported lump in the breast. On mammography, microcalcifications were noted and ultrasound imaging yielded an ill-defined hypoechoic lesion. The diagnosis was made on biopsy, so the lesion was not resected. The lesion demonstrated apple green birefringence when stained with Congo Red and stained positive with an anti-insulin antibody.
This case also had a clear history of the patient switching their insulin injection site from the abdomen to the axillary tail 7 years previously (when pregnant). The author, therefore, attributes the amyloidoma to subcutaneous injection of exogenous insulin at the site.
In our case, the lesion was found in the upper inner quadrant of the breast, and neither clinical history nor examination revealed apparent recent injection at such an unusual site. This has lead us to speculate on the aetiology of this particular lesion.
To the best of our knowledge, isolated insulin-related amyloidomas have only been reported in association with exogenous insulin injection. This can be attributed to the action of C-peptide, a by-product of insulin synthesis in vivo. It has been shown to interfere with amyloid formation in vitro, and this may be part of the mechanism which prevents endogenous insulin-forming amyloid structures.8 It seems likely, therefore, that the amyloidoma is linked to subcutaneous insulin injection.
We could consider migration of the insulin from injection site to amyloid location, however in the absence of a focus around which the amyloid can form (as happens locally with the trauma of injection), this is less likely. The possibility remains, despite history and examination indicating otherwise, that this amyloidoma was the result of a previous injection directly into the breast.
Learning points.
Consider amyloidoma as a rare cause of a breast lump, particularly in older women.
Isolated amyloidomas are uncommon, and patients should always be screened for systemic amyloidosis.
Consider amyloidoma when subcutaneous lumps are noticed in patients with diabetes who use insulin, especially at injection sites.
Footnotes
Contributors: JMM identified, drafted and revised the case, and is the corresponding author. TAKG and VK also identified the case, acquired the clinical data and revised and approved the case. TA contributed to the clinical data including images, drafted, revised and approved the case.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Sipe JD, Benson MD, Buxbaum JN, et al. . Amyloid fibril protein nomenclature: 2012 recommendations from the Nomenclature Committee of the International Society of Amyloidosis. Amyloid 2012;19:167–70. 10.3109/13506129.2012.734345 [DOI] [PubMed] [Google Scholar]
- 2.Charlot M, Seldin DC, O'hara C, et al. . Localized amyloidosis of the breast: a case series. Amyloid 2011;18:72–5. 10.3109/13506129.2011.570817 [DOI] [PubMed] [Google Scholar]
- 3.Deolekar MV, Larsen J, Morris JA. Primary amyloid tumour of the breast: a case report. J Clin Pathol 2002;55:634–5. 10.1136/jcp.55.8.634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yumlu S, Barany R, Eriksson M, et al. . Localized insulin-derived amyloidosis in patients with diabetes mellitus: a case report. Hum Pathol 2009;40:1655–60. 10.1016/j.humpath.2009.02.019 [DOI] [PubMed] [Google Scholar]
- 5.Gupta Y, Singla G, Singla R. Insulin-derived amyloidosis. Indian J Endocrinol Metab 2015;19:174–7. 10.4103/2230-8210.146879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nilsson MR. Insulin amyloid at injection sites of patients with diabetes. Amyloid 2016;23:139–47. 10.1080/13506129.2016.1179183 [DOI] [PubMed] [Google Scholar]
- 7.Kayser O, Order B, Schäfer F. Die insulininduzierte, lokale amyloidose der Brust – eine seltene Differenzialdiagnose zum DCIS und Mammakarzinom. Geburtshilfe Frauenheilkd 2011;71:606–8. 10.1055/s-0031-1279988 [DOI] [Google Scholar]
- 8.Landreh M, Stukenborg JB, Willander H, et al. . Proinsulin C-peptide interferes with insulin fibril formation. Biochem Biophys Res Commun 2012;418:489–93. 10.1016/j.bbrc.2012.01.051 [DOI] [PubMed] [Google Scholar]


