Abstract
We have developed a simple and efficient transformation system for the agaric fungus, Coprinus cinereus. Protoplasts were prepared from asexual spores that harbor one or two mutations in the structural gene for tryptophan synthetase. The protoplasts can be stably transformed using the cloned Coprinus gene at a frequency of 1 in 10(4) viable protoplasts. A variety of molecular events accompanies the formation of stable transformants, including insertion of the transforming DNA at the homologous locus. The transforming DNA is stable through cell division, mating, fruiting body formation, and meiosis.
Full text
PDF


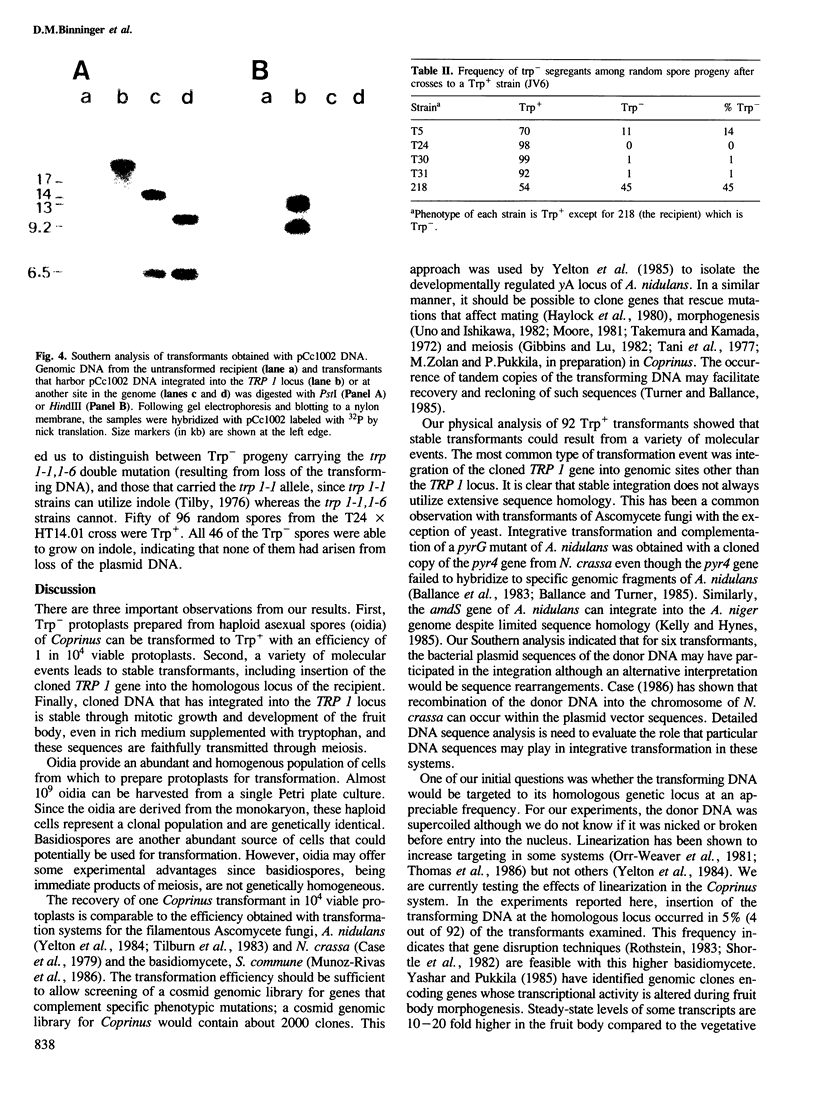


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballance D. J., Buxton F. P., Turner G. Transformation of Aspergillus nidulans by the orotidine-5'-phosphate decarboxylase gene of Neurospora crassa. Biochem Biophys Res Commun. 1983 Apr 15;112(1):284–289. doi: 10.1016/0006-291x(83)91828-4. [DOI] [PubMed] [Google Scholar]
- Ballance D. J., Turner G. Development of a high-frequency transforming vector for Aspergillus nidulans. Gene. 1985;36(3):321–331. doi: 10.1016/0378-1119(85)90187-8. [DOI] [PubMed] [Google Scholar]
- Case M. E. Genetical and molecular analyses of qa-2 transformants in Neurospora crassa. Genetics. 1986 Jul;113(3):569–587. doi: 10.1093/genetics/113.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case M. E., Schweizer M., Kushner S. R., Giles N. H. Efficient transformation of Neurospora crassa by utilizing hybrid plasmid DNA. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5259–5263. doi: 10.1073/pnas.76.10.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J. M., Hynes M. J. Transformation of Aspergillus niger by the amdS gene of Aspergillus nidulans. EMBO J. 1985 Feb;4(2):475–479. doi: 10.1002/j.1460-2075.1985.tb03653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsman A. J., Gimlich R. L., Clarke L., Chinault A. C., Carbon J. Sequence variation in dispersed repetitive sequences in Saccharomyces cerevisiae. J Mol Biol. 1981 Feb 5;145(4):619–632. doi: 10.1016/0022-2836(81)90306-5. [DOI] [PubMed] [Google Scholar]
- Lin F. L., Sperle K., Sternberg N. Recombination in mouse L cells between DNA introduced into cells and homologous chromosomal sequences. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1391–1395. doi: 10.1073/pnas.82.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B. C., Raju N. B. Meiosis in Coprinus. II. Chromosome pairing and the lampbrush diplotene stage of meiotic prophase. Chromosoma. 1970;29(3):305–316. doi: 10.1007/BF00325945. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Munoz-Rivas A., Specht C. A., Drummond B. J., Froeliger E., Novotny C. P., Ullrich R. C. Transformation of the basidiomycete, Schizophyllum commune. Mol Gen Genet. 1986 Oct;205(1):103–106. doi: 10.1007/BF02428038. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pukkila P. J., Lu B. C. Silver staining of meiotic chromosomes in the fungus, Coprinus cinereus. Chromosoma. 1985;91(2):108–112. doi: 10.1007/BF00294053. [DOI] [PubMed] [Google Scholar]
- Rao P. S., Niederpruem D. J. Carbohydrate metabolism during morphogenesis of Coprinus lagopus (sensu Buller). J Bacteriol. 1969 Dec;100(3):1222–1228. doi: 10.1128/jb.100.3.1222-1228.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Shortle D., Haber J. E., Botstein D. Lethal disruption of the yeast actin gene by integrative DNA transformation. Science. 1982 Jul 23;217(4557):371–373. doi: 10.1126/science.7046050. [DOI] [PubMed] [Google Scholar]
- Smith A. J., Berg P. Homologous recombination between defective neo genes in mouse 3T6 cells. Cold Spring Harb Symp Quant Biol. 1984;49:171–181. doi: 10.1101/sqb.1984.049.01.020. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Folger K. R., Capecchi M. R. High frequency targeting of genes to specific sites in the mammalian genome. Cell. 1986 Feb 14;44(3):419–428. doi: 10.1016/0092-8674(86)90463-0. [DOI] [PubMed] [Google Scholar]
- Tilburn J., Scazzocchio C., Taylor G. G., Zabicky-Zissman J. H., Lockington R. A., Davies R. W. Transformation by integration in Aspergillus nidulans. Gene. 1983 Dec;26(2-3):205–221. doi: 10.1016/0378-1119(83)90191-9. [DOI] [PubMed] [Google Scholar]
- Tilby M. J. Tryptophan biosynthesis in Coprinus lagopus: a genetic analysis of mutants. J Gen Microbiol. 1976 Mar;93(1):126–132. doi: 10.1099/00221287-93-1-126. [DOI] [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]
- Walz A., Ratzkin B., Carbon J. Control of expression of a cloned yeast (Saccharomyces cerevisiae) gene (trp5) by a bacterial insertion element (IS2). Proc Natl Acad Sci U S A. 1978 Dec;75(12):6172–6176. doi: 10.1073/pnas.75.12.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward V. W., De Zeeuw J. R., Srb A. M. THE SEPARATION AND ISOLATION OF PARTICULAR BIOCHEMICAL MUTANTS OF NEUROSPORA BY DIFFERENTIAL GERMINATION OF CONIDIA, FOLLOWED BY FILTRATION AND SELECTIVE PLATING. Proc Natl Acad Sci U S A. 1954 Mar;40(3):192–200. doi: 10.1073/pnas.40.3.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelton M. M., Hamer J. E., Timberlake W. E. Transformation of Aspergillus nidulans by using a trpC plasmid. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1470–1474. doi: 10.1073/pnas.81.5.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelton M. M., Timberlake W. E., Hondel C. A. A cosmid for selecting genes by complementation in Aspergillus nidulans: Selection of the developmentally regulated yA locus. Proc Natl Acad Sci U S A. 1985 Feb;82(3):834–838. doi: 10.1073/pnas.82.3.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalkin H., Yanofsky C. Yeast gene TRP5: structure, function, regulation. J Biol Chem. 1982 Feb 10;257(3):1491–1500. [PubMed] [Google Scholar]
- Zolan M. E., Pukkila P. J. Inheritance of DNA methylation in Coprinus cinereus. Mol Cell Biol. 1986 Jan;6(1):195–200. doi: 10.1128/mcb.6.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]





