Abstract
Sleep apnoea and respiratory difficulties are reported in adult-onset Alexander’s disease (AOAD), an autosomal-dominant leukodystrophy that presents mainly with progressive ataxia. We demonstrate for the first time that the respiratory symptoms can result from association of palatal tremor with a similar tremor of laryngeal and respiratory muscles that interrupts normal inspiration and expiration.
A 60-year-old woman presented with progressive ataxia, palatal tremor and breathlessness. MRI revealed medullary atrophy, bilateral T2 hyperintensities in the dentate nuclei and hypertrophic olivary degeneration (HOD). AOAD was confirmed genetically with a positive glial fibrillary acidic protein (GFAP) mutation. Electrophysiological study revealed 1.5 Hz rhythmic laryngeal and respiratory muscle activity. Her respiratory symptoms were significantly improved at night with variable positive pressure ventilation.
This case illustrates that palatal tremor in AOAD, and potentially in other conditions, may be associated with treatable breathlessness due to a similar tremor of respiratory muscles.
Keywords: Movement disorders (other than Parkinsons), Neuroimaging, Mechanical ventilation, neurogenetics
Background
Adult-onset Alexander’s disease (AOAD) is a rare autosomal-dominant leukodystrophy characterised by slowly progressive dysarthria, dysphagia, palatal tremor, cerebellar ataxia and pyramidal signs.1 2 Like the more common younger-onset forms, it results from a mutation of the gene coding for glial fibrillary acidic protein (GFAP) that deposits within astrocytic cytoplasmic inclusions and gives the astrocytes their characteristic pathological appearance as Rosenthal fibres.3 4 However, unlike the juvenile forms, adult-onset disease typically spares the frontal lobes, with atrophy and T2-weighted hyperintensity on MRI restricted to the medulla, middle cerebellar peduncles, dentate nuclei and cervical spinal cord.5
Rarely, as the disease progresses, sleep apnoea and respiratory dysfunction may appear but their nature is poorly characterised. One case of sleep apnoea has been reported to relate to epiglottic dysfunction.6 A further potential mechanism for respiratory disturbance exists in relation to palatal tremor, a feature of some patients with AOAD; palatal tremor can also result from many other diseases and breathing difficulty in these conditions sometimes results from spread of the tremor to the cricothyroid muscles, thereby interrupting airflow.7 8
This report describes a case of genetically confirmed AOAD where there was development of marked breathlessness associated with a palatal tremor-type modulation on electromyographic recording of laryngeal and respiratory muscle activity. A nocturnal variable positive pressure device, adjusted to a level found on laryngoscopy optimally to open the vocal cords during inspiration, provided considerable symptom relief. Given the disease’s genetic association is only recently discovered, its phenotypic range is not yet strongly established and so there should be general awareness of the potential for treatable respiratory difficulties in patients presenting with progressive ataxia and palatal tremor.
Case presentation
A 60-year-old woman presented with a 6-year history of progressive unsteadiness of gait, oscillopsia, speech difficulty and mild swallowing difficulty, tending to choke equally on food and drink. She had also developed breathlessness with no clear pulmonary cause, sleep apnoea described as obstructive and urinary incontinence. Her medical history included ischaemic heart disease and type II diabetes with macular degeneration. Her mother died aged 60 years of ischaemic heart disease, but also had unsteadiness. Similarly, her mother’s mother and sister had been wheelchair users from late middle age due to unsteadiness. A maternal uncle’s son is affected by unsteadiness.
On examination, she was somewhat breathless at rest and on listening carefully there were glottic interruptions of her breathing both on inspiration and expiration. Cognition was normal. She had gaze-evoked nystagmus in all directions and broken smooth pursuit. She was mildly dysarthric of cerebellar pattern. Examination of the tongue revealed a 1–2 Hz tremor and this was also present in the soft palate. There was visible movement of the skin of the anterior neck in time with this activity. The rest of the cranial nerves were normal. In the limbs, there were no pyramidal signs or fasciculations but she had mild ataxia on finger-nose pointing. Repetitive hand and finger movements were slow. She was uniformly hyporeflexic. She had a wide-based unsteady gait.
Investigations
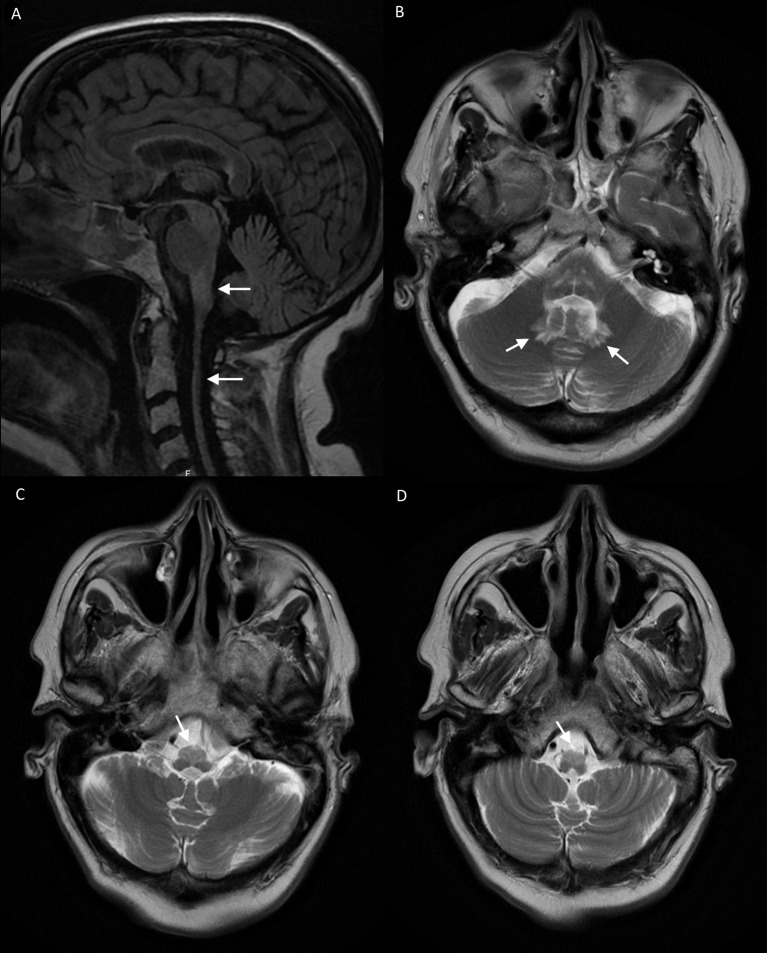
MRI revealed atrophy of the medulla and cervical spinal cord with high T2-weighted signal in the medulla and dentate nuclei (figure 1). There was particular high signal and relative tissue expansion in the region of the inferior olives indicative of hypertrophic olivary degeneration (HOD).
Figure 1.
(A) Sagittal FLAIR MRI showing medullary and spinal cord atrophy. (B–D) Contiguous 5 mm slice axial T2-weighted MRI showing bilateral dentate signal hyperintensity (B) and bilateral signal hyperintensity in the anterior medulla with relative hypertrophy (C, D).
Investigation of the patient’s breathlessness at rest included a normal CT chest, O2 saturation 94% on air, forced expiratory volume in 1 s (FEV1) of 2.1 L (96% predicted), forced vital capacity (FVC) of 2.4 L (99% predicted), peak flow of 395 L/min (114% predicted) and sniff inspiratory pressure was normal at 90 cm H2O. Echocardiography and myocardial perfusion scanning were normal. A sleep study revealed a mild to moderate deficit, with mean O2 saturation of 93%, not dipping below 80%, and an apnoea hypopnoea index of 17.3/hour.
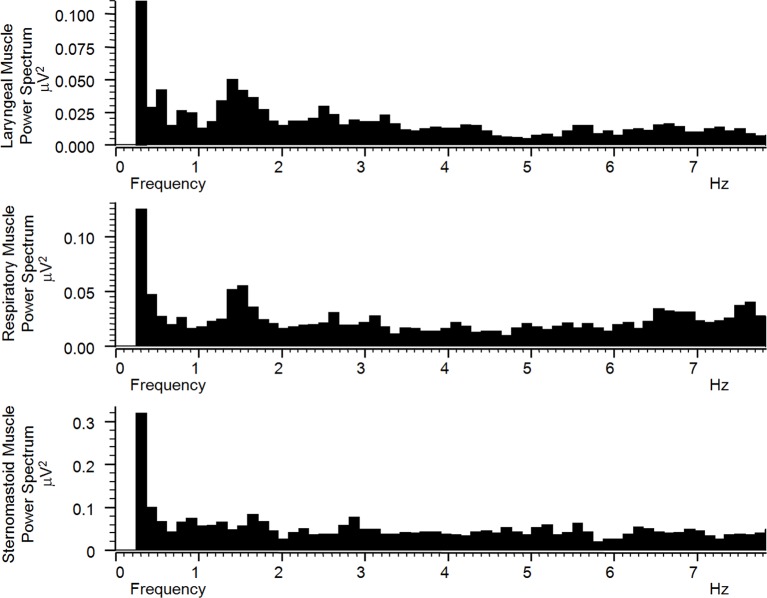
If a respiratory or glottic muscle tremor were intermittently interfering with breathing, this might not be revealed on sleep study; for example, apnoeic episodes are defined as 10 s duration and respiratory stops integrated over a 5 s period and so would not detect the brief but frequent interruptions of a respiratory tremor. Similarly, a peak sniff inspiratory pressure recording might not detect an interruption of smooth sustained inspiration, while a forced expiration might override an interruption of tidal expiration. Therefore, surface electromyogram (EMG) recordings were made of the postero-inferior rib cage overlying the lower intercostal and diaphragmatic muscles, and of the right and left omohyoid/ thyrohyoid muscles and right and left sternomastoid muscles. A 1.5 Hz rhythmicity matching the tongue and palate tremor was found on power spectra bilaterally in both laryngeal and respiratory muscles but not the sternomastoid muscles (figure 2). Lack of sternomastoid muscle involvement indicated that the spectral peaks in the other muscles were not simply artefactual or due to passive electrotonic spread. Separate sampling periods during inspiration and expiration (as distinguished by overall level of respiratory muscle activity) revealed the same 1.5 Hz peak activity. These findings indicated that the tremor of her palate was also involving the laryngeal muscles, interrupting air flow, and the respiratory muscles directly to interrupt inspiration and expiration.
Figure 2.
Power spectra of filtered and rectified surface electromyogram signal digitally sampled at 2000 Hz from right laryngeal, right respiratory and right sternomastoid muscles. There is a peak frequency in laryngeal and respiratory muscle activities at around 1.5 Hz that corresponds to the visible palatal and tongue tremor rhythm. This peak frequency is not present in sternomastoid muscle activity.
Diagnosis
In view of the progressive ataxia, dysarthria, eye movement abnormalities, palatal and tongue tremor and family history suggestive of autosomal-dominant inheritance, a clinical diagnosis of AOAD was made. She had had no sudden deteriorations suggestive of brainstem stroke as an alternative diagnosis. It later came to light that her uncle’s son had also been diagnosed on the basis of a positive GFAP gene mutation as having Alexander’s disease and subsequent GFAP genetic testing in our patient was positive.
Treatment
Various medications were used in an attempt to treat the tremor component of the patient’s condition. The patient unfortunately could not tolerate clonazepam and had a poor response to gabapentin, levetiracetam and memantine.
Specifically for breathlessness at night, a standard continuous positive airway pressure (CPAP) mask for sleep apnoea was not tolerated and a variable positive pressure device triggered by breathing was not helpful. However, it was subsequently possible using laryngoscopy to adjust the latter device to a pressure level during inspiration that minimised vocal cord closures associated with palatal tremor, and this provided significant relief of her breathlessness at night.
Outcome and follow-up
Unfortunately, the patient has followed the typical pattern of progression of symptoms of AOAD, and she is increasingly using the variable positive pressure device during the daytime to relieve breathlessness.
Discussion
This case of AOAD is the first where breathing difficulty has been demonstrated to relate to a laryngeal and respiratory muscle tremor similar to palatal tremor. Indeed, palatal tremor of any cause has never previously been linked to respiratory muscle involvement. Recognising this as the cause of her breathlessness encouraged us to pursue a special system of assisted ventilation that provided considerable relief.
Conditions other than AOAD that result in palatal tremor include idiopathic cases, with no associated features or abnormal imaging findings, and symptomatic cases that typically result from a lesion of the so-called Guillain-Mollaret triangle linking the red nucleus and the inferior olive with the contralateral dentate nucleus.9 The most common symptomatic cause is cerebrovascular insult, followed by neurodegenerative diseases (eg, polymerase gamma (POLG) mutations, surfeit locus protein 1 (SURF-1) mutations, Alexander’s disease, dark dentate disease, olivopontocerebellar atrophy), trauma, demyelination, neuroinflammation (eg, Hashimoto’s encephalitis, Whipple’s disease, Behcet’s disease and antigliadin antibody disease), alcohol toxicity and tumour.10 Many such cases will have other clinical features relating to brainstem dysfunction.
A common imaging finding in symptomatic cases of palatal tremor is hypertrophic olivary degeneration (HOD). When there is a lesion of the afferent pathway from the dentate, through the superior cerebellar peduncle, past the contralateral red nucleus and down to the contralateral inferior olive, trans-synaptic degeneration of inferior olivary neurons may occur.11 After a few months, vacuolation results in pseudo-hypertrophy on MRI which gradually subsides again after a few years to leave atrophy.12 13 Our case clearly showed HOD, and yet this is not previously described in palatal tremor resulting from AOAD.5 10 This suggests that AOAD and other neurodegenerative conditions are not unique in bypassing the trans-synaptic degenerative process; perhaps the course of onset in previous cases has simply been too gradual for the transient hypertrophic vacuolated phase of olivary degeneration to be seen on imaging.
Why palatal tremor should be the result of such degeneration is poorly understood. It has been proposed that the critical lesion lies in the inhibitory GABAergic input from the dentate to the inferior olive, an input that normally promotes closure of gap junctions between olivary dendrites.14 To result in the localised low frequency oscillation of palatal tremor, this process would have to be distinct somehow from the gap junction opening that has been proposed to result in the higher frequency more widespread oscillation of essential tremor.15 16
Our case and previous literature do indicate that there is some spread of low frequency palatal tremor oscillations in symptomatic palatal tremor. Certain associated eye movement abnormalities, such as square wave jerks and pendular nystagmus, appear by observation to be synchronous with the palatal tremor rhythm,10 but the situation is not clear in previous rare cases where palatal tremor was associated with laryngeal involvement.7 8 Occasional case reports have described a dystonic-type limb or head tremor with palatal tremor,17 18 but the latter tremors were at a different frequency and may have reflected additional pathology resulting from the same brainstem insult. Another study has reported limb muscle 3 Hz oscillation in HOD without palatal tremor.19 Isolated diaphragmatic myoclonus, or van Leeuwenhoek’s disease, is well recognised,20 21 but has not been associated with palatal tremor, with any of the other features of AOAD or with HOD. There is a single case report of essential palatal tremor also involving the face and the vocal cords, though the tremor frequency was higher than usual.22
The finding in this report of the same frequency of oscillation in the palate, the tongue, the larynx and the respiratory muscles but not the sternomastoids demonstrates clearly for the first time that the typical palatal tremor oscillation may simultaneously modulate a number of other muscle groups. Given that the affected muscles and motor nuclei are linked neither developmentally nor anatomically, this selective susceptibility perhaps relates to common olivary throughput of internuclear pathways mediating particular brainstem functions such as swallowing, breathing and vestibulo-ocular reflexes.
Learning points.
Palatal tremor is a slow regular 1–2 Hz involuntary movement of the soft palate and may be essential (isolated) or symptomatic (associated with a structural brain lesion in the Guillain-Mollaret triangle and sometimes accompanied by other neurological deficits).
Symptomatic cases have many aetiologies, one example being adult-onset Alexander’s disease (AOAD), an autosomal-dominant mutation of the gene coding for glial fibrillary acidic protein.
A characteristic imaging feature of certain causes of symptomatic palatal tremor is hypertrophic olivary degeneration (HOD), thought to be due to trans-synaptic degeneration from a lesion of the pathway from the dentate to the contralateral inferior olive. Our case of AOAD shows that HOD can be a feature of neurodegenerative causes of palatal tremor, which has not previously been described.
AOAD and other causes of symptomatic palatal tremor have been reported as being associated with breathlessness. Our case presents clinical and electrophysiological evidence that this is due to spread of the palatal tremor to laryngeal and respiratory muscles and may be treated by variable positive pressure ventilation set at a level to minimise the effect of tremulous vocal cord closures during inspiration.
Footnotes
Contributors: JA contributed substantially to managing the patient, planned the report and conducted a literature search pertaining to the report, wrote the manuscript, revised drafts of the manuscript and agrees to be accountable for the article and to ensure that all questions regarding the accuracy or integrity of the article are investigated and resolved.
RT contributed substantially to the respiratory aspects of patient management, revisions of drafts of the manuscript and approval of the submitted version and agrees to be accountable for the article and to ensure that all questions regarding the accuracy or integrity of the article are investigated and resolved.
AS contributed substantially to the respiratory aspects of patient management, revisions of drafts of the manuscript and approval of the submitted version and agrees to be accountable for the article and to ensure that all questions regarding the accuracy or integrity of the article are investigated and resolved.
SC contributed substantially to the radiological aspects of patient management, revisions of drafts of the manuscript and approval of the submitted version and agrees to be accountable for the article and to ensure that all questions regarding the accuracy or integrity of the article are investigated and resolved.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Schwankhaus JD, Parisi JE, Gulledge WR, et al. . Hereditary adult-onset Alexander’s disease with palatal myoclonus, spastic paraparesis, and cerebellar ataxia. Neurology 1995;45:2266–71. 10.1212/WNL.45.12.2266 [DOI] [PubMed] [Google Scholar]
- 2. Pareyson D, Fancellu R, Mariotti C, et al. . Adult-onset Alexander disease: a series of eleven unrelated cases with review of the literature. Brain 2008;131:2321–31. 10.1093/brain/awn178 [DOI] [PubMed] [Google Scholar]
- 3. Brenner M, Johnson AB, Boespflug-Tanguy O, et al. . Mutations in GFAP, encoding glial fibrillary acidic protein, are associated with Alexander disease. Nat Genet 2001;27:117–20. 10.1038/83679 [DOI] [PubMed] [Google Scholar]
- 4. Stumpf E, Masson H, Duquette A, et al. . Adult Alexander disease with autosomal dominant transmission: a distinct entity caused by mutation in the glial fibrillary acid protein gene. Arch Neurol 2003;60:1307–12. 10.1001/archneur.60.9.1307 [DOI] [PubMed] [Google Scholar]
- 5. Graff-Radford J, Schwartz K, Gavrilova RH, et al. . Neuroimaging and clinical features in type II (late-onset) Alexander disease. Neurology 2014;82:49–56. 10.1212/01.wnl.0000438230.33223.bc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ishikawa M, Shimohata T, Ishihara T, et al. . Sleep apnea associated with floppy epiglottis in adult-onset Alexander disease: a case report. Mov Disord 2010;25:1098–100. 10.1002/mds.23042 [DOI] [PubMed] [Google Scholar]
- 7. Toland AD, Porubsky ES, Coker NJ, et al. . Velo-pharyngo-laryngeal myoclonus: evaluation of objective tinnitus and extrathoracic airway obstruction. Laryngoscope 1984;94:691–5. [PubMed] [Google Scholar]
- 8. Sumer M. Symptomatic palatal myoclonus: an unusual cause of respiratory difficulty. Acta Neurol Belg 2001;101:113–5. [PubMed] [Google Scholar]
- 9. Deuschl G, Toro C, Valls-Solé J, et al. . Symptomatic and essential palatal tremor. 1. Clinical, physiological and MRI analysis. Brain 1994;117:775–88. [DOI] [PubMed] [Google Scholar]
- 10. Samuel M, Torun N, Tuite PJ, et al. . Progressive ataxia and palatal tremor (PAPT): clinical and MRI assessment with review of palatal tremors. Brain 2004;127:1252–68. 10.1093/brain/awh137 [DOI] [PubMed] [Google Scholar]
- 11. Goto N, Kaneko M. Olivary enlargement: chronological and morphometric analyses. Acta Neuropathol 1981;54:275–82. 10.1007/BF00697000 [DOI] [PubMed] [Google Scholar]
- 12. Goyal M, Versnick E, Tuite P, et al. . Hypertrophic olivary degeneration: metaanalysis of the temporal evolution of MR findings. AJNR Am J Neuroradiol 2000;21:1073–7. [PMC free article] [PubMed] [Google Scholar]
- 13. Konno T, Broderick DF, Tacik P, et al. . Hypertrophic olivary degeneration: a clinico-radiologic study. Parkinsonism Relat Disord 2016;28:36–40. 10.1016/j.parkreldis.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pearce JM. Palatal myoclonus (syn. palatal tremor). Eur Neurol 2008;60:312–5. 10.1159/000159929 [DOI] [PubMed] [Google Scholar]
- 15. De Zeeuw CI, Simpson JI, Hoogenraad CC, et al. . Microcircuitry and function of the inferior olive. Trends Neurosci 1998;21:391–400. 10.1016/S0166-2236(98)01310-1 [DOI] [PubMed] [Google Scholar]
- 16. McAuley JH, Marsden CD. Physiological and pathological tremors and rhythmic central motor control. Brain 2000;123:1545–67. 10.1093/brain/123.8.1545 [DOI] [PubMed] [Google Scholar]
- 17. Yanagisawa T, Sugihara H, Shibahara K, et al. . Natural course of combined limb and palatal tremor caused by cerebellar-brain stem infarction. Mov Disord 1999;14:851–4. [DOI] [PubMed] [Google Scholar]
- 18. Shaikh AG, Ghasia FF, DeLong MR, et al. . Ocular palatal tremor plus dystonia – new syndromic association. Mov Disord Clin Pract 2015;2:267–70. 10.1002/mdc3.12193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu JC, Lu CS, Ng SH, et al. . Limb myorhythmia in association with hypertrophy of the inferior olive: report of two cases. Chang Gung Med J 2000;23:630–5. [PubMed] [Google Scholar]
- 20. Chen R, Remtulla H, Bolton CF. Electrophysiological study of diaphragmatic myoclonus. J Neurol Neurosurg Psychiatry 1995;58:480–3. 10.1136/jnnp.58.4.480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Espay AJ, Fox SH, Marras C, et al. . Isolated diaphragmatic tremor: is there a spectrum in "respiratory myoclonus"? Neurology 2007;69:689–92. 10.1212/01.wnl.0000267431.16316.69 [DOI] [PubMed] [Google Scholar]
- 22. Panda AK, Kushwaha S, Kaur M. Essential palatal tremor with hemifacial and vocal cord tremor. BMJ Case Rep 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]