Abstract
The authors present a case of a 57-year-old man, who presented to the surgical clinic with a mass in the suprapubic region. A CT scan revealed a well-circumscribed lobular, heterogeneous soft tissue mass measuring 12×8.6×7.8 cm. The final histopathological diagnosis from the resection of the lesion was a myxofibrosarcoma (MFS), grade 3. The management of MFS includes surgical and oncological options which are reviewed here. These are aimed at complete excision and reducing the risk of local occurrence.
Keywords: Oncology, Surgical oncology
Background
Myxofibrosarcoma (MFS), previously known as myxoid malignant fibrous histiocytoma, is a connective tissue neoplasm and a rare form of sarcoma. It is, however, one of the most common sarcomas of the elderly and predominantly affects the extremities.1–3 MFS involves the dermis or subcutaneous tissue in over two-thirds of cases. More deep-seated tumours show a higher rate of metastasis and tumour-related death.1 4 Tumours are divided into three grades, with grade I being locally aggressive and grades II and III showing metastatic potential, as well as having more complex cytogenic aberrations.5 All grades of MFS show a high degree (50%–60%) of local recurrence following excision, often increasing in histological grade, aggressiveness and number of cytogenic abnormalities.5–8 MFS is a diagnosis of exclusion, and there is a lack of firm prognosticators to predict tumour invasion and metastatic spread.9 We describe a case of high-grade MFS of the abdominal wall in a middle-aged patient and review the surgical and oncological management options.
Case presentation
A 57-year-old man presented to the surgical clinic at a district general hospital with a history of right iliac fossa discomfort over the course of 5 months. He had lost a stone in weight and had developed night sweats and fever. He had a history of travelling to Bangladesh before the discomfort started. The patient was previously fit and well without any previous operations or trauma to the region. He took atorvastatin and amitriptyline and had no allergies. He had a significant family history of breast cancer and had previously screened positive for the breast cancer 2 gene.
On examination, the patient looked pale and there was a tender, non-reducible, non-mobile mass felt in the suprapubic region measuring 10×10 cm in size. Per rectum (PR) examination and rigid sigmoidoscopy was unremarkable. His haemoglobin was 103 and had a raised c-reactive protein of 173.
The patient underwent a CT scan of his abdomen and pelvis (figure 1). This revealed a well-circumscribed lobular, heterogeneous soft tissue mass with solid and cystic components measuring 12×8.6×7.8 cm in maximal dimensions arising from the lower right rectus abdominis muscle. It was situated superior to the urinary bladder and posteriorly displaced some small bowel loops but there was no invasion of any surrounding structures. The rest of the abdomen and pelvis was unremarkable.
Figure 1.
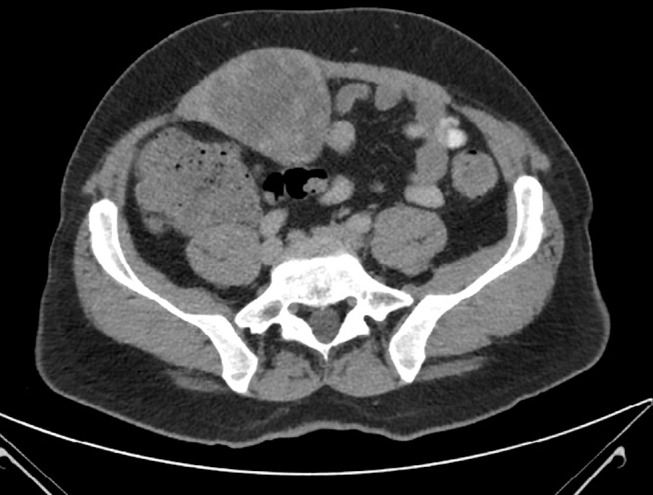
CT scan of the abdomen showing a heterogeneous soft tissue mass within the pelvis arising from the lower right rectus abdominis muscle.
The patient was discussed in a multidisciplinary meeting and referred to a tertiary centre for further management. A needle biopsy of the mass was investigated, and immunohistochemistry was performed. The tumour showed cores of a high-grade spindle cell tumour with scattered nuclear pleomorphism in a fibromyxoid stroma, with infiltration of skeletal muscle fibres and fascial-type tissue (figure 2). Staining for mooth muscle antibody (SMA), caldesmon, desmin, S100, pancytokeratin (AE1-AE3), MYOD1, CD34, CD117 and DOG1 were all negative. Cytogenetic studies followed, where interphase fluorescence in situ hybridisation was used to establish negative MDM2 gene amplification status. In theatre, the sarcoma was confirmed to have invaded the rectus abdominis muscle, as well as the posterior sheath. The bladder was uninvolved and safely moved away during the resection. The tumour was excised with a 1 cm margin (figure 3), and the abdominal wall reconstructed with a BARD (Bard Davol Inc. ltd.) mesh. After resection of the tumour, histology confirmed complete excision of a MFS, grade 3. The tumour was surrounded by skeletal muscle and fibro-fatty tissue and focally extends to within 1.5 mm of the inked margin, that is, the resected specimen had negative but narrow margins. Following this, he underwent a 6-week course of adjuvant radiotherapy.
Figure 2.
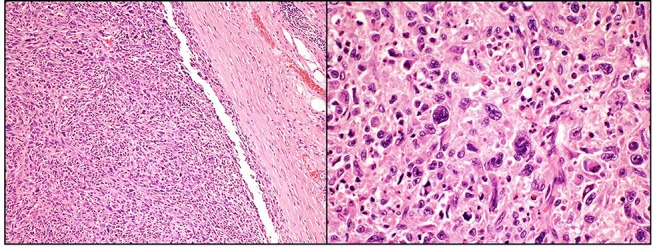
Well-defined intramuscular tumour composed of sheets and fascicles of highly pleomorphic spindle cells, cellular areas and myxoid background (H&E 100x and 400x).
Figure 3.
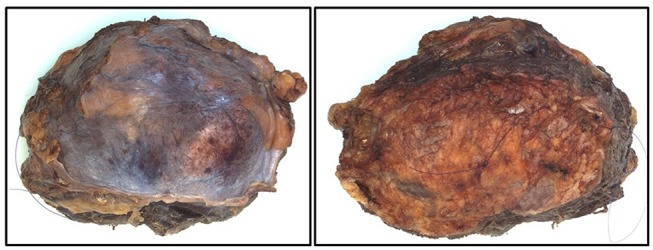
The resected sarcoma, measuring 13×10×6 cm.
Discussion
Intermediate and high-grade sarcomas present a significant difficulty in management, as they carry a much higher risk of recurrence and distant metastasis versus low-grade sarcomas. Ten per cent of the patient population who present with sarcoma will have distant lung metastasis, having a significant effect on survival.10 Because of this, it is of great importance to excise the tumour with wide margins to ensure the best chance of curative treatment.7 11 The complete management of sarcomas demands input from a multidisciplinary team of medical oncologists, clinical oncologists, radiologists, pathologists and surgeons to produce an effective treatment plan for patients.12
Surgical management
Abdominal wall sarcoma surgery involves two stages. First, the tumour must be resected and second, the abdominal wall needs to be reconstructed. For tumour removal, there is no consensus on the width of surrounding normal tissue that should be removed to improve outcomes.13 Negative tumour margins lower local recurrence rates and improve long-term survival.14 15 Indeed, the wider these resection margins the better, especially with poorly differentiated tumours, which may have satellite lesions and a greater predisposition to local recurrence.16 In the event of tumour invasion or compression of an important adjacent structure, the surgeon must consider the risks and benefits of either partial resection of the adjacent structure or careful dissection around the tumour with a higher likelihood of leaving a positive resection margin.13 In the later instance, preoperative or postoperative radiotherapy can be offered.
The two operative stages are not mutually exclusive as the position and wound status of the abdominal wall defect created as well as the histology of the resected tumour alters the reconstructive options. For position, a resected tumour can leave either a partial or a complete abdominal wall defect depending on its depth. Reconstructive surgeons classify partial defects as those involving either loss of the skin and subcutaneous tissue or loss of just the myofascial tissue. Complete defects are classified as those that involve full-thickness loss of both the superficial (skin and subcutaneous tissue) and myofascial layers.17 18
Superficial partial defects are treated according to their size. Defects less than 5 cm can be treated with primary closure. Larger defects can be treated with skin grafts, vacuum assisted closure, tissue expanders, local and distant flaps.17 Myofascial partial defects can either be closed or bridged. Large defects are sometimes bridged with prosthetic mesh. Reconstructive surgeons try to avoid a bridged repair as these have higher complication rates, in particular higher postoperative herniation rates.19
Complete closure of the myofascial defect can be aided by open or endoscopic component separation (with or without mesh augmentation), local and distant pedicle tissue flaps and free flaps. Recently more advanced techniques include subcutaneous tissue expanders20 and transverse abdominis release.21
Complete defects or full thickness defects can be separated out according to whether fascial and skin closure can or cannot be achieved. As a general rule defects with a width less than 15 cm can be closed especially if the defect is in the midline.17 For large lateral defects and for defects greater than 15 cm in the midline, the lost tissue needs to be replaced and a variety of flaps can be used. The position of the defect in relation to the midline, the rib cage and the pelvis affects the reconstruction technique and type of flap that can be used (table 1).
Table 1.
A summary of surgical techniques used for sarcomas, based on type and location
| Defect characteristic | Subgroup | Dimensions | Surgical technique |
| Position | |||
| Partial defect | Superficial | <5 cm wide | Primary closure |
| >5 cm wide | Skin graft | ||
| Vacuum dressing | |||
| Tissue expansion (TE) | |||
| Local/distant flap/ free tissue transfer (FTT) |
|||
| Myofascial | |||
| Central | <10 cm wide | Primary closure | |
| Component separation (open/endoscopic) | |||
| Transverse abdominis release | |||
| Local flaps (rectus/ext./int. oblique) | |||
| >10 cm wide | Distant flaps: transverse fascia Lata (TFL) | ||
| Rectus femoris | |||
| Vastus lateralis (anterolateral thigh) | |||
| TE/FTT | |||
| Lateral | <5 cm wide | Primary closure | |
| Component separation (open/endoscopic) | |||
| Transverse abdominis release | |||
| Local flaps (rectus/ext./int. oblique) | |||
| >5 cm wide | Distant flaps: TFL | ||
| Rectus femoris | |||
| Vastus lateralis (anterolateral thigh) | |||
| TE/FTT | |||
| Complete defect | Upper abdomen | <10 cm wide | Adequate skin—see partial myofascial options |
| >10 cm wide | Local flaps: superior rectus abdominis | ||
| External oblique | |||
| Distant flaps: ext. latissimus dorsi | |||
| Ext. TFL | |||
| TE/FTT | |||
| Mid abdomen | <10 cm wide | Adequate skin—see partial myofascial options | |
| >10 cm wide | Local flaps: rectus abdominis | ||
| External oblique | |||
| Distant flaps: TFL | |||
| Rectus femoris | |||
| TE/FTT | |||
| Lower abdomen | <10 cm wide | Adequate skin—see partial myofascial options | |
| >10 cm wide | Local flaps: inferior rectus flap | ||
| Internal oblique | |||
| Distant flaps: TFL | |||
| Rectus femoris | |||
| Vastus lateralis | |||
| TE/FTT | |||
| Wound status | Local infection | If infected, a temporary repair with a bridging mesh is advised. After treatment of the wound infection and a sterile defect is obtained, assess according to defect position. | |
| Histology | Possible positive margins | If positive margins are likely, a temporary repair with a bridging mesh is advised. After re-excision of positive margins and/or remission of disease is established, assess according to defect position. | |
The wound status of an open defect needs to be examined prior to reconstruction. If there is infection or inflammation (eg, from an enterocutaneous fistula (ECF)) or surrounding cellulitis, delayed reconstruction after a prolonged course of intravenous antibiotics may be beneficial as local wound complications are much less likely with a sterile operating field.17 Another consideration is whether preoperative radiotherapy has been used. Radiotherapy may affect the surrounding abdominal wall tissue and the surgeon may be more inclined to consider reconstruction using healthy, vascularised tissue from a regional or distant source to replace radiated local tissues. Vascularised tissue should lower local wound complications.22
Finally, the reconstructive surgeon must consider the risk of local sarcoma recurrence. This may occur because of high-grade histology,16 because the tumour may not be encapsulated but possess finger-like projections into the surrounding tissue23 or because of a high likelihood of positive resection margins. If local recurrence is likely, a temporary method of abdominal wall reconstruction may be used. Although this is less permanent, it facilitates re-excision of local recurrences and positive resection margins if required. A stronger more permanent reconstruction of the abdominal wall can be undertaken at a later stage once remission of primary disease has been established.24
Oncological management
While the first-line treatment of soft tissue sarcomas remains wide margin surgery, postoperative radiotherapy is often used as adjuvant therapy to improve curative rates. Radiotherapy shows a large benefit in reducing local recurrence, especially when surgical excision is not complete such as in cases where limbs need to be salvaged.12 External beam intensity modulated therapy (IMRT) is commonly used and shows a better profile in controlling local disease following surgical excision compared with control and brachytherapy both in high-grade and low-grade sarcomas.25 26While brachytherapy has a lower efficacy ratio versus IMRT, it significantly reduces local recurrence versus control, and the benefit of fewer side effects due to less irradiation of nearby structures should be considered.27 Other techniques such as proton beam therapy has been restricted to the use of areas where surgical excision with negative margins is uncommon, such as spinal sarcomas28 and retroperitoneal sarcomas,29 showing promising results.
While postoperative radiotherapy has traditionally been used, preoperative is also an option, having a similar local recurrence rate.30While it does incur higher acute wound complications, it can lead to fewer long-term soft tissue side effects such as fibrosis.31 In cases where preoperative radiotherapy has been given, there is little evidence to suggest that an adjuvant postoperative dose is needed in the case of positive margins when compared with re-excision surgery and planned conservative management.32 33 Postoperative radiotherapy has a larger role when surgery is unable to achieve negative margins while maintaining acceptable function, which may be the case in sarcomas of the extremities.34
The use of chemotherapy in aiding the treatment of localised disease is controversial and has classically not showed any tangible benefit on survival, with only marginal effects on overall recurrence.35 It is, however, important to point out that histological subtypes of sarcoma are associated with different outcomes following chemotherapy.36 Studies with negative results often include a heterogeneous patient cohort in terms of demographics and tumour characteristics.37 When focusing analysis on high-risk individuals with high-grade sarcomas, chemotherapy has suggested benefit to overall survival.38 39 The regimen most often used is doxorubicin or epirubicin together with ifosfamide in either a neoadjuvant or adjuvant context depending on the degree of disease, but this regimen may significantly change in the future, as chemotherapy becomes more targeted towards the molecular structure of the tumour.37 With further available evidence, clinicians will be able to tailor regimens on the specific subtype and grade of sarcoma, optimising benefit and reducing the associated risks of chemotherapy.
Learning points.
High-grade myxofibrosarcomas of the abdominal wall are rare presentations of sarcoma.
The main treatment options are surgical and oncological.
A multidisciplinary team approach to treatment is essential to ensure high curative rate and low recurrence rate.
Footnotes
Contributors: All authors provided substantial contributions to the conception or design of the work, the acquisition, analysis and interpretation of data, were involved in drafting the work or revising it critically for important intellectual content and gave final approval of the version published. They also agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Castronovo C, Arrese JE, Quatresooz P, et al. Myxofibrosarcoma: a diagnostic pitfall. Rare Tumors 2013;5:15–1. 10.4081/rt.2013.e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mentzel T, Calonje E, Wadden C, et al. Myxofibrosarcoma. clinicopathologic analysis of 75 cases with emphasis on the low-grade variant. Am J Surg Pathol 1996;20:391–405. [DOI] [PubMed] [Google Scholar]
- 3.Jo VY, Fletcher CD. WHO classification of soft tissue tumours: an update based on the 2013 (4th) edition. Pathology 2014;46:95–104. 10.1097/PAT.0000000000000050 [DOI] [PubMed] [Google Scholar]
- 4.Clarke LE, Zhang PJ, Crawford GH, et al. Myxofibrosarcoma in the skin. J Cutan Pathol 2008;35:935–40. 10.1111/j.1600-0560.2007.00922.x [DOI] [PubMed] [Google Scholar]
- 5.Willems SM, Debiec-Rychter M, Szuhai K, et al. Local recurrence of myxofibrosarcoma is associated with increase in tumour grade and cytogenetic aberrations, suggesting a multistep tumour progression model. Mod Pathol 2006;19:407–16. 10.1038/modpathol.3800550 [DOI] [PubMed] [Google Scholar]
- 6.Roland CL, Wang WL, Lazar AJ, et al. Myxofibrosarcoma. Surg Oncol Clin N Am 2016;25:775–88. 10.1016/j.soc.2016.05.008 [DOI] [PubMed] [Google Scholar]
- 7.Pencavel T, Strauss DC, Thomas JM, et al. The surgical management of soft tissue tumours arising in the abdominal wall. Eur J Surg Oncol 2010;36:489–95. 10.1016/j.ejso.2010.03.007 [DOI] [PubMed] [Google Scholar]
- 8.Riouallon G, Larousserie F, Pluot E, et al. Superficial myxofibrosarcoma: assessment of recurrence risk according to the surgical margin following resection. A series of 21 patients. Orthop Traumatol Surg Res 2013;99:473–7. 10.1016/j.otsr.2012.11.020 [DOI] [PubMed] [Google Scholar]
- 9.Lee JC, Li CF, Fang FM, et al. Prognostic implication of MET overexpression in myxofibrosarcomas: an integrative array comparative genomic hybridization, real-time quantitative PCR, immunoblotting, and immunohistochemical analysis. Mod Pathol 2010;23:1379–92. 10.1038/modpathol.2010.128 [DOI] [PubMed] [Google Scholar]
- 10.Williams KJ, Hayes AJ. A guide to oncological management of soft tissue tumours of the abdominal wall. Hernia 2014;18:91–7. 10.1007/s10029-013-1156-x [DOI] [PubMed] [Google Scholar]
- 11.Baldini EH, Goldberg J, Jenner C, et al. Long-term outcomes after function-sparing surgery without radiotherapy for soft tissue sarcoma of the extremities and trunk. J Clin Oncol 1999;17:3252–9. 10.1200/JCO.1999.17.10.3252 [DOI] [PubMed] [Google Scholar]
- 12.Ahmad R, Jacobson A, Hornicek F, et al. The width of the Surgical margin does not influence outcomes in extremity and truncal soft tissue sarcoma treated with radiotherapy. Oncologist 2016;21:1269–76. 10.1634/theoncologist.2015-0534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kandel R, Coakley N, Werier J, et al. Surgical margins and handling of soft-tissue sarcoma in extremities: a clinical practice guideline. Curr Oncol 2013;20:247–54. 10.3747/co.20.1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Youssef E, Fontanesi J, Mott M, et al. Long-term outcome of combined modality therapy in retroperitoneal and deep-trunk soft-tissue sarcoma: analysis of prognostic factors. Int J Radiat Oncol Biol Phys 2002;54:514–9. 10.1016/S0360-3016(02)02942-5 [DOI] [PubMed] [Google Scholar]
- 15.Trovik CS, Bauer HC, Alvegård TA, et al. Surgical margins, local recurrence and metastasis in soft tissue sarcomas: 559 surgically-treated patients from the scandinavian Sarcoma Group Register. Eur J Cancer 2000;36:710–6. [DOI] [PubMed] [Google Scholar]
- 16.Lintz F, Moreau A, Odri GA, et al. Critical study of resection margins in adult soft-tissue sarcoma surgery. Orthop Traumatol Surg Res 2012;98(4 Suppl):S9–S18. 10.1016/j.otsr.2012.04.006 [DOI] [PubMed] [Google Scholar]
- 17.Rohrich RJ, Lowe JB, Hackney FL, et al. An algorithm for abdominal wall reconstruction. Plast Reconstr Surg 2000;105:202–16. 10.1097/00006534-200001000-00036 [DOI] [PubMed] [Google Scholar]
- 18.Khansa I, Janis JE. Modern reconstructive techniques for abdominal wall defects after oncologic resection. J Surg Oncol 2015;111:587–98. 10.1002/jso.23824 [DOI] [PubMed] [Google Scholar]
- 19.Holihan JL, Askenasy EP, Greenberg JA, et al. Component separation vs. Bridged Repair for large ventral hernias: a Multi-Institutional Risk-Adjusted comparison, systematic review, and Meta-Analysis. Surg Infect 2016;17:17–26. 10.1089/sur.2015.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carlson GW, Elwood E, Losken A, et al. The role of tissue expansion in abdominal wall reconstruction. Ann Plast Surg 2000;44:147–53. 10.1097/00000637-200044020-00004 [DOI] [PubMed] [Google Scholar]
- 21.Novitsky YW, Elliott HL, Orenstein SB, et al. Transversus abdominis muscle release: a novel approach to posterior component separation during complex abdominal wall reconstruction. Am J Surg 2012;204:709–16. 10.1016/j.amjsurg.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 22.Tukiainen E, Leppäniemi A. Reconstruction of extensive abdominal wall defects with microvascular tensor fasciae latae flap. Br J Surg 2011;98:880–4. 10.1002/bjs.7489 [DOI] [PubMed] [Google Scholar]
- 23.Yang F. Radical tumor excision and immediate abdominal wall reconstruction in patients with aggressive neoplasm compromised full-thickness lower abdominal wall. Am J Surg 2013;205:15–21. 10.1016/j.amjsurg.2012.04.007 [DOI] [PubMed] [Google Scholar]
- 24.Siegel GW, Kuzon WM, Hasen JM, et al. Staged soft tissue reconstruction following sarcoma excision with anticipated large cutaneous defects: an Oncologically Safe Alternative. Iowa Orthop J 2016;36:104–8. [PMC free article] [PubMed] [Google Scholar]
- 25.Alektiar KM, Brennan MF, Singer S. Local control comparison of adjuvant brachytherapy to intensity-modulated radiotherapy in primary high-grade sarcoma of the extremity. Cancer 2011;117:3229–34. 10.1002/cncr.25882 [DOI] [PubMed] [Google Scholar]
- 26.Beane JD, Yang JC, White D, et al. Efficacy of adjuvant radiation therapy in the treatment of soft tissue sarcoma of the extremity: 20-year follow-up of a randomized prospective trial. Ann Surg Oncol 2014;21:2484–9. 10.1245/s10434-014-3732-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pisters PW, Harrison LB, Leung DH, et al. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J Clin Oncol 1996;14:859–68. 10.1200/JCO.1996.14.3.859 [DOI] [PubMed] [Google Scholar]
- 28.DeLaney TF, Liebsch NJ, Pedlow FX, et al. Long-term results of phase II study of high dose photon/proton radiotherapy in the management of spine chordomas, chondrosarcomas, and other sarcomas. J Surg Oncol 2014;110:115–22. 10.1002/jso.23617 [DOI] [PubMed] [Google Scholar]
- 29.Yoon SS, Chen YL, Kirsch DG, et al. Proton-beam, intensity-modulated, and/or intraoperative electron radiation therapy combined with aggressive anterior surgical resection for retroperitoneal sarcomas. Ann Surg Oncol 2010;17:1515–29. 10.1245/s10434-010-0935-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Sullivan B, Davis AM, Turcotte R, et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 2002;359:2235–41. 10.1016/S0140-6736(02)09292-9 [DOI] [PubMed] [Google Scholar]
- 31.Davis AM, O'Sullivan B, Turcotte R, et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 2005;75:48–53. 10.1016/j.radonc.2004.12.020 [DOI] [PubMed] [Google Scholar]
- 32.Al Yami A, Griffin AM, Ferguson PC, et al. Positive surgical margins in soft tissue sarcoma treated with preoperative radiation: is a postoperative boost necessary? Int J Radiat Oncol Biol Phys 2010;77:1191–7. 10.1016/j.ijrobp.2009.06.074 [DOI] [PubMed] [Google Scholar]
- 33.Pan E, Goldberg SI, Chen YL, et al. Role of post-operative radiation boost for soft tissue sarcomas with positive margins following pre-operative radiation and surgery. J Surg Oncol 2014;110:817–22. 10.1002/jso.23741 [DOI] [PubMed] [Google Scholar]
- 34.Kaushal A, Citrin D. The role of radiation therapy in the management of sarcomas. Surg Clin North Am 2008;88:629–46. 10.1016/j.suc.2008.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pervaiz N, Colterjohn N, Farrokhyar F, et al. A systematic meta-analysis of randomized controlled trials of adjuvant chemotherapy for localized resectable soft-tissue sarcoma. Cancer 2008;113:573–81. 10.1002/cncr.23592 [DOI] [PubMed] [Google Scholar]
- 36.Gronchi A, Frustaci S, Mercuri M, et al. Short, full-dose adjuvant chemotherapy in high-risk adult soft tissue sarcomas: a randomized clinical trial from the Italian Sarcoma Group and the Spanish Sarcoma Group. J Clin Oncol 2012;30:850–6. 10.1200/JCO.2011.37.7218 [DOI] [PubMed] [Google Scholar]
- 37.Saponara M, Stacchiotti S, Casali PG, et al. (Neo)adjuvant treatment in localised soft tissue sarcoma: the unsolved affair. Eur J Cancer 2017;70:1–11. 10.1016/j.ejca.2016.09.030 [DOI] [PubMed] [Google Scholar]
- 38.Woll PJ, Reichardt P, Le Cesne A, et al. Adjuvant chemotherapy with doxorubicin, Ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol 2012;13:1045–54. 10.1016/S1470-2045(12)70346-7 [DOI] [PubMed] [Google Scholar]
- 39.Brodowicz T, Schwameis E, Widder J, et al. Intensified Adjuvant IFADIC Chemotherapy for adult Soft tissue sarcoma: a Prospective Randomized Feasibility Trial. Sarcoma 2000;4:151–60. 10.1155/2000/126837 [DOI] [PMC free article] [PubMed] [Google Scholar]


