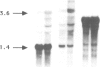Abstract
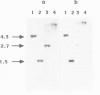
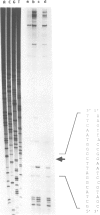
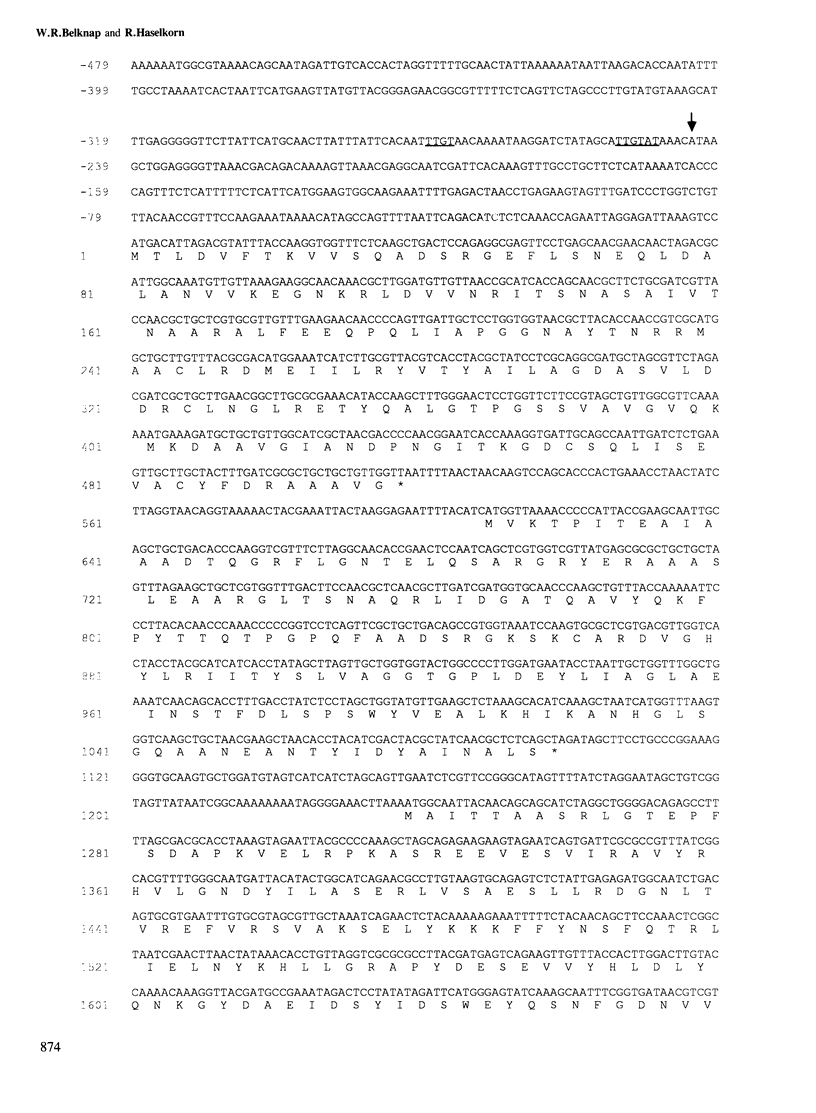
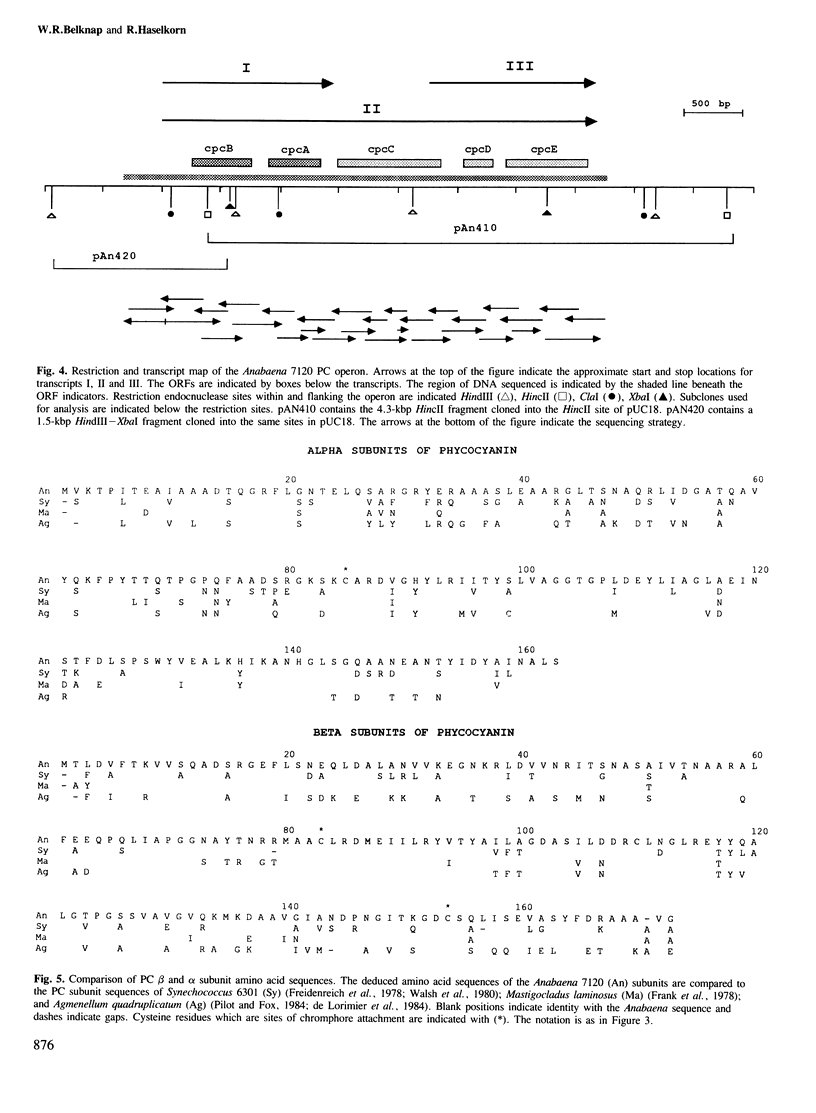
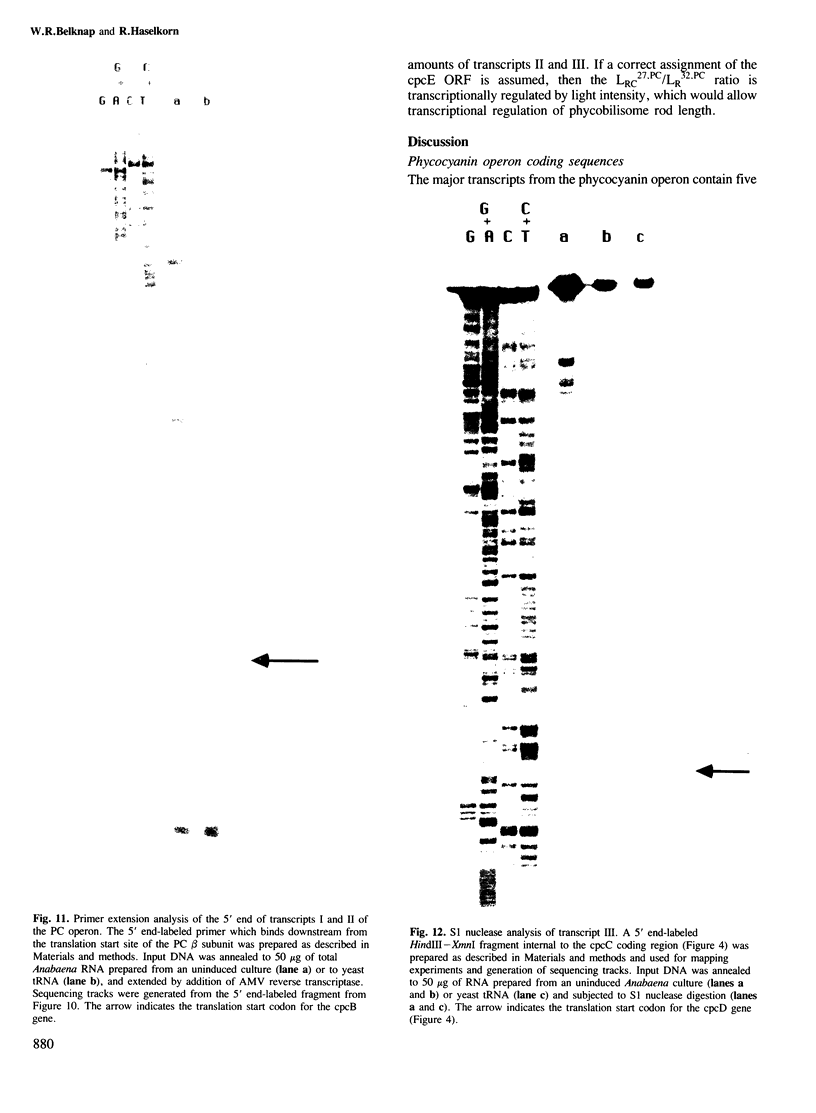
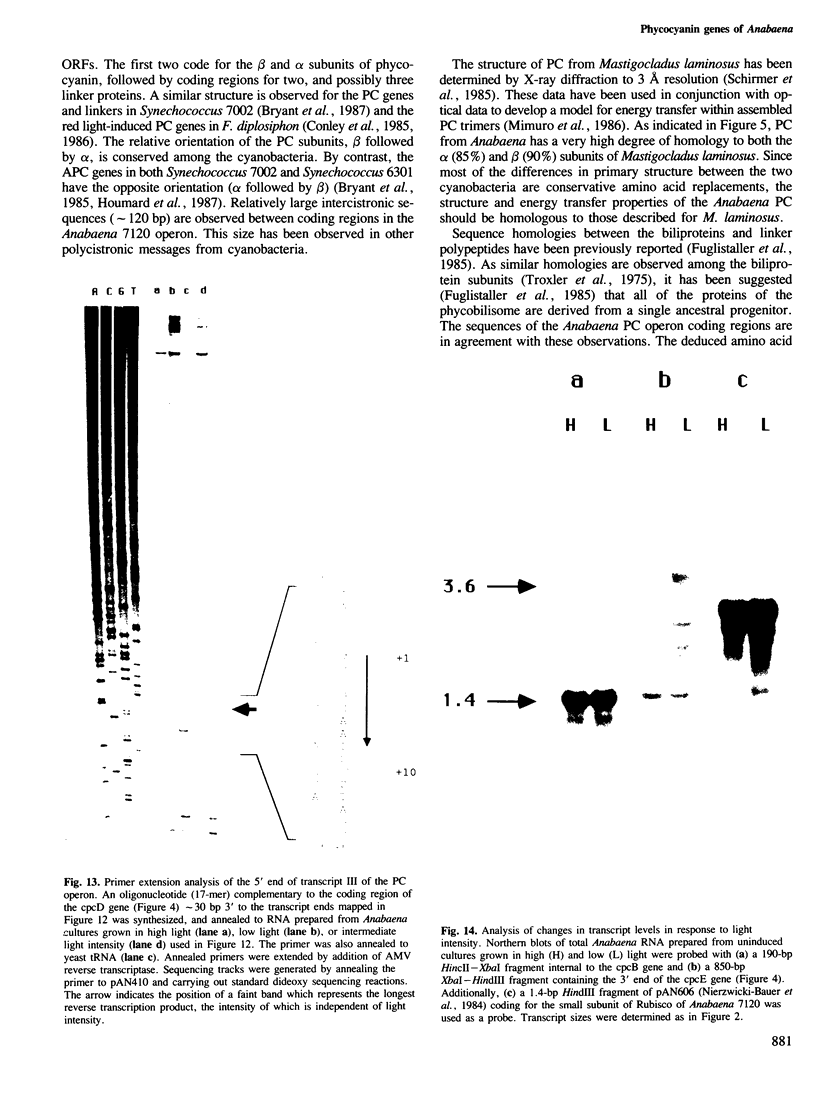
The biliprotein phycocyanin (PC) is a major constituent of the light-harvesting apparatus of cyanobacteria and red algae. A DNA fragment encoding the beta and alpha subunits of PC was isolated from a genomic library of the cyanobacterium Anabaena 7120 DNA. The single-copy PC genes are part of a larger operon which consists of five open reading frames (ORFs) encoding, in order, the beta and alpha subunits of PC, two linker polypeptides associated with PC in phycobilisome rods, and a fifth ORF, which may encode a linker polypeptide involved in attachment of the phycobilisome rod to the core of the structure. The operon yields three major transcripts, the first of which (1.4 kb) encodes only the PC subunits. A second (3.6 kb) encodes all five ORFs, and appears to arise from partial read-through of a terminator following the PC subunit genes. The third transcript (1.4 kb) encodes the last two ORFs. The relative levels of the three transcripts in vivo are modulated by light intensity, but they are not altered by the removal of fixed nitrogen from the growth medium. The site of light regulation appears to be the terminator following the PC genes, rather than a promoter.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belasco J. G., Beatty J. T., Adams C. W., von Gabain A., Cohen S. N. Differential expression of photosynthesis genes in R. capsulata results from segmental differences in stability within the polycistronic rxcA transcript. Cell. 1985 Jan;40(1):171–181. doi: 10.1016/0092-8674(85)90320-4. [DOI] [PubMed] [Google Scholar]
- Biggin M. D., Gibson T. J., Hong G. F. Buffer gradient gels and 35S label as an aid to rapid DNA sequence determination. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3963–3965. doi: 10.1073/pnas.80.13.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley P. B., Lemaux P. G., Grossman A. R. Cyanobacterial light-harvesting complex subunits encoded in two red light-induced transcripts. Science. 1985 Nov 1;230(4725):550–553. doi: 10.1126/science.3931221. [DOI] [PubMed] [Google Scholar]
- Conley P. B., Lemaux P. G., Lomax T. L., Grossman A. R. Genes encoding major light-harvesting polypeptides are clustered on the genome of the cyanobacterium Fremyella diplosiphon. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3924–3928. doi: 10.1073/pnas.83.11.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R., Tuli R., Haselkorn R. A cloned cyanobacterial gene for glutamine synthetase functions in Escherichia coli, but the enzyme is not adenylylated. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3393–3397. doi: 10.1073/pnas.78.6.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank G., Sidler W., Widmer H., Zuber H. The complete amino acid sequence of both subunits of C-phycocyanin from the cyanobacterium Mastigocladus laminosus. Hoppe Seylers Z Physiol Chem. 1978 Nov;359(11):1491–1507. doi: 10.1515/bchm2.1978.359.2.1491. [DOI] [PubMed] [Google Scholar]
- Freidenreich P., Apell G. S., Glazer A. N. Structural studies on phycobiliproteins II. C-phycocyanin: amino acid sequence of the beta subunit. Specific cleavage of the alpha subunit. J Biol Chem. 1978 Jan 10;253(1):212–219. [PubMed] [Google Scholar]
- Füglistaller P., Suter F., Zuber H. Linker polypeptides of the phycobilisome from the cyanobacterium Mastigocladus laminosus. I. Isolation and characterization of phycobiliprotein-linker-polypeptide complexes. Biol Chem Hoppe Seyler. 1986 Jul;367(7):601–614. doi: 10.1515/bchm3.1986.367.2.601. [DOI] [PubMed] [Google Scholar]
- Füglistaller P., Suter F., Zuber H. Linker polypeptides of the phycobilisome from the cyanobacterium Mastigocladus laminosus. II. Amino-acid sequences and functions. Biol Chem Hoppe Seyler. 1986 Jul;367(7):615–626. doi: 10.1515/bchm3.1986.367.2.615. [DOI] [PubMed] [Google Scholar]
- Füglistaller P., Suter F., Zuber H. Linker polypeptides of the phycobilisome from the cyanobacterium Mastigocladus laminosus: amino-acid sequences and relationships. Biol Chem Hoppe Seyler. 1985 Oct;366(10):993–1001. doi: 10.1515/bchm3.1985.366.2.993. [DOI] [PubMed] [Google Scholar]
- Glazer A. N. Light harvesting by phycobilisomes. Annu Rev Biophys Biophys Chem. 1985;14:47–77. doi: 10.1146/annurev.bb.14.060185.000403. [DOI] [PubMed] [Google Scholar]
- Grossman A. R., Lemaux P. G., Conley P. B. Regulated synthesis of phycobilisome components. Photochem Photobiol. 1986 Dec;44(6):827–837. doi: 10.1111/j.1751-1097.1986.tb05543.x. [DOI] [PubMed] [Google Scholar]
- Grosveld F. G., Lund T., Murray E. J., Mellor A. L., Dahl H. H., Flavell R. A. The construction of cosmid libraries which can be used to transform eukaryotic cells. Nucleic Acids Res. 1982 Nov 11;10(21):6715–6732. doi: 10.1093/nar/10.21.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Houmard J., Mazel D., Moguet C., Bryant D. A., Tandeau de Marsac N. Organization and nucleotide sequence of genes encoding core components of the phycobilisomes from Synechococcus 6301. Mol Gen Genet. 1986 Dec;205(3):404–410. doi: 10.1007/BF00338074. [DOI] [PubMed] [Google Scholar]
- Kassavetis G. A., Geiduschek E. P. Bacteriophage T4 late promoters: mapping 5' ends of T4 gene 23 mRNAs. EMBO J. 1982;1(1):107–114. doi: 10.1002/j.1460-2075.1982.tb01132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaux P. G., Grossman A. R. Major light-harvesting polypeptides encoded in polycistronic transcripts in a eukaryotic alga. EMBO J. 1985 Aug;4(8):1911–1919. doi: 10.1002/j.1460-2075.1985.tb03870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaux P. G., Grossman A. Isolation and characterization of a gene for a major light-harvesting polypeptide from Cyanophora paradoxa. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4100–4104. doi: 10.1073/pnas.81.13.4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundell D. J., Yamanaka G., Glazer A. N. A terminal energy acceptor of the phycobilisome: the 75,000-dalton polypeptide of Synechococcus 6301 phycobilisomes--a new biliprotein. J Cell Biol. 1981 Oct;91(1):315–319. doi: 10.1083/jcb.91.1.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nierzwicki-Bauer S. A., Curtis S. E., Haselkorn R. Cotranscription of genes encoding the small and large subunits of ribulose-1,5-bisphosphate carboxylase in the cyanobacterium Anabaena 7120. Proc Natl Acad Sci U S A. 1984 Oct;81(19):5961–5965. doi: 10.1073/pnas.81.19.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Peterson R. B., Dolan E., Calvert H. E., Ke B. Energy transfer from phycobiliproteins to photosystem I in vegetative cells and heterocysts of Anabaena variabilis. Biochim Biophys Acta. 1981 Feb 12;634(2):237–248. doi: 10.1016/0005-2728(81)90142-0. [DOI] [PubMed] [Google Scholar]
- Pilot T. J., Fox J. L. Cloning and sequencing of the genes encoding the alpha and beta subunits of C-phycocyanin from the cyanobacterium Agmenellum quadruplicatum. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6983–6987. doi: 10.1073/pnas.81.22.6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T. Transcription termination and the regulation of gene expression. Annu Rev Biochem. 1986;55:339–372. doi: 10.1146/annurev.bi.55.070186.002011. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer T., Bode W., Huber R., Sidler W., Zuber H. X-ray crystallographic structure of the light-harvesting biliprotein C-phycocyanin from the thermophilic cyanobacterium Mastigocladus laminosus and its resemblance to globin structures. J Mol Biol. 1985 Jul 20;184(2):257–277. doi: 10.1016/0022-2836(85)90379-1. [DOI] [PubMed] [Google Scholar]
- Searle G. F., Barber J., Porter G., Tredwell C. J. Picosecond time-resolved energy transfer in Porphyridium cruentum. Part II. In the isolated light harvesting complex (phycobilisomes). Biochim Biophys Acta. 1978 Feb 9;501(2):246–256. doi: 10.1016/0005-2728(78)90030-0. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Berk A. J., Berget S. M. Transcription maps of adenovirus. Methods Enzymol. 1980;65(1):750–768. doi: 10.1016/s0076-6879(80)65071-x. [DOI] [PubMed] [Google Scholar]
- Troxler R. F., Foster J. A., Brown A. S., Franzblau C. The alpha and beta subunits of Cyanidium caldarium phycocyanin: properties and amino acid sequences at the amino terminus. Biochemistry. 1975 Jan 28;14(2):268–274. doi: 10.1021/bi00673a012. [DOI] [PubMed] [Google Scholar]
- Wood N. B., Haselkorn R. Control of phycobiliprotein proteolysis and heterocyst differentiation in Anabaena. J Bacteriol. 1980 Mar;141(3):1375–1385. doi: 10.1128/jb.141.3.1375-1385.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yu M. H., Glazer A. N. Cyanobacterial phycobilisomes. Role of the linker polypeptides in the assembly of phycocyanin. J Biol Chem. 1982 Apr 10;257(7):3429–3433. [PubMed] [Google Scholar]
- Yu M. H., Glazer A. N., Williams R. C. Cyanobacterial phycobilisomes. Phycocyanin assembly in the rod substructures of anabaena variabilis phycobilisomes. J Biol Chem. 1981 Dec 25;256(24):13130–13136. [PubMed] [Google Scholar]
- Zhu Y. S., Kiley P. J., Donohue T. J., Kaplan S. Origin of the mRNA stoichiometry of the puf operon in Rhodobacter sphaeroides. J Biol Chem. 1986 Aug 5;261(22):10366–10374. [PubMed] [Google Scholar]
- de Lorimier R., Bryant D. A., Porter R. D., Liu W. Y., Jay E., Stevens S. E., Jr Genes for the alpha and beta subunits of phycocyanin. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7946–7950. doi: 10.1073/pnas.81.24.7946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Marsac N. T., Cohen-bazire G. Molecular composition of cyanobacterial phycobilisomes. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1635–1639. doi: 10.1073/pnas.74.4.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]