Abstract
We report the case of a previously healthy man who presented with subacute dyspnoea after a long drive. He developed hypoxic respiratory failure, thought secondary to a massive pulmonary embolism and was treated with tissue plasminogen activator but died in the hospital despite aggressive medical measures. Autopsy revealed pulmonary tumour thrombotic microangiopathy (PTTM) from papillary renal cell carcinoma. PTTM is a rare clinicopathological syndrome that clinically results in symptoms of dyspnoea and right heart failure. Pathologically, a localised paraneoplastic process evolves from tumour microemboli in the pulmonary arterioles, resulting in fibrocellular proliferation and narrowing of the vessels, causing subacute right heart failure. To our knowledge, this is the first case of PTTM due to papillary renal cell carcinoma.
Keywords: Adult Intensive Care, Urological Cancer, Pulmonary Hypertension, Heart Failure
Background
This case demonstrates several important principles in caring for patients with respiratory failure. It illustrates an example of rapidly progressive hypoxic respiratory failure that was incompletely evaluated. When the patient presented to his primary care physician and was noted to be hypoxic, additional testing could have been obtained at that time, such as an echocardiogram or ventilation–perfusion scan to evaluate for chronic thromboembolic pulmonary hypertension (which, clinically, presents similarly to pulmonary tumour thrombotic microangiopathy (PTTM)). It also demonstrates the errors in clinical reasoning that his physicians at both institutions made, most notably, failure to consider alternative diagnosis and focusing almost exclusively on pulmonary embolism (PE). Finally, PTTM is a rare condition associated with malignancy but with increasing recognition worldwide. To our knowledge, this is the first case of PTTM secondary renal cell carcinoma.
Case presentation
A 64-year-old man with a history of lymph node negative malignant melanoma 5 years prior status post resection and with no evidence of recurrence presented to local emergency department for a 2-week history of progressive shortness of breath. He recently had a 10-hour road trip. Review of systems was notable for mild abdominal distention, poor appetite and 3 kg weight loss over the last 2–3 weeks. CT with angiography (CTA) of the chest was negative for PE or acute lung parenchyma disease, and D-dimer was elevated to 4502 ng/mL (normal 0–500 ng/mL). He was still dyspnoeic with an oxygen saturation of 88% on room air 4 days later at a follow-up, and he was urgently referred to the University of Iowa Pulmonary clinic.
Two days later, he was admitted to the local hospital for hypoxaemic respiratory failure. Due to high concern for PE given rapid progression, severity of symptoms and recent travel history, chest CTA was repeated and showed a right lower lobe subsegmental PE. An acute deep venous thrombosis (DVT) of the right lower leg was also found. Anticoagulation was started but the patient continued to have high oxygen requirements (5 L) and was transferred to our institution for further evaluation and management.
In our intensive care unit, examination revealed a diaphoretic, acutely ill appearing male in respiratory distress with accessory muscle use. His respiratory rate was 26 breaths/min and his oxygen saturation was 95% on high-flow oxygen. Physical examination revealed tachycardia (heart rate 118) without a murmur, decreased air flow in the posterior lung bases without crackles or wheezes and a mildly distended abdomen.
Investigations
Laboratory investigations revealed haemoglobin 12.5 g/dL (13.2–17.7 g/dL), white blood cells 18.3x109/L (3.7–10.5 x109/L) with neutrophil predominance, creatinine 0.9 mg/dL (0.6–1.2 mg/dL) and bicarbonate 19 mEq/L (22–29 mEq/L). Arterial blood gas demonstrated respiratory alkalosis consistent with tachypnoea. Liver tests, coagulation markers and troponin were within normal limits. Chest radiograph did not demonstrate any acute cardiopulmonary disease. ECG revealed sinus tachycardia with an S1Q3T3 finding. Formal echocardiogram revealed a hyperdynamic left ventricle with an ejection fraction of 75% but severely decreased right ventricular systolic function, dilated right ventricle, septal flattening in systole (figure 1A–C).
Figure 1.
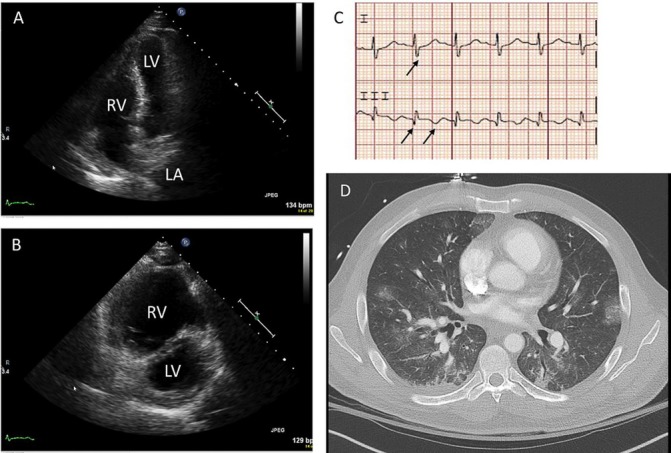
(A) Transthoracic echocardiography, apical four chamber view illustrating bowing of the interventricular septum towards the left ventricle during diastole. (B) Transthoracic echocardiography, parasternal short-axis view with massive RV and septal flattening (‘D-sign’). (C) ECG with S wave in lead I, Q wave and inverted T wave in lead III. (D) CT angiography shows right heart strain with septal flattening and reflux of contrast into IVC, lack of new pulmonary emboli and scattered ground glass infiltrates in the lung bases, concerning for pulmonary haemorrhage. LA, left atrium; LV, left ventricle; RV, right ventricle; IVC, inferior vean cava.
Treatment
Clinically, his course was extremely concerning for interval development of massive PE with obstructive shock. This conclusion was supported by recent travel history, known acute DVT, ECG evidence of right ventricular strain (S1Q3T3 finding present in 10% of cases with massive PE), echocardiographic evidence of pulmonary hypertension, septal collapse and severely decreased right ventricle function and acute progression of hypoxaemic respiratory failure. The patient was felt to be too critically ill to leave the intensive care unit for repeat chest CTA.
With the patient’s consent, intravenous tissue plasminogen activator was administered in attempt to resolve the obstructive shock. Over the next several hours, his mental status began to decline and he was intubated for airway protection and worsening hypoxaemia. Vasopressors were required shortly thereafter for hypotension. Extracorporeal membranous oxygenation (ECMO) was considered and a CT of the head and CTA of chest were performed prior to initiating ECMO. CT of the brain showed acute intraparenchymal haemorrhage and chest CTA showed ground glass opacities at the bases (figure 1D) but no other abnormalities. Despite aggressive medical measures, the patient died 30 hours after transfer. Consent for autopsy was obtained from his brother.
Outcome and follow-up
Autopsy revealed a papillary renal cell carcinoma, WHO/International Society of Urological Pathology grade 3/4, involving both kidneys, 1.5 cm in greatest dimension, with metastasis to the liver, lungs and brain. Tumour cells were positive for CK7, pancytokeratin, vimentin and PAX8 on immunohistochemical staining. The morphological pattern and strong expression of CK7 with some nuclear PAX8 positivity is consistent with a diagnosis of papillary renal cell carcinoma rather than clear cell carcinoma (figure 2A–D).
Figure 2.
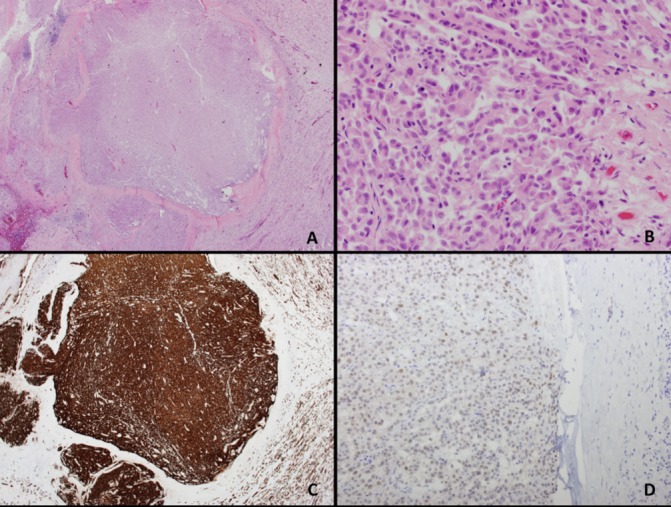
Right renal mass histology. (A–B) Papillary renal cell carcinoma (H&E; original magnification; 2×; 40×). (C) Tumour cells positive for CK7 (CK7 IHC; original magnification; 2×) (D) Tumour cells positive for PAX8 (PAX8 IHC; original magnification; 20×). IHC, immunohistochemistry.
Microscopic evaluation of the lungs showed occlusion of pulmonary arteries and arterioles by tumour emboli (figure 3A). The intraluminal tumour cells showed strong positivity for CK7, consistent with metastatic renal cell carcinoma (figure 3B). Tumour cells showed strong positive osteopontin expression and partial expression of vascular endothelial growth factor A on immunohistochemical staining (figures 3C and 2D). Associated concentric fibrocellular intimal proliferation and organised thrombi were identified (figure 3E–F).
Figure 3.
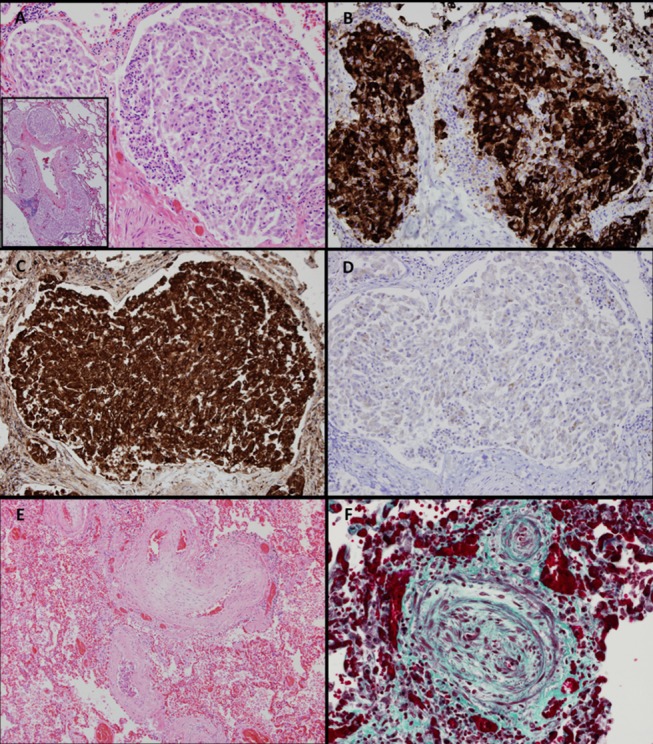
Lung histology. (A) Peripheral pulmonary artery occluded by tumour emboli (H&E; original magnification; 20×; inset 4×). (B) Peripheral pulmonary artery with intraluminal tumour cells positive for CK7 (CK7 IHC; original magnification; 20×). (C) Peripheral pulmonary artery with intraluminal tumour cells positive for osteopontin (osteopontin IHC; original magnification; 20×). (D) Peripheral pulmonary artery with intraluminal tumour cells partially positive for VEGFa (VEGFa IHC; original magnification; 20×). (E) Pulmonary arteriole with concentric fibrocellular intimal proliferation (H&E; original magnification; 10×). (F) Pulmonary arteriole with concentric fibrocellular intimal proliferation (Elastichrome; original magnification; 40×). IHC, immunohistochemistry; VEGFa, vascular endothelial growth factor A.
There was widely metastatic disease indicated by tumour emboli in the brain with associated intraparenchymal haemorrhages. Sections of heart showed a focus of tumour emboli within the small vessels of the lateral left ventricle. There was cardiomegaly with right ventricular dilatation, consistent with hypertensive cardiac disease complicated by right heart strain due to pulmonary hypertension.
Discussion
PTTM is a rare, rapidly progressive clinicopathological entity that causes acute to subacute pulmonary hypertension and subsequent right heart failure. Pathophysiology is secondary to tumour microthrombi causing local paraneoplastic changes in the pulmonary vasculature, resulting in intimal hyperplasia and fibrocellular proliferation. The lumen of the arteriolar vessels become narrowed secondary to the vessel thickening and further blocked by tumour and fibrin thrombi.1 PTTM has a spectrum of severity, with some cases being discovered incidentally at autopsy and others presenting with rapid right heart failure. Generally the progression of pulmonary hypertension is rapid, resulting in a clinical syndrome of progressive dyspnoea, subacute respiratory failure and sudden death.2 Pulmonary tumour embolism is a separate clinical entity resulting from mechanical blockage of the right heart or pulmonary vessels by large pieces of solid tumour. Tumour embolism is a well-known complication of renal cell carcinoma (RCC)3 but to our knowledge, this is the first case of PTTM caused by papillary RCC described in the literature.
Antemortem diagnosis of PTTM is extremely challenging and requires clinicians to have a high index of suspicion in patients with known malignancy who present with dyspnoea and hypoxia. CT findings are generally non-specific and can show non-specific interstitial infiltrates, ground glass opacities or reticular-nodular findings. Patients may develop disseminated intravascular coagulation, microangiopathic haemolytic anaemia and/or thrombotic microangiopathy, which may lead clinicians astray and result in treatment with plasma exchange for presumptive diagnosis of thrombotic thrombocytopenic purpura.2 4 Gold standard for diagnosis is with lung biopsy, although usually this is not possible given the severity of the patient’s condition. Cytology obtained by aspirating blood from a right heart catheterisation has been described as an alternative method for diagnosis.5 Case reports have described antemortem diagnosis of PTTM in patients with cancer by recognising the rapid development of hypoxaemia and evidence of pulmonary hypertension without pulmonary embolism.5 6 If diagnosed early enough, treatment of PTTM with tyrosine kinase inhibitors7 8 and chemotherapy targeted at the underlying malignancy has been described, and one report describes full recovery of a woman with PTTM and gastric adenocarcinoma.9
In this case, our patient was not previously known to have papillary RCC, which made antemortem diagnosis nearly impossible. Although all the historical and clinical findings were consistent with massive pulmonary embolism, his clinicians at both hospitals failed to consider alternative causes for pulmonary hypertension and right heart failure, which lead to misdiagnosis.
Patient’s perspective.
Written by the patient’s brother.
On Saturday morning, [his local] hospital calls me and says that [the patient] is a very sick man and that they can’t figure out how to help him, and if they don’t send him to the University of Iowa Hospital immediately he will surely die. They said they were preparing to airlift him to the University of Iowa Hospital shortly. I felt very good about him being transferred, because the University of Iowa Hospital would be like what Vanderbilt Hospital is in our area. The best place to be for something serious. I believed that once there, that would be able to run some tests, figure out what was wrong and [the patient] would be back on his feet in no time. I called [the patient’s] cell phone Saturday night, and he answered the phone. I only talked to him for a minute, because he was struggling so hard to breath. We said we loved each other, and that was the last time I talked to him. A couple of hours after talking to him, the hospital called and it became clear to me that he wasn’t going to make it. Early Sunday morning, my son and I started travelling to the hospital [From Tennessee]. We made it there a couple of hours before he died. An hour or so after [the patient] died, and we had already started driving to his home, a doctor called and asked if they could do an autopsy so they could figure out what caused his death. At first, I really didn’t want to allow an autopsy. The doctor said information that would come from the autopsy might help save someone’s life in the future. That’s when I knew [the patient] would’ve wanted an autopsy, so I consented to it. I’m glad that an autopsy was performed and that I know what caused his death. It was such a shock to lose him so quickly, but knowing what was wrong with him, I’m glad that he went so quickly, and didn’t have to suffer for a long time.
Learning points.
Pulmonary tumour thrombotic microangiopathy is a rare complication of advanced malignancy that is being increasingly recognised antemortem.
History and physical exam reveal hypoxaemic respiratory failure and subacute cor pulmonale secondary to pulmonary hypertension.
Antemortem diagnosis can be made by classic echocardiogram findings, aspiration by right heart catheterisation with cytology or lung biopsy. A high index of suspicion must be maintained in patients with known or newly diagnosed malignancy.
Evaluation of new onset hypoxia should include echocardiogram to evaluate for cardiac dysfunction and pulmonary hypertension.
Premature closure on the diagnosis of massive pulmonary embolism prevented these physicians from adequately considering alternative diagnoses for hypoxaemic respiratory failure.
Acknowledgments
The authors would like to acknowledge Mariah R Leidinger for her significant contributions in performing the staining in the pathology research laboratory.
Footnotes
Contributors: MS took care of the patient, developed the idea for the case report, researched PTTM and wrote the majority of the manuscript. SK performed the autopsy, researched PTTM and wrote the pathology portion of the manuscript. RR assisted with the diagnosis of PTTM based on gross and pathological autopsy findings and reviewed/edited the pathology portion of the manuscript. SF assisted with manuscript preparation, editing and reviewed the manuscript for content and accuracy.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.von Herbay A, Illes A, Waldherr R, et al. Pulmonary tumor thrombotic microangiopathy with pulmonary hypertension. Cancer 1990;66:587–92. [DOI] [PubMed] [Google Scholar]
- 2.McAnearney S, Drain M. A case of pulmonary tumour thrombotic microangiopathy. Respir Med Case Rep 2015;16:7–10. 10.1016/j.rmcr.2015.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal A, Sahni S, Iftikhar A, et al. Pulmonary manifestations of renal cell carcinoma. Respir Med 2015;109:1505–8. 10.1016/j.rmed.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 4.Gainza E, Fernández S, Martínez D, et al. Pulmonary tumor thrombotic microangiopathy: report of 3 cases and review of the literature. Medicine 2014;93:359–63. 10.1097/MD.0000000000000219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price LC, Wells AU, Wort SJ. Pulmonary tumour thrombotic microangiopathy. Curr Opin Pulm Med 2016;22:421–8. 10.1097/MCP.0000000000000297 [DOI] [PubMed] [Google Scholar]
- 6.Moon SY, Lee KH, Lee JS, et al. Acute cor pulmonale due to pulmonary tumor thrombotic microangiopathy in two patients with breast cancer. Korean J Intern Med 2017;32:190–4. 10.3904/kjim.2015.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takahashi Y, Uruga H, Fujii T, et al. Antemortem diagnosis of pulmonary tumor thrombotic microangiopathy in a patient with recurrent breast Cancer: a case report. BMC Cancer 2016;16:666 10.1186/s12885-016-2721-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banno A, Chiba K, Kasai H, et al. Ante-mortem diagnosis of pulmonary tumour thrombotic microangiopathy in a patient with unrecognised extramammary Paget's disease. BMJ Case Rep 2016;2016 10.1136/bcr-2016-216666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katayama D, Kuriyama K, Kinoshita T, et al. Pulmonary tumor thrombotic microangiopathy caused by prostate carcinoma. Acta Radiol Open 2016;5:1–4. 10.1177/2058460116662300 [DOI] [PMC free article] [PubMed] [Google Scholar]


