Abstract
Down syndrome is a frequent clinical entity, being considered one of the most frequent chromosomal aberrations. It is characterised by a typical clinical phenotype and is associated with a heterogeneous group of organ and system-specific abnormalities. The cardiovascular system is commonly affected and if so, it may be associated with an increased morbidity and mortality. Cerebrovascular events in patients with Down syndrome are multifactorial, being possibly related to congenital heart disease, vascular malformations and traditional cardiovascular risk factors. Moyamoya disease is a rare chronic occlusive vascular disease causing stenosis of the distal portion of the internal carotid artery, which has been associated with Down syndrome. The authors report the case of a 26-year-old woman with Down syndrome who presented with an acute stroke secondary to Moyamoya disease. The case is noteworthy for the rarity of this clinicopathological entity, and serves as a reminder for the possible association between these two conditions.
Keywords: Genetics, Neurology, Stroke, Neuroimaging
Background
Down syndrome (DS) is the most prevalent chromosomal disorder, affecting 1 in 800 live births.1 It is caused by trisomy of chromosome 21 and is one of the most frequent causes of intellectual disability. Increased morbidity and mortality are cardinal traits in DS, with the risk of premature death being significantly higher in the first year of life. Overall life expectancy is estimated to be 49 years.1 Although patients with DS are thought to suffer from premature ageing, cardiovascular disease is not traditionally considered to be a major determinant in the mortality of patients with DS.2 Rather, dementia, mobility restrictions, visual impairment and epilepsy are the most important disorders related to mortality in these patients. A recent meta-analysis has reported a higher incidence of cerebrovascular disease in DS compared with the general population (2.2% vs 1%; p<0001). Both ischaemic and haemorrhagic strokes tend to occur at an earlier age (41.8 vs 57.1 years) despite the lower prevalence of traditional cardiovascular risk factors in patients with DS (except perhaps obesity). Atherosclerosis is an unusual finding in these patients, with coronary artery disease and atherosclerotic cerebrovascular disease being unusual. Cardioembolic strokes, arising in the setting of congenital heart disease, pulmonary hypertension and cardiac arrhythmias, account for the majority of cerebrovascular events in DS.3 Moyamoya disease (MMD) is a rare form of cerebrovascular disease for which DS has been considered to be a risk factor. The authors report on a case of a patient with DS having a haemorrhagic stroke secondary to MMD.
Case presentation
A 26-year-old Caucasian woman with a known medical history of DS, primary hypothyroidism, obesity and surgically corrected ventricular septal defect (16 months of age), treated with enalapril and levothyroxine, presented to the emergency department with left-sided hemiparesis, hemihyperaesthesia, headache and vomiting.
Investigations
Cranial CT revealed a thalamocapsular haematoma (29×22 mm) with intraventricular extension. Contrast-enhanced MRI was remarkable for the absence of supraclinoid flow of the right internal carotid, middle, posterior and anterior cerebral arteries, along with intrinsic posterior cerebral artery parietal irregularities. Cerebral angiography was performed, being highly suggestive of MMD (figures 1–4).
Figure 1.
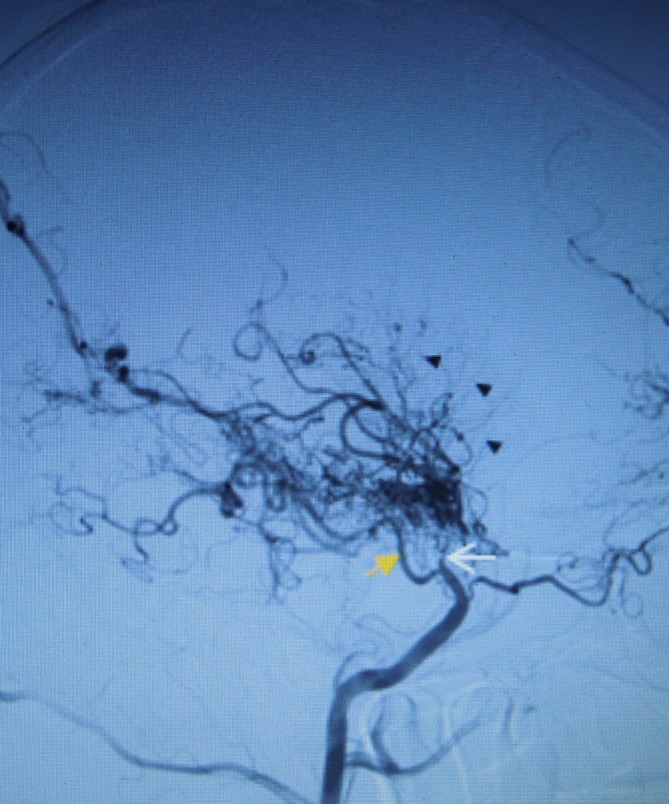
Digital subtraction angiography through femoral retrograde catheterisation where we can see the occlusion of the distal (supraclinoid) right internal carotid artery (white arrow) in sagittal plane keeping regular perfusion of posterior communicating branch (orange arrow) where there is typical Moyamoya pattern with prominent deep-seated lenticulostriate and thalamoperforator collaterals, forming the ‘puff of smoke’ (black triangles).
Figure 2.
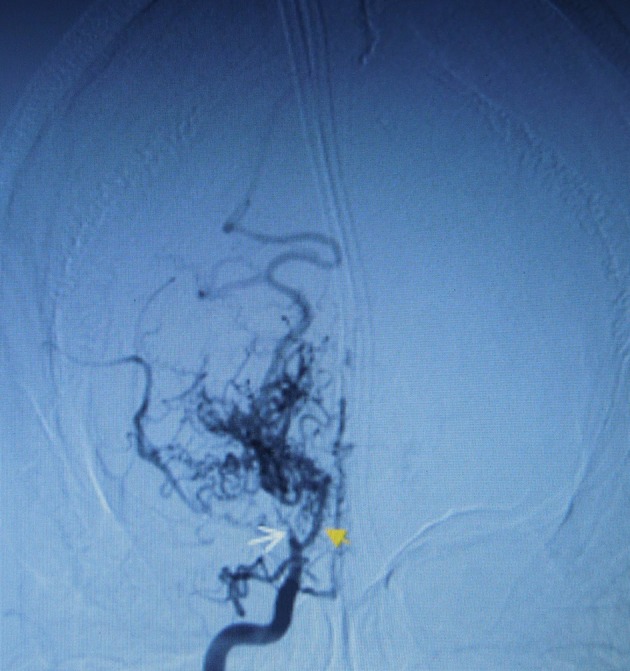
Digital subtraction angiography through femoral retrograde catheterisation where we can see the occlusion of the distal (supraclinoid) right internal carotid artery (white arrow) in coronal plane keeping regular perfusion of posterior communicating branch (orange arrow) where there is typical Moyamoya pattern with prominent deep-seated lenticulostriate and thalamoperforator collaterals, forming the ‘puff of smoke’ (black triangles).
Figure 3.
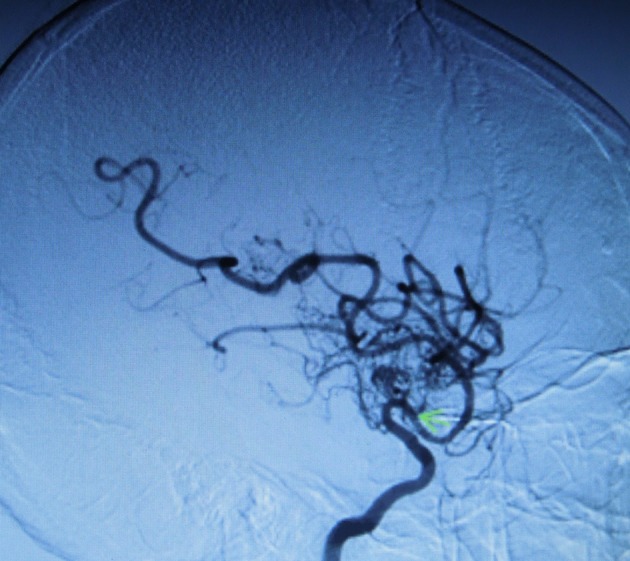
Stenosis of the left internal carotid artery (green arrow) in sagittal view showing the abnormal capillary formation of lenticulostriate and thalamoperforator collaterals also with ‘puff of smoke’ pattern (black triangles).
Figure 4.
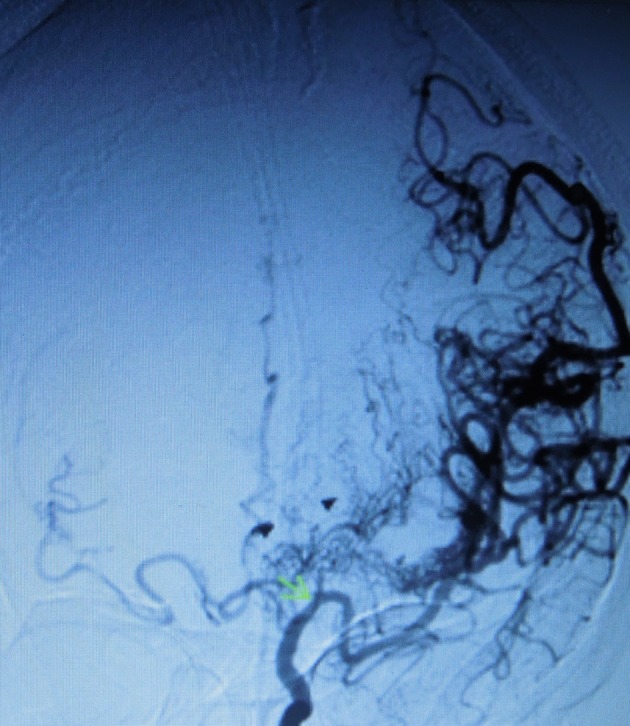
Stenosis of the left internal carotid artery (green arrow) also in coronal view showing the abnormal capillary formation of lenticulostriate and thalamoperforator collaterals also with ‘puff of smoke’ pattern (black triangles).
Differential diagnosis
In the absence of the imaging studies, cardioembolic acute ischaemic stroke in patients with DS should be kept in mind due to the frequent occurrence of atrial fibrillation and atrial cushion defects. The haemorrhagic nature in a location distinct from the typical hypertensive haemorrhagic strokes was more suggestive of another underlying aetiology.
Treatment
The patient was managed conservatively with statins due to an underlying hyperlipidaemia. No surgery was needed due to the observed indolent nature of the disease, paucity of clinical manifestations and absent cerebrovascular accident recurrence.
Outcome and follow-up
Eight months after the event, the patient maintained only left-sided hemihyperaesthesia with no additional neurological sequelae. Currently, 28 months after the event, the patient has fully recovered with no additional events or sequelae.
Discussion
MMD is a rare, chronic, occlusive cerebrovascular disease first described in 1957 as hypoplasia of the bilateral internal carotid arteries with a characteristic angiographic pattern resembling a ‘puff of cigarette smoke’ (moyamoya in Japanese).4 5 It is more common in the Asian population having an estimated prevalence of 3/100 000 and an incidence of 0.0861/100 000.6 7 It has a bimodal age distribution with the highest peak in the first decade and a smaller peak in the fourth to fifth decades, and women are more frequently affected than men (1.8:1).6 It may be rarely associated with other conditions such as: thyrotoxicosis, leptospirosis, tuberculosis, aplastic anaemia, Fanconi anaemia, sickle cell anaemia, lupus anticoagulant, Apert syndrome, DS, Marfan syndrome, tuberous sclerosis, Turner syndrome, coarctation of aorta, fibromuscular dysplasia, cranial trauma, radiation injury, parasellar tumours and hypertension.8
It is characterised by stenosis occurring in the distal internal carotid arteries often involving the proximal anterior and middle cerebral arteries, along with an abnormal vascular collateral vessel network at the base of the brain. Disease is often bilateral; however, a rising number of unilateral cases have been reported. Stenosed vessels do not have evidence of inflammatory or atherosclerotic disease, showing rather a combination of smooth muscle cell hyperplasia, luminal thrombosis and tunica media thinning. Collateral vessels are dilated perforating arteries possibly representing a combination of pre-existing and newly formed vessels, and tend to have evidence of stress related to increased blood flow (fragmented elastic lamina, thinned media and microaneurysms).8
Underlying pathophysiological mechanisms are unknown; however, the disease may be caused by an increased expression of growth factors/enzymes/peptides (basic fibroblast growth factor, transforming growth factor β-1, hepatocyte growth factor, vascular endothelial growth factor, matrix metalloproteinases, intracellular adhesion molecules and hypoxia-inducing factor 1α), genetic predisposition (mostly polygenic, despite recent interest in TIMP-2 mutation—Cr. 17q25) and/or environmental factors.9 The combination of stenosis with a network of weakened collateral vessels gives rise to a very risky situation regarding both ischaemic and haemorrhagic cerebrovascular events.
The natural history is quite variable ranging from slow progression with an uneventful clinical course to a fulminant, rapidly progressive disease. Children are more frequently affected by ischaemic, and adults by haemorrhagic events. Progressive arterial stenosis creates a haemodynamic compromise mostly in the anterior cerebral circulation (middle and anterior cerebral arteries) favouring ischaemic episodes. Haemorrhagic events are the initial presentation of 30% of patients with MMD and they differ from hypertensive intracranial haemorrhage in that intraventricular and lobar haemorrhages are way more frequent in MMD (37.6% and 23.7%, respectively).10
The gold standard for diagnosis is cerebral angiography that delineates cerebral vasculature and estimates disease severity. Magnetic resonance angiography is less invasive and can be reliably used to diagnose bilateral disease.
Medical treatment outcomes have been largely disappointing and include antiplatelet agents and anticoagulants (acetylsalicylic acid and low molecular weight heparin) to prevent thrombotic diathesis and calcium channel blockers, which have been associated with a reduction of the frequency and severity of refractory transient ischaemic attacks and amelioration of intractable headaches/migraines commonly seen in MMD. Medical therapy is indicated in mild disease or when surgery has been considered to present an unacceptable high risk. Statin use in MMD has been employed by some authors as a secondary prevention strategy based on the theoretical principle of statin-induced increase of circulating endothelial progenitor cells that would allow adequate collateral vessel development. The results of the studies have been disappointing and therefore statin use should not be routinely advocated unless there is a suspicion of coexistent atherosclerotic disease, like in our patient.11
Surgical treatment includes direct and indirect revascularisation. Direct techniques involve the anastomosis of a branch of the external carotid artery (usually the superficial temporal artery) to a cortical artery. Indirect techniques consist of the placement of vascularised tissue supplied by the external carotid artery (dura, temporalis muscle or superficial temporal artery itself) in direct contact with the brain, which leads to the ingrowth of new blood vessels to the underlying underperfused cerebral cortex (pial synangiosis, encephaloduromyoarteriosynangiosis and encephaloduroarteriosynangiosis). Pial synangiosis has been shown to be safe in the long run reducing the long-term rate of strokes to 4% (compared with 67% rate before treatment).12
Prognosis will depend more on the neurological status at the time of treatment than on the patient’s age, therefore an early diagnosis and timely surgical treatment should be offered to improve clinical outcomes.
Moyamoya is more common in DS than in the general population. Some reports have suggested a 26-fold difference, and the prevalence of DS in patients admitted with Moyamoya is estimated to be 3.8%.13 However, cerebrovascular events in patients with DS and MMD differ from the ‘traditional’ Moyamoya presentation in many ways: DS tends to present at a younger age (16.2 vs 33 years), is mostly affected by ischaemic events and tends to have a better neurological outcome. Regarding possible links between the two diseases, many theories have been proposed without enough scientific evidence: chromosome 21 normally encodes many proteins involved in vascular physiology (cystathionine B-synthetase, interferon-gamma receptor, superoxide dismutase and chains of collagen type VI) that upon trisomy are inadequately expressed. This anomaly may induce vascular dysplasia and structural defects that may give rise to Moyamoya; additionally, the presence of antiphospholipid antibodies can be found in both DS and Moyamoya, and this may be a possible link between both conditions.
The reported case is noteworthy for the later age of presentation (26 years instead of the mean 16.2 years), haemorrhagic nature of the event and remarkable neurological outcome achieved with conservative treatment.
Learning points.
Down syndrome (DS) is a frequent disease associated with an increased morbidity and mortality.
The excess mortality cannot be attributed to cardiovascular disease; however, cerebrovascular disease, namely cardioembolic, is more common in DS than in the general population.
Moyamoya disease, despite rare, is also a possible aetiology of stroke in DS.
The reported case is noteworthy due to the rarity of the clinical pathological entity itself and the possible association with DS.
The reported case aims to remind clinicians of non-embolic causes of stroke in DS, namely Moyamoya disease.
Footnotes
Contributors: CTB, writing of the article. IG, neuroradiologist who revised and provided the angiography figures. CB, article revision. CV, patient's attending physician and article revision.
Competing interests: None declared.
Patient consent: Guardian consent obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Mundakel GT. Down syndrome: practice essentials, background, pathophysiology [online]. 2017. http://emedicine.medscape.com/article/943216-overview#a6.
- 2.Coppus AM, Evenhuis HM, Verberne GJ, et al. Survival in elderly persons with down syndrome. J Am Geriatr Soc 2008;56:2311–6. 10.1111/j.1532-5415.2008.01999.x [DOI] [PubMed] [Google Scholar]
- 3.Sobey CG, Judkins CP, Sundararajan V, et al. Risk of Major cardiovascular events in people with down syndrome. PLoS One 2015;10:e0137093 10.1371/journal.pone.0137093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takeuchi K, Shimizu K. Hypoplasia of the bilateral internal carotid arteries. Brain Nerve 1957;9:37–43. [Google Scholar]
- 5.Suzuki J, Takaku A. Cerebrovascular "Moyamoya" disease. Disease showing abnormal net-like vessels in base of brain. Arch Neurol 1969;20:288–99. [DOI] [PubMed] [Google Scholar]
- 6.Baba T, Houkin K, Kuroda S. Novel epidemiological features of moyamoya disease. J Neurol Neurosurg Psychiatry 2008;79:900–4. 10.1136/jnnp.2007.130666 [DOI] [PubMed] [Google Scholar]
- 7.Uchino K, Johnston SC, Becker KJ, et al. Moyamoya disease in Washington state and California. Neurology 2005;65:956–8. 10.1212/01.wnl.0000176066.33797.82 [DOI] [PubMed] [Google Scholar]
- 8.Ajimi Y, Uchida K, Kawase T, et al. [A case of Turner's syndrome associated with Moyamoya disease]. No Shinkei Geka 1992;20:1021–4. [PubMed] [Google Scholar]
- 9.Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med 2009;360:1226–37. 10.1056/NEJMra0804622 [DOI] [PubMed] [Google Scholar]
- 10.Nah HW, Kwon SU, Kang DW, et al. Moyamoya disease-related versus primary intracerebral hemorrhage: [corrected] location and outcomes are different. Stroke 2012;43:1947–50. 10.1161/STROKEAHA.112.654004 [DOI] [PubMed] [Google Scholar]
- 11.Rafat N, Beck GCh, Peña-Tapia PG, et al. Increased levels of circulating endothelial progenitor cells in patients with Moyamoya disease. Stroke 2009;40:432–8. 10.1161/STROKEAHA.108.529420 [DOI] [PubMed] [Google Scholar]
- 12.Scott RM, Smith JL, Robertson RL, et al. Long-term outcome in children with moyamoya syndrome after cranial revascularization by pial synangiosis. J Neurosurg 2004;100:142–9. 10.3171/ped.2004.100.2.0142 [DOI] [PubMed] [Google Scholar]
- 13.See AP, Ropper AE, Underberg DL, et al. Down syndrome and moyamoya: clinical presentation and surgical management. J Neurosurg Pediatr 2015;16:58–63. 10.3171/2014.12.PEDS14563 [DOI] [PubMed] [Google Scholar]


