Abstract
A man aged 33 years with previous heroin substance abuse was found unconscious lying in a bush. The patient had been without heroin for some time but had just started to use intravenous heroin again, 0.5–2 g daily. The patient had almost complete paraplegia and a sensory loss for all modalities below the mamillary level and a urine retention of 1.5 L. Acute MRI of the spine revealed an expanded spinal cord with increased intramedullary signal intensity, extending from C7–T9. The cerebrospinal fluid showed extremely high levels of nerve injury markers particularly glial fibrillar acidic protein (GFAP): 2 610 000/ng/L (ref. <750). The patient was empirically treated with intravenous 1 g methylprednisolone daily for 5 days and improved markedly. Very few diseases are known to produce such high levels of GFAP, indicating a toxic effect on astrocytes. Measuring GFAP could possibly aid in the diagnosis of heroin-induced myelopathy.
Keywords: Neurology (drugs and medicines), Immunology, Neuroimaging, Spinal cord, Drug misuse (including addiction)
Background
Heroin-induced myelopathy is an unusual cause of acute myelopathy, and possibly underdiagnosed.
Acute heroin myelopathy usually appears after heroin reuse after period of abstinence, which supports the idea of hypersensitivity on re-exposure to the drug. Otherwise, it has an unknown pathogenic mechanism.
Compared with the subacute onset of inflammatory myelitis, heroin-induced myelopathy has an acute presentation and presents as spinal shock, with flaccid paralysis, absent deep tendon reflexes, urinary retention and diminished rectal tone.
Glial acidic protein (GFAP) is a structural component of astrocytes and raised GFAP levels in cerebrospinal fluid are observed in diseases with astrocytic damage, for example, neuromyelitis optica and Alexander disease.
Case presentation
A man aged 33 years with insulin-treated diabetes type I, hepatitis C and previous substance abuse (benzodiazepines, amphetamine and heroin) was found unconscious lying in a bush. He was brought unconscious with pinpoint pupils to the nearest emergency department, where he was obtunded but not bradypneic and had normal saturation (95%) with no extra oxygen. He was afebrile, blood pressure of 120/60 mm Hg and heart rate was 80–100 beats per minute. Three doses of 0.4 mg intravenous naloxone were needed for the patient to regain consciousness. He was found to be paraplegic and had urine retention of 1.5 L. The patient had been without heroin for some time but had just started to use intravenous heroine again, 0.5–2 g daily and benzodiazepines. The patient had almost a complete paraplegia (only slight movement in the right big toe) and a sensory loss for all modalities below the mamillary level. Reflexes were retained in the arms but not present in the legs. There was no clonus and plantar responses were flexor. The patient was moved the same day to our hospital. On arrival he was awake and orientated. Neurological examination was as described above and there was a markedly reduced sphincter tonus and only mild sensation sacrally.
Investigations
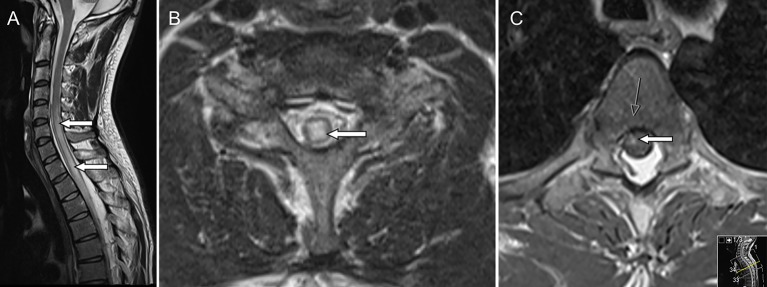
Urine toxicology was positive for opioids and benzodiazepines. Acute MRI (figure 1) of the spine revealed an expanded spinal cord with increased intramedullary signal intensity on T2-weighted imaging, extending from C7 to T9, most pronounced between levels T1–T4 (figure 1A-B) There was also some patchy gadolinium enhancement on T1 imaging (figure 1C). The finding was judged as a longitudinally extensive transverse myelitis. MRI of the brain was normal. Lumbar puncture showed a clear fluid. There were only two monocytes 106/L, albumin was mildly elevated 680 mg/L, the albumin quotient was increased 18.5 (ref. <7.0) and no oligoclonal bands were detected. Antinuclear antibodies and HIV were negative. The cerebrospinal fluid (CSF) tested negative for neurotropic viruses (herpes simplex 1 and 2, varicella zoster and enterovirus). Furthermore, the CSF was negative for Lyme disease, syphilis and the bacterial culture was negative. The CSF showed extremely high levels of nerve injury markers particularly glial fibrillar acidic protein (GFAP): 2 610 000/ng/L (ref. <750). Even neurofilament light (NFL) was markedly elevated 7 740 ng/L (ref. <560).
Figure 1.
(A) Sagittal T2-weighted image showing extensive intramedullary oedema from level C5 and caudally with slight swelling of the cord (arrows). Diffusion-weighted imaging did not show restricted diffusion (not shown). (B) Transverse T2-weighted image with centrally located intramedullary oedema at C7 level (arrow). (C) Transverse T1-weighted image after gadolinium shows enhancement in the thoracic cord (arrows).
Differential diagnosis
Neuromyelitis optica because of the longitudinally extensive medullary lesion, but unusual with only two monocytes and the hyperacute presentation.
Acute disseminated encephalomyelitis (ADEM), also unusual with only two monocytes and the patient was not encephalopathic after he woke up and did not have multifocal lesions in brain and spinal cord, as often seen in ADEM.
Hepatitis C-associated myelopathy. The patient had hepatitis C but the acute presentation makes that diagnosis highly unlikely.
Spinal cord infarction is in line with the acuteness of the presentation and having few CSF cells and high neural tissue injury markers. At the same time, the spinal lesion did not look like an infarction on imaging for the following reasons: diffusion-weighted imaging did not show any reduced diffusion and the lesion showed some enhancement. The lesion was both long and transverse, which is uncommon in a spinal infarction, where the lesion usually follows the anatomy of a specific artery such as the anterior spinal artery. Furthermore, the clinical improvement was not in line with a spinal infarction and there was no spinal atrophy (common after spinal infarction) on follow-up images.
A combined effect of ‘recreational cocktails’ including heroin is not to be excluded as a possible cause. The patient was known to use other drugs and also tested positive for benzodiazepines.
Treatment
After treatment with intravenous naloxone, the patient was empirically treated with intravenous acyclovir and 1 g intravenous methylprednisolone daily for 5 days. After a few days, he improved markedly and the urine catheter could be drawn.
Outcome and follow-up
After 10 days the patient was discharged for rehabilitation. Three months later, he was able to walk but had to use intermittent catheterisation and had difficulties with erection. At a return visit to the clinic (4 months after admission), the patient could walk stairs and 3 km. He still had some unsteadiness and difficulties walking on uneven ground. On examination, he had mildly lowered strength in the lower extremities and the reflexes in the lower extremities were still absent besides the left patella. Furthermore, there were no signs of spasticity and the plantar responses were flexor. A new spinal fluid analysis showed again two monocytes 106/L, albumin 418 mg/L and albumin quotient mildly elevated 9.3. Once more, the patient had high levels of NFL 6740 ng/L (which takes many months to subside), but GFAP had normalised (240 ng/L). Seven months after admission, MRI of the spine showed considerable regression of intramedullary oedema with some residual T2 signal abnormality at T1–T2 level (figure 2). Ten months after the injury, the patient could walk even longer distances despite mild leg weakness, some unsteadiness and erection problems.
Figure 2.
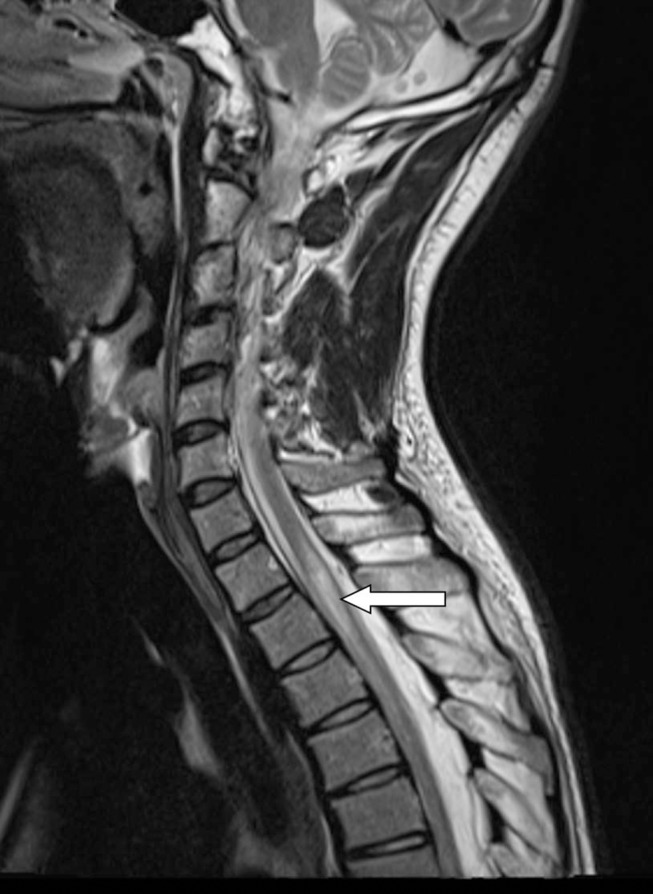
Sagittal T2-weighted images months after admission shows considerable regression of intramedullary oedema with some residual T2 signal abnormality at T1–T2 level (arrow).
Discussion
Heroin can cause diverse neurological problems such as stroke, acute inflammatory demyelinating polyradiculoneuropathy and mononeuropathy.1 Acute transverse myelopathy or acute transverse myelitis was first described in 1968 as a complication of heroin overdose.2 In most of the cases, like in our patient, the myelopathy appears after a single or few doses of heroin after a period of abstinence. This led to speculation of a hypersensitivity mechanism, but the cause of heroin-induced myelopathy is largely unknown. Suggested mechanisms have included direct toxicity, embolism, hypotension and as mentioned before, hypersensitivity.3–6 The observation that most reported cases have occurred following reuse of heroin after a period of heroin abstinence can speak for an immunopathological cause, that is, some sort of sensitisation beforehand which then results in a hypersensitivity reaction on re-exposure to the drug. Spinal cord enhancement, like in our case, speaks also for inflammation.5 Furthermore, our patient seemed also to respond to intravenous methylprednisolone. However, what distinguishes acute heroin myelopathy from inflammatory myelitis is the extreme acute onset and usually low number of monocytes. Most studies4 5 report a relative normal CSF but nerve injury markers have not been previously measured to our knowledge. In addition, we are not aware of an inflammatory myelitis that can produce such high levels of GFAP, which points towards an extreme astrocyte injury. GFAP is a structural component of astrocytes and raised GFAP levels in CSF are observed in cases with astrocytic damage.7 Neuromyelitis optica is known to produce high numbers of GFAP but not in this range, as far as we know. The only known disease to produce these numbers is Alexander disease, which the patient obviously did not have.
GFAP in blood and CSF is proposed to be used for evaluating grade of traumatic brain injury, differentiating between haemorrhagic and ischaemic stroke, and assessing grade of astrogliosis in patients with multiple sclerosis (MS). The levels of GFAP correlate with the expanded disability status scale (EDSS) in patients with MS and it is considered a marker of actual astroglial lesion load.8 Currently, most efforts in biomarker research are towards measuring tissue markers from the CNS in blood. We argue that in a clinical setting, where there is concern of false-positive or false-negative results, a lumbar puncture is warranted as this is the closest to a biopsy we can reach.
In most reports of heroin-induced myelopathy, the patients have had a similar clinical picture to our patient, that is, with spinal shock in a similar fashion as seen in traumatic spinal cord injury with flaccid paralysis, absent deep tendon reflexes, urinary retention and diminished rectal tone. The lesion has most often been located in the thoracic cord5 or ‘watershed’ area of the spinal cord. Despite that location, it is difficult in our view to see that systemic hypotension could be grave enough to damage the cord without brain damage, especially since the patient did not have hypotension in the ambulance or on arrival to the emergency room. In one of the best reviews of neurological consequences of heroin abuse,9 the authors could not conclusively decide which pathological mechanism is at work in heroin myelopathy.
It is interesting how well our patient has recovered considering the extreme high levels of nerve injury markers and that NFL was still relatively high 4 months later (but it takes NFL many months to lower again). We have no other explanation than that the toxic effect on the astrocytes was short lived and a possible helpful effect from the high dose intravenous methylprednisolone treatment. The relatively young age of the patient might also mean a high nerve fibre reserve capacity in the spinal cord. Heroin-induced myelopathy is not an easy diagnosis to make not the least considering a ‘cocktail’ of drugs often being used. At the same time, after reviewing the literature and our case we see a certain phenotype, being an individual using heroin again after a period of abstinence, suffering an acute myelopathy most often in the thoracic cord with a clinical picture of almost spinal shock. High levels of GFAP can possibly aid in the diagnosis of heroin-induced myelopathy but more research is evidently needed. Research possibilities include measuring GFAP levels in patients suspected of the disorder and even testing the effect of heroin on astrocytes in vitro.
Learning points.
The acute myelopathy usually appears after heroin reuse after period of abstinence.
The myelopathy appears as spinal chock, with flaccid paralysis, absent deep tendon reflexes, urinary retention and diminished rectal tone.
The spinal cord lesion is usually predominately located in the thoracic cord.
Despite an unknown pathogenic mechanism, high-dose methylprednisolone might improve outcome.
Measuring glial fibrillar acidic protein could possibly aid in the diagnosis of heroin-induced myelopathy.
Footnotes
Contributors: OS and AH are responsible for conception and design. AH and OS treated the patient. OS wrote the first draft and performed the literature search. LH contributed with analysis, data interpretation and reviewing the article. AH reviewed the article.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Pascual Calvet J, Pou A, Pedro-Botet J, et al. [Non-infective neurologic complications associated to heroin use]. Arch Neurobiol 1989;52(Suppl 1):155–61. [PubMed] [Google Scholar]
- 2.Richter RW, Rosenberg RN. Transverse myelitis associated with heroin addiction. JAMA 1968;206:1255–7. 10.1001/jama.1968.03150060029005 [DOI] [PubMed] [Google Scholar]
- 3.Malik MM, Woolsey RM. Acute myelopathy following intravenous heroin: a case report. J Am Paraplegia Soc 1991;14:182–3. 10.1080/01952307.1991.11735851 [DOI] [PubMed] [Google Scholar]
- 4.Ell JJ, Uttley D, Silver JR. Acute myelopathy in association with heroin addiction. J Neurol Neurosurg Psychiatry 1981;44:448–50. 10.1136/jnnp.44.5.448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCreary M, Emerman C, Hanna J, et al. Acute myelopathy following intranasal insufflation of heroin: a case report. Neurology 2000;55:316–7. 10.1212/WNL.55.2.316-a [DOI] [PubMed] [Google Scholar]
- 6.Kumar R, West DM, Jingree M, et al. Unusual consequences of heroin overdose: rhabdomyolysis, acute renal failure, paraplegia and hypercalcaemia. Br J Anaesth 1999;83:496–8. 10.1093/bja/83.3.496 [DOI] [PubMed] [Google Scholar]
- 7.Guéz M, Hildingsson C, Rosengren L, et al. Nervous tissue damage markers in cerebrospinal fluid after cervical spine injuries and whiplash trauma. J Neurotrauma 2003;20:853–8. 10.1089/089771503322385782 [DOI] [PubMed] [Google Scholar]
- 8.Axelsson M, Malmeström C, Nilsson S, et al. Glial fibrillary acidic protein: a potential biomarker for progression in multiple sclerosis. J Neurol 2011;258:882–8. 10.1007/s00415-010-5863-2 [DOI] [PubMed] [Google Scholar]
- 9.Büttner A, Mall G, Penning R, et al. The neuropathology of heroin abuse. Forensic Sci Int 2000;113:435–42. 10.1016/S0379-0738(00)00204-8 [DOI] [PubMed] [Google Scholar]