Abstract
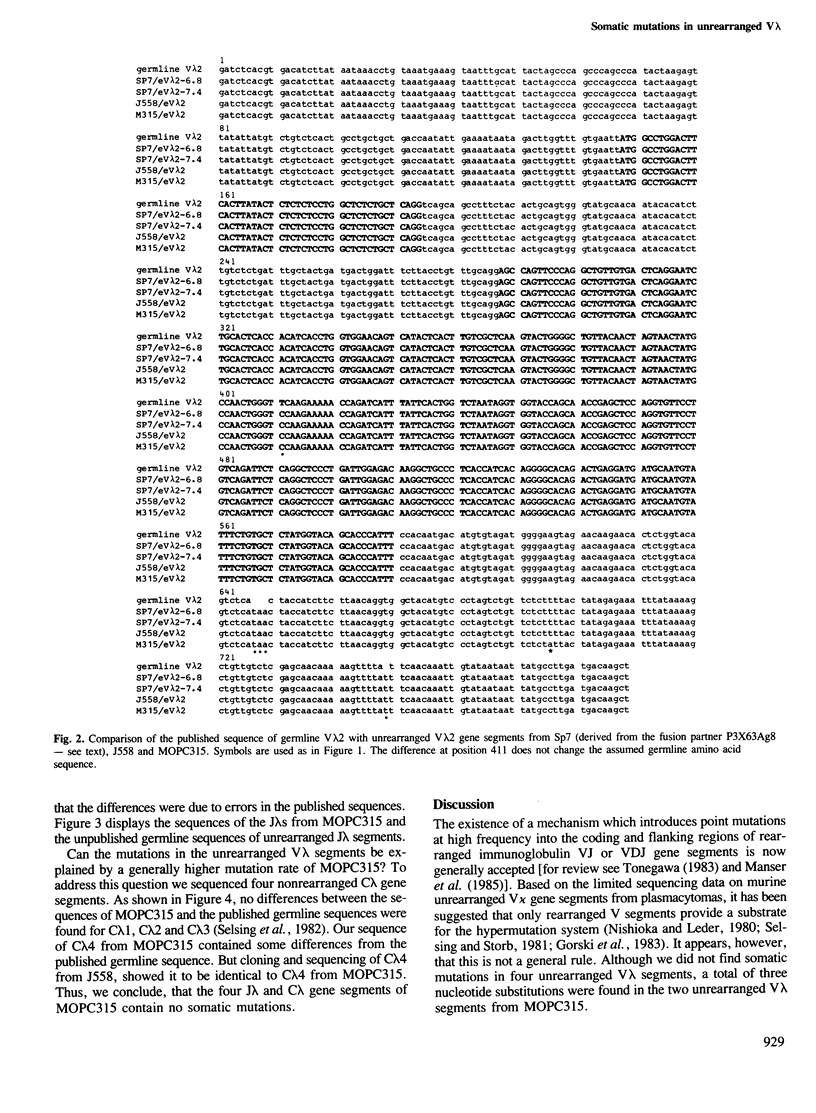
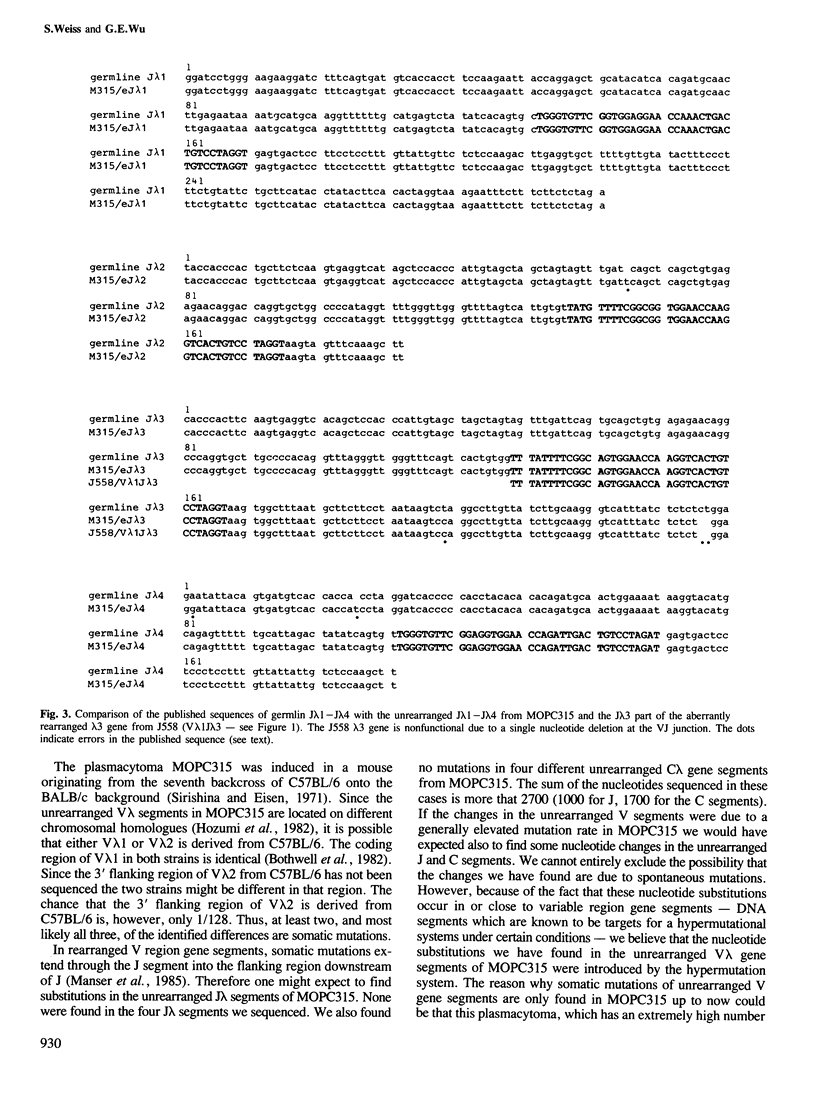
Somatic point mutations are usually found in the coding and flanking regions of functionally and aberrantly rearranged immunoglobulin variable region gene segments. Mutations in the unrearranged V gene segments of myelomas or hybridomas have not been described so far. We have cloned and sequenced unrearranged V lambda gene segments from several cell lines. There were no nucleotide changes in four unrearranged V lambda segments: one V lambda 1 from a lambda 3-producing hybridoma and one V lambda 2 from a lambda 1-producing myeloma (J558) and two V lambda 2 from a kappa-producing myeloma (P3X63). However, we found somatic mutations in the unrearranged V lambda segments from the lambda 2-producing myeloma MOPC315. The unrearranged V lambda 1 gene segment had two mutations in the coding region and the unrearranged V lambda 2 had one mutation in the 3' flanking region. We also cloned and sequenced the unrearranged J lambda and C lambda gene segments of MOPC315 and found no sequence alterations. This is consistent with the notion that the overall mutation rate is not higher in this cell line. Therefore, we suggest that the somatic hypermutation system can use unrearranged V gene segments as substrates. The extensive sequencing required for this work revealed a number of errors in the reported nucleotide sequences of the Ig lambda locus in BALB/c mice.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernard O., Hozumi N., Tonegawa S. Sequences of mouse immunoglobulin light chain genes before and after somatic changes. Cell. 1978 Dec;15(4):1133–1144. doi: 10.1016/0092-8674(78)90041-7. [DOI] [PubMed] [Google Scholar]
- Blomberg B., Tonegawa S. DNA sequences of the joining regions of mouse lambda light chain immunoglobulin genes. Proc Natl Acad Sci U S A. 1982 Jan;79(2):530–533. doi: 10.1073/pnas.79.2.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Somatic variants of murine immunoglobulin lambda light chains. Nature. 1982 Jul 22;298(5872):380–382. doi: 10.1038/298380a0. [DOI] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Schwartz R. C., Sonenshein G. E., Gefter M. L., Baltimore D. Dual expression of lambda genes in the MOPC-315 plasmacytoma. Nature. 1981 Mar 5;290(5801):65–67. doi: 10.1038/290065a0. [DOI] [PubMed] [Google Scholar]
- Carè A., Cianetti L., Giampaolo A., Sposi N. M., Zappavigna V., Mavilio F., Alimena G., Amadori S., Mandelli F., Peschle C. Translocation of c-myc into the immunoglobulin heavy-chain locus in human acute B-cell leukemia. A molecular analysis. EMBO J. 1986 May;5(5):905–911. doi: 10.1002/j.1460-2075.1986.tb04302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J., Rollini P., Mach B. Somatic mutations of immunoglobulin variable genes are restricted to the rearranged V gene. Science. 1983 Jun 10;220(4602):1179–1181. doi: 10.1126/science.6857243. [DOI] [PubMed] [Google Scholar]
- Hamlyn P. H., Gait M. J., Milstein C. Complete sequence of an immunoglobulin mRNA using specific priming and the dideoxynucleotide method of RNA sequencing. Nucleic Acids Res. 1981 Sep 25;9(18):4485–4494. doi: 10.1093/nar/9.18.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozumi N., Wu G. E., Murialdo H., Roberts L., Vetter D., Fife W. L., Whiteley M., Sadowski P. RNA splicing mutation in an aberrantly rearranged immunoglobulin lambda I gene. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7019–7023. doi: 10.1073/pnas.78.11.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozumi N., Wu G., Murialdo H., Baumal R., Mosmann T., Winberry L., Marks A. Arrangement of lambda light chain genes in mutant clones of the MOPC 315 mouse myeloma cells. J Immunol. 1982 Jul;129(1):260–266. [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Derivation of specific antibody-producing tissue culture and tumor lines by cell fusion. Eur J Immunol. 1976 Jul;6(7):511–519. doi: 10.1002/eji.1830060713. [DOI] [PubMed] [Google Scholar]
- McKean D., Huppi K., Bell M., Staudt L., Gerhard W., Weigert M. Generation of antibody diversity in the immune response of BALB/c mice to influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1984 May;81(10):3180–3184. doi: 10.1073/pnas.81.10.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J., Selsing E., Storb U. Structural alterations in J regions of mouse immunoglobulin lambda genes are associated with differential gene expression. Nature. 1982 Feb 4;295(5848):428–430. doi: 10.1038/295428a0. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Leder P. Organization and complete sequence of identical embryonic and plasmacytoma kappa V-region genes. J Biol Chem. 1980 Apr 25;255(8):3691–3694. [PubMed] [Google Scholar]
- Pech M., Höchtl J., Schnell H., Zachau H. G. Differences between germ-line and rearranged immunoglobulin V kappa coding sequences suggest a localized mutation mechanism. Nature. 1981 Jun 25;291(5817):668–670. doi: 10.1038/291668a0. [DOI] [PubMed] [Google Scholar]
- Rabbitts T. H., Hamlyn P. H., Baer R. Altered nucleotide sequences of a translocated c-myc gene in Burkitt lymphoma. Nature. 1983 Dec 22;306(5945):760–765. doi: 10.1038/306760a0. [DOI] [PubMed] [Google Scholar]
- Sablitzky F., Wildner G., Rajewsky K. Somatic mutation and clonal expansion of B cells in an antigen-driven immune response. EMBO J. 1985 Feb;4(2):345–350. doi: 10.1002/j.1460-2075.1985.tb03635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling J., Clevinger B., Davie J. M., Hood L. Amino acid sequence of homogeneous antibodies to dextran and DNA rearrangements in heavy chain V-region gene segments. Nature. 1980 Jan 3;283(5742):35–40. doi: 10.1038/283035a0. [DOI] [PubMed] [Google Scholar]
- Selsing E., Miller J., Wilson R., Storb U. Evolution of mouse immunoglobulin lambda genes. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4681–4685. doi: 10.1073/pnas.79.15.4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsing E., Storb U. Somatic mutation of immunoglobulin light-chain variable-region genes. Cell. 1981 Jul;25(1):47–58. doi: 10.1016/0092-8674(81)90230-0. [DOI] [PubMed] [Google Scholar]
- Showe L. C., Ballantine M., Nishikura K., Erikson J., Kaji H., Croce C. M. Cloning and sequencing of a c-myc oncogene in a Burkitt's lymphoma cell line that is translocated to a germ line alpha switch region. Mol Cell Biol. 1985 Mar;5(3):501–509. doi: 10.1128/mcb.5.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirisinha S., Eisen H. N. Autoimmune-like antibodies to the ligand-binding sites of myeloma proteins. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3130–3135. doi: 10.1073/pnas.68.12.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J. DNA sequence analysis by primed synthesis. Methods Enzymol. 1980;65(1):560–580. doi: 10.1016/s0076-6879(80)65060-5. [DOI] [PubMed] [Google Scholar]
- Tonegawa S., Maxam A. M., Tizard R., Bernard O., Gilbert W. Sequence of a mouse germ-line gene for a variable region of an immunoglobulin light chain. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1485–1489. doi: 10.1073/pnas.75.3.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Reiteration frequency of immunoglobulin light chain genes: further evidence for somatic generation of antibody diversity. Proc Natl Acad Sci U S A. 1976 Jan;73(1):203–207. doi: 10.1073/pnas.73.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Wabl M., Burrows P. D., von Gabain A., Steinberg C. Hypermutation at the immunoglobulin heavy chain locus in a pre-B-cell line. Proc Natl Acad Sci U S A. 1985 Jan;82(2):479–482. doi: 10.1073/pnas.82.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walfield A., Selsing E., Arp B., Storb U. Misalignment of V and J gene segments resulting in a nonfunctional immunoglobulin gene. Nucleic Acids Res. 1981 Mar 11;9(5):1101–1109. doi: 10.1093/nar/9.5.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigert M. G., Cesari I. M., Yonkovich S. J., Cohn M. Variability in the lambda light chain sequences of mouse antibody. Nature. 1970 Dec 12;228(5276):1045–1047. doi: 10.1038/2281045a0. [DOI] [PubMed] [Google Scholar]
- Weiss S., Meyer J., Wabl M. R. V lambda 2 rearranges with all functional J lambda segments in the mouse. Eur J Immunol. 1985 Aug;15(8):765–768. doi: 10.1002/eji.1830150805. [DOI] [PubMed] [Google Scholar]
- Wu G. E., Govindji N., Hozumi N., Murialdo H. Nucleotide sequence of a chromosomal rearranged lambda 2 immunoglobulin gene of mouse. Nucleic Acids Res. 1982 Jul 10;10(13):3831–3843. doi: 10.1093/nar/10.13.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Regulation of the assembly and expression of variable-region genes. Annu Rev Immunol. 1986;4:339–368. doi: 10.1146/annurev.iy.04.040186.002011. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]


