Abstract
Haemolytic anaemia caused by a paravalvular leak presenting as progressively worsening red urine. Haemoglobinuria was easily mistaken for gross haematuria, resulting in extensive invasive urological investigation that proved to be futile. Further investigation following an emergency admission led to the realisation that intravascular haemolysis secondary to a paravalvular leak—presenting 43 years following metallic valve insertion—was the cause of discoloured urine and newly presenting symptomatic anaemia. This case highlights that there remains other causes of what often appears to be haematuria, and further exploration of alternative causes should be considered when no urological cause is found.
Keywords: Hematuria, Urological surgery, Cardiothoracic surgery
Background
A case of clinically evident haemolytic anaemia secondary to a paravalvular leak is reported. Paravalvular leaks are a well-documented complication following valve replacement surgery; the vast majority of leaks are small and non-progressive, and the patients remain clinically asymptomatic.1 Patients with substantial leaks are rare but can develop symptomatic haemolytic anaemia and typically present within the first year after surgery, with later presentations becoming progressively less common as time progresses.2 The below case report describes a patient presenting 43 years following valve replacement—an interval of which is currently unique in the literature. Given such an unprecedented late presentation, this important differential was overlooked, and haemoglobinuria was presumed to be haematuria, resulting in extensive investigation via urological services to no avail, before a later emergency admission prompted haemolytic anaemia and paravalvular leak being diagnosed.
Case presentation
A 74-year-old woman with a history of Bjork-Shirley mechanical mitral valve replacement 43 years previously, for which she was on long-term warfarin, presented to a haematuria outpatient’s clinic with 1 week of what appeared to be moderate haematuria. Investigation included flexible cystoscopy, abdominal ultrasound and CT urogram, revealing no evident abnormalities or sources of bleeding. Haemoglobin levels were seen to be stable for the patient at 10.6 g/dL. Three weeks later, the patient presented to emergency inpatient urological services with apparent gross haematuria. Physical examination at this time showed a mild tachycardia of 103 beats/min, blood pressure 113/64 mm Hg, a soft non-tender abdomen and an unremarkable cardiorespiratory examination—apart from the expected metallic first heart sound. No audible murmur heard. The patient complained of presyncope and significantly increased shortness of breath. Urine samples were intensely red in colour, but no clots were present. Urine dipstick results were greatly positive for ‘blood’ but negative for all markers of infection. Laboratory analysis showed a haemoglobin of 7.9 g/dL, haematocrit 0.235 L/L, international normalised ratio of 3.6 (patient’s target range 2.5–3.5), with normal renal function and infection markers. After discussion with a haematologist regarding whether to continue with warfarin given the persistent haematuria despite normal urological investigation 3 weeks previously, the possibility of haemolysis secondary to the mitral valve replacement was introduced.
Investigations
Further laboratory analysis showed bilirubin 40 µmol/L, elevated reticulocytes 131×109/L, haptoglobin <0.07 g/L, serum lactate dehydrogenase >3000 IU/L, evidence of haemolysis on blood film, negative Coombs tests and no red blood cells seen on urine microscopy. Transoesophageal echocardiogram was arranged following discussion with cardiology, which showed good left ventricular size and function with a well-seated valve replacement in situ. There was an extensive paravalvular leak that extended across 25% of the sewing ring, causing severe paravalvular mitral regurgitation (figure 1). An intraoperative transoesophageal echocardiogram was also performed following decision to proceed with surgical intervention, which evidences with greater precision the severe paravalvular mitral regurgitation (figure 2).
Figure 1.
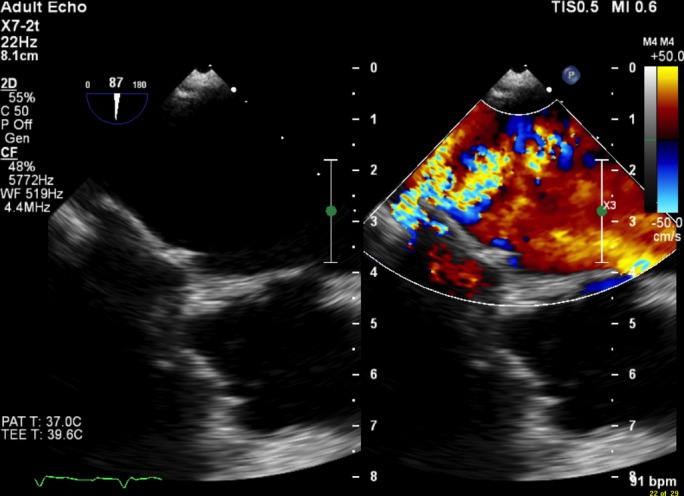
Paravalvular leak around mechanical mitral valve replacement as seen via preoperative transoesophageal echocardiogram.
Figure 2.
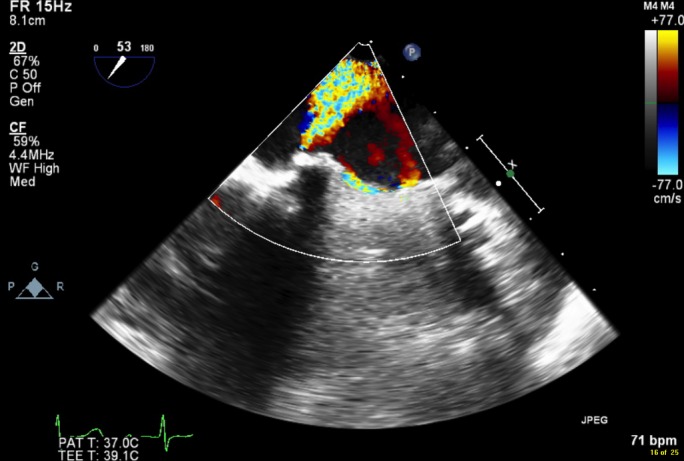
Paravalvular leak around mechanical mitral valve replacement as seen via intraoperative transoesophageal echocardiogram.
Outcome and follow-up
Following diagnosis, the patient was discussed with both cardiology and cardiothoracics with regard to the most appropriate approach to take with this patient. The case was discussed at a ‘complex patient’ meeting—the outcome being that swift surgical intervention to replace the valve prosthesis would be desirable. Surgical intervention was successful, and the patient has made a full recovery to premorbid condition.
Discussion
The mechanism of erythrocyte breakdown in such cases is due to shearing forces and turbulent blood flow caused by paravalvular leaks, as opposed to direct mechanical impact of the cells on the metallic valve.3 Damaged cells release haemoglobin into the blood, and if levels of haemoglobin exceed the binding capacity of haptoglobin and evade subsequent breakdown by the reticuloendothelial system, free haemoglobin is filtered through the glomerulus—resulting in haemoglobinuria.4 An important potential complication to be aware of in such cases is the risk of renal insufficiency as a result of pigment nephropathy; renal iron deposits following intravascular haemolysis are a well-documented phenomenon.5 While paravalvular leaks often result in haemolytic anaemia, the progression to clinically evident haemoglobinuria is rare. The bloody appearance of this patient’s urine was presumed to be haematuria; however, further targeted investigation showed it to be a result of haemoglobinuria instead. The patient had previously undergone a battery of urological testing when she initially presented as an outpatient with mildly red urine; she would no doubt have been subjected to replicated invasive, resource dependant and potentially harmful investigations as an inpatient had the possibility of paravalvular leak not been suggested at an early stage due to a coincidental discussion with haematology regarding warfarin dosing.
Learning points.
Intravascular haemolysis is an important differential to consider in patients with previous valve replacements presenting with apparent haematuria.
A simple set of blood tests and urine microscopy are able to alert the medical team to the likelihood of haemolysis and therefore prevent extensive invasive investigation and enable prompt recognition of valvular dysfunction.
Paravalvular leaks can occur as late as 43 years following metallic valve insertion.
In such patients, early asymptomatic disease can progress rapidly to severely symptomatic and potentially life-threatening disease, making early recognition essential.
Footnotes
Contributors: SM: collecting patient information and clinical details, consenting patient, writing case report, reviewing existing literature and following up patient progress. PG: identifying patient as a unique case, advice with planning and reviewing draft report. RI: advice with planning and reviewing draft report.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Rallidis LS, Moyssakis IE, Ikonomidis I, et al. . Natural history of early aortic paraprosthetic regurgitation: a five-year follow-up. Am Heart J 1999;138:351–7. 10.1016/S0002-8703(99)70124-9 [DOI] [PubMed] [Google Scholar]
- 2.Genoni M, Franzen D, Vogt P, et al. . Paravalvular leakage after mitral valve replacement: improved long-term survival with aggressive surgery? Eur J Cardiothorac Surg 2000;17:14–19. 10.1016/S1010-7940(99)00358-9 [DOI] [PubMed] [Google Scholar]
- 3.Rubinson RM, Morrow AG, Gebel P. Mechanical destruction of erythrocytes by incompetent aortic valvular prosthesis; clinical, hemodynamic, and hematologic findings. Am Heart J 1966;71:179–86. [DOI] [PubMed] [Google Scholar]
- 4.Eyster E, Mayer K, McKenzie S. Traumatic hemolysis with iron deficiency anemia in patients with aortic valve lesions. Ann Intern Med 1968;68:995–1004. 10.7326/0003-4819-68-5-995 [DOI] [PubMed] [Google Scholar]
- 5.Lathem W. The renal excretion of hemoglobin: regulatory mechanisms and the differential excretion of free and protein-bound hemoglobin. J Clin Invest 1959;38:652–8. 10.1172/JCI103843 [DOI] [PMC free article] [PubMed] [Google Scholar]


