Abstract
Inferior vena cava (IVC) filters are useful adjuncts to prevent venous thromboembolism to the pulmonary circulation in the setting of contraindication for anticoagulation. Despite their proven decreased rate of pulmonary embolism, IVC filters are not without complications. We herein present the case of a 22-year-old man with a history of antiphospholipid antibody syndrome who was sent to our institution for evaluation with Budd-Chiari and post-thrombotic syndromes associated to a chronic retrohepatic complete IVC occlusion secondary to an IVC filter placed 5 years earlier. Via common femoral, transjugular and transhepatic accesses, we performed a successful endovascular recanalisation and reconstruction of the IVC with a 16 mm×60 mm covered stent; the hepatic outflow was restored with an 8×20 mm Palmaz stent. At 12-month follow-up, his symptoms have resolved, and his liver tests are within normal limits. He remains on systemic anticoagulation.
Keywords: Cardiovascular medicine, Interventional cardiology, Venous thromboembolism, Haematology (drugs and medicines), Drugs and medicines
Background
Inferior vena cava (IVC) filters are useful adjuncts to prevent thromboembolic disease in the setting of contraindication for anticoagulation. Pulmonary embolism (PE) may be fatal in as many as 25% of patients1; IVC filter placement should be considered in such clinical situation. Despite their widespread use, long-term benefits of this intraluminal device remain controversial, particularly in complex cases such as patients with prothrombotic disorders as antiphospholipid antibody syndrome (APS).2 We herein present the case of a young man with known history of APS and IVC filter placement in a retrohepatic position, who developed a chronic occlusion in the IVC and progressed to Budd-Chiari and post-thrombotic syndromes. We describe the successful endovascular recanalisation and reconstruction of the IVC and restoration of the hepatic venous outflow.
Case presentation
The patient was a 22-year-old man who first presented 5 years prior to our encounter with bilateral oedema of the lower extremities to an outside facility. Subsequent work-up identified bilateral iliac and caval thrombosis with no inciting factor. For this reason, he was initiated on warfarin, and at that time his primary team decided to place an IVC filter, and this device was placed in the retrohepatic position. After fulfilling serologic criteria, the patient was diagnosed with primary APS.
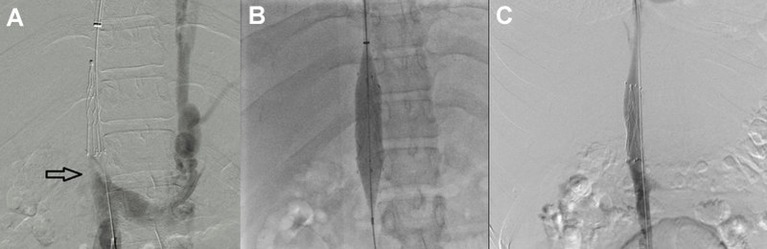
In 2014, he presented at our institution and was found to have visible collateral circulation over the anterior wall of his abdomen and ascites, severe post-thrombotic syndrome in both lower extremities characterised by the presence of limb oedema, discolouration, lipodermatosclerosis and venous ulcers. The liver function tests (LFTs) showed mild elevation of the unconjugated hyperbilirubinaemia; the remaining laboratory value parameters were within normal limits. An abdominal CT revealed a complete chronic occlusion of the retrohepatic portion of the IVC, patency of renal veins, abundant venous collateral network and absence of flow from the hepatic veins. Based on these findings, the diagnosis of Budd-Chiari syndrome was made. The patient initially refused invasive treatment for his condition and decided to continue only with oral anticoagulants. During his follow-up, an endoscopy was performed by the Gastroenterology Service, which showed oesophageal varices. After discussion of the therapeutic options with the patient and his family, the decision was made to restore the flow through the IVC by endovascular means and angiographically evaluate the venous outflow from the liver circulation. Venous access was established, both from the right internal jugular and the right common femoral veins, and complete retrohepatic IVC occlusion was demonstrated by ascending venography (figure 1A). The occlusion was crossed with a Glidewire (Terumo Medical, Somerset, NJ, USA), and a sheath was introduced from the jugular vein. The guidewire coming from the femoral vein was snared through the sheath in the right internal jugular vein, making it a through-and-through guidewire. Aided by the extra support provided by this guidewire, a balloon was introduced from the femoral access and used to dilate the infrahepatic IVC first, followed by the retrohepatic portion displacing and compressing the IVC filter. Once IVC lumen was restored, a 16 mmx60 mm balloon-expandable covered stent was placed at the retrohepatic portion of the IVC (Atrium ADVANTA V12 Covered Stent, Maquet, Germany) (figure 1B). Completion venography demonstrated flow through the IVC (figure 1C). Hepatic vein access was then established in a transhepatic fashion, aided by ultrasound and fluoroscopy. Another through-and-through guidewire was established from the hepatic vein to the previously obtained jugular access. Contrast venography demonstrated a stenotic lesion in the hepatic outflow (figure 2); subsequently, balloon angioplasty of the middle hepatic vein was performed, and an 8 mmx×20 mm Palmaz stent was placed at the confluence of the hepatic veins (figure 3A). Completion venography was performed, which showed patency of two hepatic veins, a compressed IVC filter against the IVC wall and complete patency of the IVC (figure 3B).
Figure 1.
Ascending venography demonstrated the inferior vena cava (IVC) occlusion at the level of the IVC filter (black arrow); the presence of collateral flow through the hemiazygos vein is observed (A). A balloon was introduced from the common femoral access and used to dilate the retrohepatic portion displacing and compressing the IVC filter; the infrahepatic IVC was also balloon angioplastied. Once IVC lumen was restored, a 16 mm×60 mm balloon-expandable covered stent was placed at the retrohepatic portion of the IVC (Atrium ADVANTA V12 Covered Stent, Maquet, Germany) (B). Completion venography demonstrated flow through the IVC (C).
Figure 2.
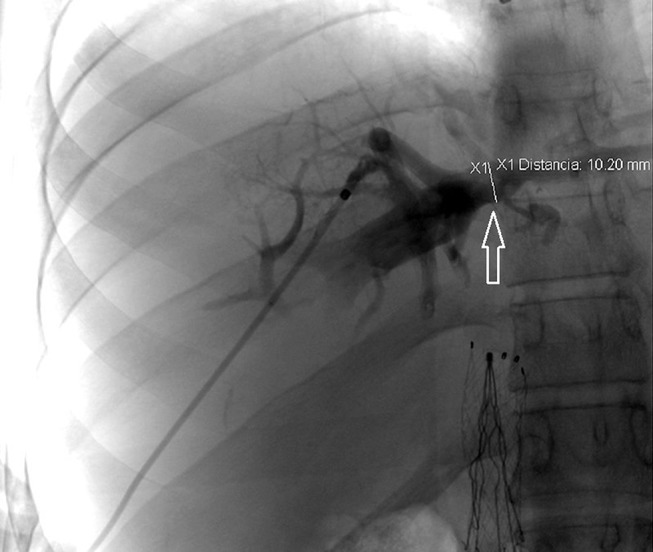
Contrast venography via transhepatic access demonstrated a stenotic lesion in the hepatic outflow (white arrow).
Figure 3.
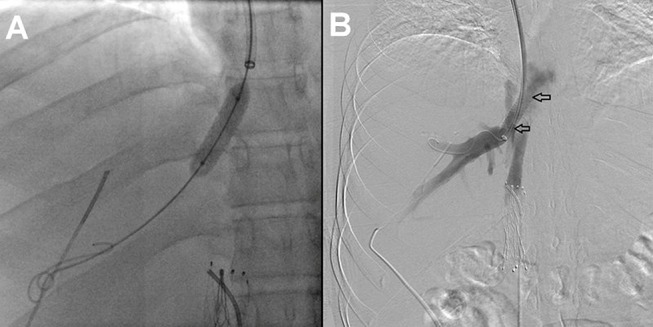
Balloon angioplasty of the middle hepatic vein was performed, and an 8 mm× 20 mm Palmaz stent was placed at the confluence of the hepatic veins (A). Completion venogram was performed demonstrating patency of the hepatic vein in the stented area (between black arrows) (B).
After 2 days, the patient was discharged home on 10 mg of apixaban twice a day for 7 days, followed by 10 mg daily (5 mg twice a day).
Differential diagnosis
protein C deficiency
factor V Leiden thrombophilia
secondary antiphospholipid syndrome associated to systemic lupus erythematosus.
Outcome and follow-up
His hospitalisation course was uneventful, and he was discharged home in stable condition. A follow-up visit 2 months later showed significant improvement of the abdominal collateral network, completely healed lower extremity ulcers, significant improvement of the post-thrombotic symptoms, with no ascites, and normalisation of the LFTs. At 12-month follow-up, he remains in good health, with no recurrence of the prior symptoms.
Discussion
Venous thromboembolism (VTE) affects approximately 1 per 1000 of the population, with an annual estimate of 600 000 cases of clinically significant PE, and about 200 000 deaths annually in the USA alone.3 Because of this high incidence, and given the fact that in as many as 25% PE may be fatal, the concept of vena cava interruption has long been part of the treatment algorithm for VTE, particularly in the setting of contraindication to anticoagulation. Since Bottini reported the first successful IVC ligation to prevent PE in 1893,4 numerous techniques have been proposed for this same purpose, including partial interruption of the IVC with staples, plastic clips or surgical plication.5 In 1973, the permanent Greenfield filter (Boston Scientific, Natick, Massachusetts, USA) was introduced, which became the gold standard for treatment.1 In later years, designs that allow endovascular placement and subsequent retrieval of filters have been developed.
The APS is a well-known prothrombotic disorder, and this is defined as the classic triad of arterial and/or venous thrombosis, recurrent fetal loss and thrombocytopenia, in the presence of antiphospholipid antibodies.6 These antibodies have been associated with thrombosis in unusual vascular beds, such as the hepatic veins, causing Budd-Chiari syndrome.7 The latter is a relatively infrequent complication of APS, associated in particular with a higher proportion of positive IgG Anticardiolipin (aCL) or antiphospholipid (aPL) antibodies levels than healthy controls.7
Although interruption of the IVC by means of an IVC filter has well established indications, less clear is the benefit of offering such treatment in the setting of IVC thrombosis that would require suprarenal placement of the device. Reported complications of IVC filters include IVC penetration associated with visceral perforation, aortic dissection and chronic IVC occlusions.8 Interestingly, conical filters are associated with the highest risk of penetration, and cylindrical/umbrella filters have the highest risk of IVC thrombosis and subsequent occlusion of the vessel.9
Endovascular recanalisation of chronic total occlusions of the IVC has previously been shown to be feasible and effective,10 and less invasive than open surgical reconstruction or bypass.11 However, current experience with this technique is limited to a small series of cases. Although experience continues to enlarge, results become more acceptable: a series of 982 patients with venous outflow obstruction, published by Neglén et al,12 was treated by endovascular means, with 86% cumulative secondary patency rates at 72 months and ulcer healing of 58% at 5 years. However, experience of endovascular recanalisation of chronic total occlusions of the IVC in patients with APS remains scarce and limited only to case reports. In this case, we opted for a covered stent to isolate from the circulation the compressed IVC filter; the diameter of the selected device was smaller (16 mm) to the commonly reported in the literature for IVC stenting. We consider that this calibre would be sufficient to recanalise successfully the vessel and to displace and compress the filter. Additionally in the case of central venous occlusion covered stents have demonstrated better technical results and longer patency rates than bare metal stents,13 and reports have shown the utility of these devices for haemorrhage control in the case of vein rupture.14 15
In conclusion, this case highlights the need for a careful and thorough analysis before placing any IVC filter, particularly in the setting of prothrombotic conditions and removing the device when patients can be initiated on anticoagulation therapy to avoid long-term complications as the case presented. Although IVC filter has been shown to prevent PE cases in patients with APS, the potential for significant thrombotic complications inherent to the location where the filter is placed must be considered. In this case, multiple endovascular adjuncts, such as the use of the through-and-through guidewire technique and transhepatic venous access, made the IVC and hepatic vein recanalisation possible, with excellent angiographic and clinical results.
Learning points/take home messages.
Inferior vena cava (IVC) filters are useful adjuncts to prevent thromboembolic disease in the setting of contraindication for anticoagulation.
Careful and thorough analysis is necessary before placing any IVC filter, particularly in the setting of a prothrombotic condition.
Retrieval of IVC filters must be performed once anticoagulation can be initiated to prevent long-term complications.
Endovascular recanalisation of a chronic total occlusion of the IVC secondary to an IVC filter and restoration of venous circulation is feasible and clinically effective; it represents a less invasive modality than open surgical reconstruction or bypass.
Patient’s perspective.
My quality of life improved significantly following the intervention; my abdomen reduced in size, and the sensation of heaviness is gone. Also my legs wounds healed completely, and swelling is remarkably improved.
Footnotes
Contributors: CAH contributed to the conception and design, planning, writing the manuscript and final approval.
RL contributed to the acquisition of data, writing the manuscript and final approval.
JEAA contributed to the acquisition of data, writing the manuscript, critical review and final approval.
HLE contributed to the acquisition of data and final approval.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016;149:315–52. 10.1016/j.chest.2015.11.026 [DOI] [PubMed] [Google Scholar]
- 2. Cherian J, Gertner E. Recurrent pulmonary embolism despite inferior vena cava filter placement in patients with the antiphospholipid syndrome. J Clin Rheumatol 2005;11:56–8. 10.1097/01.rhu.0000152150.01274.1b [DOI] [PubMed] [Google Scholar]
- 3. Heit JA, Silverstein MD, Mohr DN, et al. The epidemiology of venous thromboembolism in the community. Thromb Haemost 2001;86:452–63. [PubMed] [Google Scholar]
- 4. Dale WA. Ligation of the inferior vena cava for thromboembolism. Surgery 1958;43:24–44. [PubMed] [Google Scholar]
- 5. Lindenauer SM. Prophylactic staple plication of the inferior vena cava. Arch Surg 1973;107:669–75. 10.1001/archsurg.1973.01350230027007 [DOI] [PubMed] [Google Scholar]
- 6. Qi X, De Stefano V, Su C, et al. Associations of antiphospholipid antibodies with splanchnic vein thrombosis. Medicine 2015;94:e496 10.1097/MD.0000000000000496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aggarwal R, Ravishankar B, Misra R, et al. Significance of elevated IgG anticardiolipin antibody levels in patients with Budd-Chiari syndrome. Am J Gastroenterol 1998;93:954–7. 10.1111/j.1572-0241.1998.00286.x [DOI] [PubMed] [Google Scholar]
- 8. Deso SE, Idakoji IA, Kuo WT. Evidence-based evaluation of inferior vena cava filter complications based on filter type. Semin Intervent Radiol 2016;33:93–100. 10.1055/s-0036-1583208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hinojosa CA, Torres-Machorro A, Lizola R, et al. Open removal of a retained retrohepatic inferior vena cava filter with a residual primary neuroectodermal renal tumoral Thrombus. BMJ Case Rep 2015;2015:bcr2015212190 10.1136/bcr-2015-212190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adam L, Wyss TR, Do DD, et al. Endovascular stent reconstruction of a chronic total occlusion of the inferior vena cava using bidirectional wire access and a balloon puncture by a re-entry device. J Vasc Surg Venous Lymphat Disord 2015;3:442–5. 10.1016/j.jvsv.2015.04.006 [DOI] [PubMed] [Google Scholar]
- 11. Anaya-Ayala JE, Johnson BA, Smolock CJ, et al. Inferior vena cava bypass for the treatment of obliterative hepatocavopathy with five-year follow-up. Vascular 2011;19:282–6. 10.1258/vasc.2011.cr0262 [DOI] [PubMed] [Google Scholar]
- 12. Neglén P, Hollis KC, Olivier J, et al. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg 2007;46:979–90. 10.1016/j.jvs.2007.06.046 [DOI] [PubMed] [Google Scholar]
- 13. Anaya-Ayala JE, Smolock CJ, Colvard BD, et al. Efficacy of covered stent placement for central venous occlusive disease in hemodialysis patients. J Vasc Surg 2011;54:754–9. 10.1016/j.jvs.2011.03.260 [DOI] [PubMed] [Google Scholar]
- 14. Anaya-Ayala JE, Charlton-Ouw KM, Kaiser CL, et al. Successful emergency endovascular treatment for superior vena cava injury. Ann Vasc Surg 2009;23:139–41. 10.1016/j.avsg.2008.02.016 [DOI] [PubMed] [Google Scholar]
- 15. Adams MK, Anaya-Ayala JE, Davies MG, et al. Endovascular management of iliac vein rupture during percutaneous interventions for occlusive lesions. Ann Vasc Surg 2012;26:575.e5–9. 10.1016/j.avsg.2011.08.025 [DOI] [PubMed] [Google Scholar]