Abstract
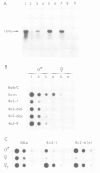
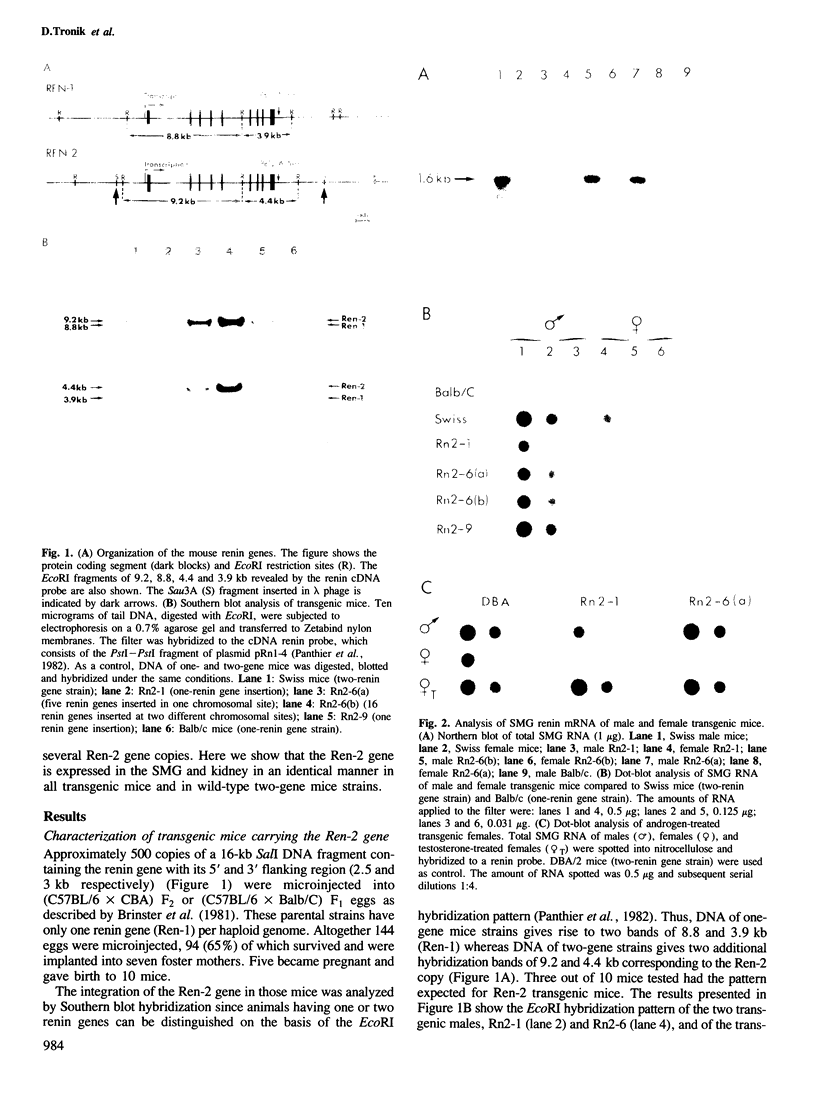
The Ren-2 gene encoding the mouse submaxillary gland (SMG) renin was microinjected into the pronuclei of fertilized eggs from mice carrying only the Ren-1 gene. In addition to the whole transcription unit, the injected DNA contained 2.5 and 3 kb of upstream and downstream flanking sequences, respectively. Three independent transgenic mice lines were obtained; two of them had integrated one copy of the Ren-2 gene, the last one had integrated five and eleven copies at two independent sites. Independently of the number of Ren-2 copies integrated, the pattern of Ren-2 gene expression in all the transgenic mice was identical to that observed in wild-type animals in which Ren-1 and Ren-2 are closely linked on chromosome 1. In particular, the exogenous Ren-2 gene was only transcribed in the kidney and in the SMG. In the kidney, Ren-1 and Ren-2 mRNAs were present at a comparable level, whereas in the SMG Ren-2 mRNA was at least 100-fold more abundant than Ren-1 mRNA. Moreover, Ren-2 expression in the SMG was positively regulated by androgens. Only one difference between transgenic mice and wild-type mice carrying the Ren-2 gene has been observed: the basal level of Ren-2 transcription in the SMG of transgenic females was lower than in two-gene strain females. Androgen treatment of transgenic females induced SMG renin mRNA to a level identical to that of transgenic males. This suggests that the basal level of SMG renin mRNA is dependent upon cis-acting elements which are not present in the microinjected fragment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Banerji J., Olson L., Schaffner W. A lymphocyte-specific cellular enhancer is located downstream of the joining region in immunoglobulin heavy chain genes. Cell. 1983 Jul;33(3):729–740. doi: 10.1016/0092-8674(83)90015-6. [DOI] [PubMed] [Google Scholar]
- Bing J., Poulsen K., Hackenthal E., Rix E., Taugner R. Renin in the submaxillary gland: a review. J Histochem Cytochem. 1980 Aug;28(8):874–880. doi: 10.1177/28.8.7003007. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M., Senear A. W., Warren R., Palmiter R. D. Somatic expression of herpes thymidine kinase in mice following injection of a fusion gene into eggs. Cell. 1981 Nov;27(1 Pt 2):223–231. doi: 10.1016/0092-8674(81)90376-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson D. P., Gross K. W., Piccini N., Wilson C. M. Evolution and variation of renin genes in mice. Genetics. 1984 Nov;108(3):651–667. doi: 10.1093/genetics/108.3.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. Control of eukaryotic messenger RNA synthesis by sequence-specific DNA-binding proteins. 1985 Aug 29-Sep 4Nature. 316(6031):774–778. doi: 10.1038/316774a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Field L. J., Gross K. W. Ren-1 and Ren-2 loci are expressed in mouse kidney. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6196–6200. doi: 10.1073/pnas.82.18.6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field L. J., Philbrick W. M., Howles P. N., Dickinson D. P., McGowan R. A., Gross K. W. Expression of tissue-specific Ren-1 and Ren-2 genes of mice: comparative analysis of 5'-proximal flanking regions. Mol Cell Biol. 1984 Nov;4(11):2321–2331. doi: 10.1128/mcb.4.11.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Holm I., Ollo R., Panthier J. J., Rougeon F. Evolution of aspartyl proteases by gene duplication: the mouse renin gene is organized in two homologous clusters of four exons. EMBO J. 1984 Mar;3(3):557–562. doi: 10.1002/j.1460-2075.1984.tb01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krumlauf R., Hammer R. E., Tilghman S. M., Brinster R. L. Developmental regulation of alpha-fetoprotein genes in transgenic mice. Mol Cell Biol. 1985 Jul;5(7):1639–1648. doi: 10.1128/mcb.5.7.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S., Hammer R. E., Kuenzel E. A., Brinster R. L. Expression of the chicken transferrin gene in transgenic mice. Cell. 1983 Sep;34(2):335–341. doi: 10.1016/0092-8674(83)90368-9. [DOI] [PubMed] [Google Scholar]
- Morello D., Moore G., Salmon A. M., Yaniv M., Babinet C. Studies on the expression of an H-2K/human growth hormone fusion gene in giant transgenic mice. EMBO J. 1986 Aug;5(8):1877–1883. doi: 10.1002/j.1460-2075.1986.tb04439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins J. J., Burt D. W., Windass J. D., McTurk P., George H., Brammar W. J. Molecular cloning of two distinct renin genes from the DBA/2 mouse. EMBO J. 1982;1(11):1461–1466. doi: 10.1002/j.1460-2075.1982.tb01338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ondetti M. A., Cushman D. W. Enzymes of the renin-angiotensin system and their inhibitors. Annu Rev Biochem. 1982;51:283–308. doi: 10.1146/annurev.bi.51.070182.001435. [DOI] [PubMed] [Google Scholar]
- Overbeek P. A., Lai S. P., Van Quill K. R., Westphal H. Tissue-specific expression in transgenic mice of a fused gene containing RSV terminal sequences. Science. 1986 Mar 28;231(4745):1574–1577. doi: 10.1126/science.3006249. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L. Germ-line transformation of mice. Annu Rev Genet. 1986;20:465–499. doi: 10.1146/annurev.ge.20.120186.002341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D., Chen H. Y., Brinster R. L. Differential regulation of metallothionein-thymidine kinase fusion genes in transgenic mice and their offspring. Cell. 1982 Jun;29(2):701–710. doi: 10.1016/0092-8674(82)90186-6. [DOI] [PubMed] [Google Scholar]
- Panthier J. J., Dreyfus M., Roux T. L., Rougeon F. Mouse kidney and submaxillary gland renin genes differ in their 5' putative regulatory sequences. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5489–5493. doi: 10.1073/pnas.81.17.5489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panthier J. J., Holm I., Rougeon F. The mouse Rn locus: S allele of the renin regulator gene results from a single structural gene duplication. EMBO J. 1982;1(11):1417–1421. doi: 10.1002/j.1460-2075.1982.tb01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccini N., Knopf J. L., Gross K. W. A DNA polymorphism, consistent with gene duplication, correlates with high renin levels in the mouse submaxillary gland. Cell. 1982 Aug;30(1):205–213. doi: 10.1016/0092-8674(82)90026-5. [DOI] [PubMed] [Google Scholar]
- Rougeon F., Chambraud B., Foote S., Panthier J. J., Nageotte R., Corvol P. Molecular cloning of a mouse submaxillary gland renin cDNA fragment. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6367–6371. doi: 10.1073/pnas.78.10.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward T. A., Wagner E. F., Mintz B. Human beta-globin gene sequences injected into mouse eggs, retained in adults, and transmitted to progeny. Science. 1982 Sep 10;217(4564):1046–1048. doi: 10.1126/science.6287575. [DOI] [PubMed] [Google Scholar]
- Theisen M., Stief A., Sippel A. E. The lysozyme enhancer: cell-specific activation of the chicken lysozyme gene by a far-upstream DNA element. EMBO J. 1986 Apr;5(4):719–724. doi: 10.1002/j.1460-2075.1986.tb04273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Wilson C. M., Cherry M., Taylor B. A., Wilson J. D. Genetic and endocrine control of renin activity in the submaxillary gland of the mouse. Biochem Genet. 1981 Jun;19(5-6):509–523. doi: 10.1007/BF00484623. [DOI] [PubMed] [Google Scholar]
- Wilson C. M., Erdös E. G., Wilson J. D., Taylor B. A. Location on chromosome 1 of Rnr, a gene that regulates renin in the submaxillary gland of the mouse. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5623–5626. doi: 10.1073/pnas.75.11.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C. M., Taylor B. A. Genetic regulation of thermostability of mouse submaxillary gland renin. J Biol Chem. 1982 Jan 10;257(1):217–223. [PubMed] [Google Scholar]
- Yamamoto K. R. Steroid receptor regulated transcription of specific genes and gene networks. Annu Rev Genet. 1985;19:209–252. doi: 10.1146/annurev.ge.19.120185.001233. [DOI] [PubMed] [Google Scholar]