Abstract
Timely diagnosis of diencephalic syndrome is not often the case for patients presenting with failure to thrive (FTT) because of its rarity and lack of specific symptoms. Herein, we report two cases of diencephalic syndrome (2-year-old girl and 10-month-old boy) presenting with severe emaciation. Both patients had histories of poor weight gain for months despite having good appetites prior to diagnosis. Initial work-up did not reveal the diagnosis. Horizontal nystagmus was noted in both patients: by a neurologist in the first patient and by a family member in the second patient. MRI of the brain showed large suprasellar mass and pilocytic astrocytoma was confirmed by pathology in each case. The patients were started on appropriate chemotherapy with interval improvements in weight gain. These cases illustrate the importance of cranial imaging and consideration of diencephalic syndrome for children presenting with FTT despite normal or increased caloric intake.
Keywords: failure to thrive, infant health
Background
Diencephalic syndrome is characterised by progressive weight loss despite normal or increased caloric intake, and preservation of linear growth. It is usually associated with a mass lesion in the diencephalon. Delay in diagnosis is common because of its rarity and non-specific symptoms, which may have a devastating outcome. Herein, we report two cases of diencephalic syndrome presenting with failure to thrive (FTT).
Case presentation
Case 1
A 2-year-old girl, a recent immigrant from Mexico, presented with poor weight gain starting at 8 months of age. She was born full term with unremarkable prenatal and perinatal courses. She had appropriate weight gain during the first 8 months of life. No inciting event was identified as a potential trigger prior to the onset of poor weight gain. At the time of presentation, she had a normal appetite and was eating regular table foods. The treatment for an unknown parasitic infection diagnosed 6 months prior to the presentation did not help in gaining weight. No diarrhoea, vomiting or constipation was reported. Developmental milestones were appropriate for her age and her past medical history was otherwise unremarkable.
Case 2
A 10-month-old boy baby presented with poor weight gain starting at 6 months of age (figure 1). He was born full term with unremarkable prenatal and perinatal courses. He was breastfed for the first few months, followed by Similac Sensitive, due to presence of gastro-oesophageal reflux. He had appropriate weight gain until he reached 6 months of age. He then developed intermittent diarrhoea and food-induced vomiting. Initial work-up for FTT did not reveal a diagnosis. Upper gastrointestinal (GI) endoscopy and flexible sigmoidoscopy were consistent with food allergy. He was diagnosed with cow’s milk protein allergy and switched to soy formula without any improvement. A diagnosis of salmonella infection was made during the work-up for diarrhoea, but no treatment was given as the diarrhoea improved spontaneously. His development was appropriate for his age. Horizontal nystagmus, which was first noted by a family member, prompted the medical team to obtain MRI of the brain, which revealed the diagnosis.
Figure 1.

The patient’s picture before and after onset of symptoms.
Investigations
Case 1
On examination, she was noted to have severe emaciation (weight z=−6.53, height z=−3.34 SD, BMI z=−4.64 SD). Three-day calorie count showed caloric intake of ~160 kcal/kg/day (Reference range for age: 75–90 kcal/kg/day).
Initial work-up on admission included the followings:
Complete blood count (CBC), basic metabolic panel (BMP): Normal
Sweat chloride: Normal
Erythrocyte sedimentation rate and C-reactive protein: Normal
HIV and stool-reducing substance: Negative
Plasma aminoacids and acylcarnitine analysis: Normal
Karyotype and microarray: Normal
Hypercalcaemia, diabetes mellitus and diabetes insipidus are excluded as endocrine causes of FTT with normal laboratory test results (serum calcium, glucose and sodium) and lack of associated symptoms. Additional work-up for endocrine causes of FTT to exclude hyperthyroidism, vitamin D deficiency, growth hormone deficiency and adrenal insufficiency included the following:
Thyroid function tests (TFTs): Normal
25-OH Vitamin D: 41.1 ng/mL (RR>=21: Optimum level)
IGFBP-3: 2.7 mg/L (RR:0.8–3.9)
Insulin: 3.6 mU/L (RR: 0.7–29.6)
8-AM Cortisol: 7.8 µg/dL (RR: 3–21 for 1–16-year old)
1 µg cosyntropin stimulation testing: Normal.
A fine horizontal nystagmus was noted during neurology evaluation, and MRI of the brain was obtained, which showed a large hypothalamic/chiasmatic tumour measuring approximately 4×4×6 cm (figure 2). Several metastases outlining the spinal cord from the cervical to the conus were noted on MRI myelogram. Pilocytic astrocytoma (WHO grade I) diagnosis was confirmed by pathology.
Figure 2.
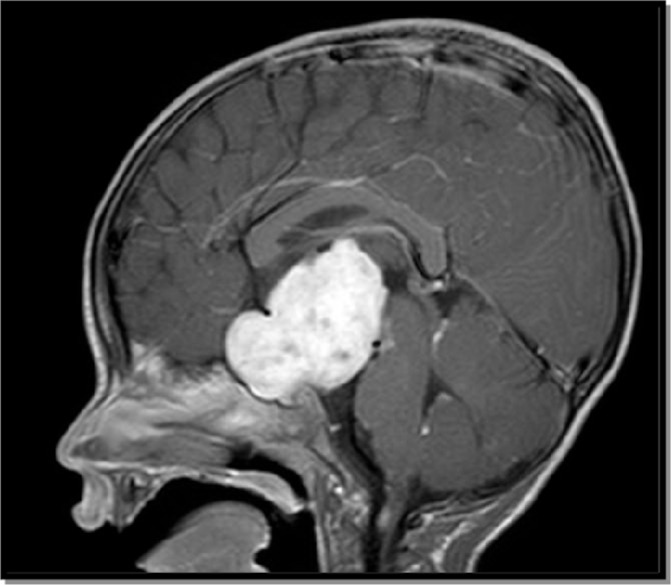
Brain MRI postcontrast T1W sagittal image.
Case 2
On examination, he was noted to have severe emaciation (weight z=−3.48, length z=−1.22 SD, weigh-for-length z=−3.40 SD). Three-day calorie count showed caloric intake of ~101 kcal/kg/day (Reference range for age: 75–90 kcal/kg/day).
Previous work up prior to admission included the following:
CBC and BMP: Normal
TFTs: Normal
Sweat chloride test: Normal
ESR and CRP: Normal
Liver function tests: Normal
Occult blood in stool and stool-reducing substance: Negative
MRI of the brain showed 4.1×6.8×2.4 cm enhancing suprasellar mass (figure 3). Pilocytic astrocytoma (WHO grade I) diagnosis was confirmed by pathology.
Figure 3.
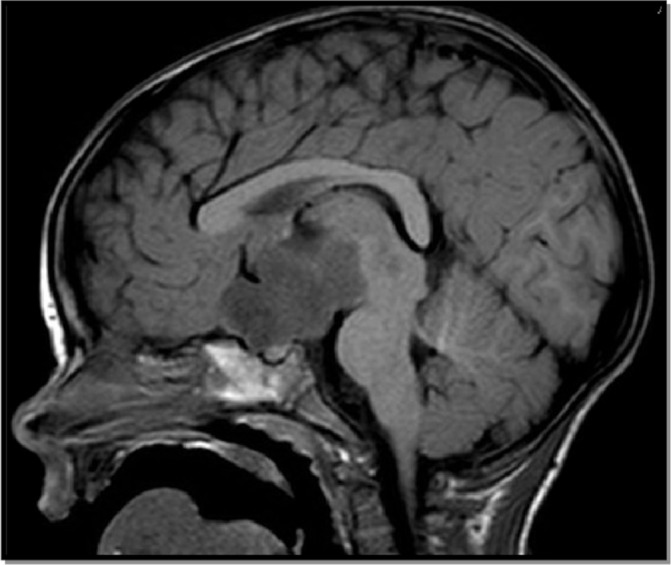
Brain MRI precontrast T1W sagittal image.
Endocrine work-up to rule out pituitary hormonal deficiencies and shed light on possible pathophysiology of diencephalic syndrome included the following:
TFTs and 1 µg cosyntropin stimulation testing: Normal
IGF-1: 68 ng/mL (RR: 16–142)
IGFBP-3: 3.3 mg/L (RR: 0.7–3.6)
Random growth hormone: 4.2 ng/mL (RR:≤10.1)
Leptin: 0.6 ng/mL (No reference range established for this age group; RR: 0.6–16.8 for age 5.0–9.9 years)
Insulin: 1.2 mU/L (RR: 1.3–28.7, serum glucose 71 mg/dL)
MRI of the brain showed 4.1×6.8×2.4 cm enhancing suprasellar mass. Pilocytic astrocytoma (WHO grade I) diagnosis was confirmed by pathology.
Outcome and follow-up
In case 1, she was started on individualised institutional protocol with carboplatin and vincristine. She completed 80 weeks of chemotherapy with decrease in the bulk of her hypothalamic tumour. In addition to visual disturbances, she developed central precocious puberty requiring histrelin implant placement. Her thyroid function tests, morning cortisol and growth factors were all within normal limits at 1 year after the diagnosis. There was no follow-up hormone levels on insulin, leptin or random growth hormone. Her most recent weight was 14.3 kg (z=−1.1) at 4 years of age (18 months into the initiation of treatment).
In case 2, he was also started on individualised institutional protocol with carboplatin and vincristine. He was placed a gastrostomy tube secondary to the need of significant enteral nutritional supplementation, which was later converted to gastrojejunal tube. The chemotherapy protocol was adjusted at week 60 to weekly vinblastine treatment due to gradual progression of the tumour on MRI of the brain. He required intermittent tumour cyst drainage and later ventriculoperitoneal shunt placement. He developed tumour associated bitemporal hemianopsia. Although his weight improved up to 9.5 kg (z=−2.12) at 2 years of age, his most recent weight was 9.9 kg (z=−3.22) at 2 years and 8 months of age. No further hormonal testing available for this patient.
Discussion
Diencephalic syndrome was first described by Russell in 1951 and characterised by progressive weight loss despite normal or increased caloric intake, and preservation of linear growth.1 2 It is usually associated with a mass lesion in the diencephalon, but posterior fossa brain tumours also have been reported. The most striking feature of diencephalic syndrome is severe emaciation despite normal or high caloric intake.3 Affected children are usually mentally alert and happy, as in our two cases.
Vomiting and nystagmus are reported in the majority of case reports. A case series of 11 patients with diencephalic syndrome from Children’s Hospital of Boston and the Dana Farber Cancer Institute showed a median age of onset at 18 months and a reduced incidence of hyperkinesia (9%), nystagmus (27%) and emesis (36%) compared with previous reports. All patients had heights within normal range for age and all met their age-appropriate developmental milestones.4 Neither of our patients had hyperkinesia, one of them had intermittent food-induced vomiting (case 2), and both had nystagmus. Whereas linear growth was preserved in case 2, it was greatly affected in case 1. We speculate that this may be attributed to delay in diagnosis and more severely affected weight gain (weight z scores –6.53 vs –3.48).
In another series of eight patients with Diencephalic syndrome who were evaluated between 1995 and 2013 at Seoul National University Children’s Hospital, all patients presented with a chief complaint of poor weight gain. Mean age for symptom onset was 18±10.5 months. Diagnosis is delayed about 11±9.7 months on average. Other associated symptoms included hydrocephalus (n=5), recurrent vomiting (n=5), strabismus (n=2), hyperactivity (n=1) and nystagmus (n=1). Only three of them showed improvement and remained stable, while two patients were still requiring chemotherapy and other three patients were discharged for palliative care.5
Pathophysiology is still unclear despite previous postulations of increased growth hormone (GH) and ghrelin, and decreased insulin and leptin levels. However, these hormonal changes were claimed to be related to patients’ nutritional status, rather than causality.4 6 Our patients had normal GH markers (case 1 and 2), normal random GH (case 2), normal insulin level (case 1), and low-normal leptin and insulin levels (case 2). Furthermore, increased energy expenditure has been previously shown in patients with diencephalic syndrome, and it was proposed as a mechanism for FTT in these patients.7
Although the mass lesion was located at the level of the hypothalamic region and in close proximity to the pituitary gland, no endocrine abnormality was found in either case except central precocious puberty in case 1. In a previous report, there was only GH deficiency following radiation treatment and the remainder of the pituitary hormonal secretion remained intact at 2-year follow-up after the diagnosis.8 Further studies with long-term follow-up are needed not only to understand the mechanisms of the diencephalic syndrome but also for surveillance of pituitary hormonal functions.
Learning points.
Diencephalic syndrome is a rare cause of failure to thrive associated with significant intracranial pathology.
In children presenting with unexplained FTT despite normal or high caloric intake, a detailed neurological examination is warranted.
A cranial imaging should be considered as a next step in the diagnostic evaluation, especially in those with abnormal findings on the neurological examination.
The roles of different hormones (insulin, growth hormone, leptin or ghrelin) on FTT in diencephalic syndrome remain to be elucidated.
Endocrine testing usually appears to be surprisingly normal in patients with diencephalic syndrome.
Footnotes
Contributors: MT involved in patient care of case 1, AT in case 2, and DLP in both cases. MT drafted the initial case report. AT and DLP critically reviewed and edited the manuscript. All authors approved the final version of the manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Russell A. A diencephalic syndrome of emaciation in infancy and childhood. Arch Dis Child 1951;26:274–80. [Google Scholar]
- 2.Fleischman A, Brue C, Poussaint TY, et al. . Diencephalic syndrome: a cause of failure to thrive and a model of partial growth hormone resistance. Pediatrics 2005;115:e742–e748. 10.1542/peds.2004-2237 [DOI] [PubMed] [Google Scholar]
- 3.Poussaint TY, Barnes PD, Nichols K, et al. . Diencephalic syndrome: clinical features and imaging findings. AJNR Am J Neuroradiol 1997;18:1499–505. [PMC free article] [PubMed] [Google Scholar]
- 4.Brauner R, Trivin C, Zerah M, et al. . Diencephalic syndrome due to hypothalamic tumor: a model of the relationship between weight and puberty onset. J Clin Endocrinol Metab 2006;91:2467–73. 10.1210/jc.2006-0322 [DOI] [PubMed] [Google Scholar]
- 5.Kim A, Moon JS, Yang HR, et al. . Diencephalic syndrome: a frequently neglected cause of failure to thrive in infants. Korean J Pediatr 2015;58:28–32. 10.3345/kjp.2015.58.1.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Velasco P, Clemente M, Lorite R, et al. . The role of leptin in diencephalic syndrome. Pediatrics 2014;133:e263–e266. 10.1542/peds.2012-3196 [DOI] [PubMed] [Google Scholar]
- 7.Vlachopapadopoulou E, Tracey KJ, Capella M, et al. . Increased energy expenditure in a patient with diencephalic syndrome. J Pediatr 1993;122:922–4. 10.1016/S0022-3476(09)90021-X [DOI] [PubMed] [Google Scholar]
- 8.Drop SL, Guyda HJ, Colle E. Inappropriate growth hormone release in the diencephalic syndrome of childhood: case report and 4 year endocrinological follow-up. Clin Endocrinol 1980;13:181–7. 10.1111/j.1365-2265.1980.tb01040.x [DOI] [PubMed] [Google Scholar]


