Abstract
Major depression (MD) is associated with peripheral inflammation and increased cardiovascular risk. Regular physical exercise can have anti-inflammatory effects. The present study examined whether behavioral activation with exercise affects inflammatory processes in MD. Ninety-eight patients with MD were randomly assigned to cognitive-behavioral therapy (CBT) emphasizing exercise during behavioral activation (CBT-E), CBT with pleasurable low-energy activities as an active control condition (CBT-C) or a passive waiting list control group (WL). Plasma levels of C-reactive protein (CRP), interleukin (IL)-6, IL-10, lipopolysaccharide (LPS)-stimulated IL-6 production, and blood immune cell counts were analyzed at baseline and weeks 8 (post-behavioral activation) and 16 (post-treatment). Thirty non-depressed age- and sex-matched controls were included to examine potential immunological alterations in MD at baseline. Patients with MD exhibited higher levels of CRP, higher neutrophil and monocyte counts, lower IL-10 levels and reduced LPS-stimulated IL-6 production compared to controls (P<0.001−0.045). Multilevel modeling indicated that CBT-E was associated with increased anti-inflammatory IL-10 at weeks 8 and 16 compared to CBT-C and WL (P=0.004−0.018). CBT-E did not significantly affect other immunological makers in the total sample. A subgroup analysis including patients with potentially higher cardiovascular risk (CRP ⩾1 μg ml−1) indicated that CRP was reduced in CBT-E compared to CBT-C (P<0.007) and marginally reduced compared to WL (P<0.085) after week 16. The present findings provide new insights into immunological effects of behavioral treatments against depression. Behavioral activation in conjunction with exercise may have the potential to reverse, in part, immunological alterations in MD.
Introduction
Major depression (MD) is associated with low-grade inflammation as evident from elevated levels of pro-inflammatory cytokines (for example, interleukin (IL)-6, tumor necrosis factor (TNF)-α) and acute-phase proteins (that is, C-reactive protein (CRP)) in the circulation.1, 2 Longitudinal observations in humans and studies in experimental animals document a bidirectional loop in which peripheral inflammatory signals can induce depressive symptoms and vice versa.3, 4 Mechanistically, elevated peripheral inflammatory signaling leads to dysregulation of several mood-relevant neural processes via molecular, cellular and neural routes.5 In this context, it has been suggested that reduced levels of anti-inflammatory cytokines, in particular IL-10, might also increase the risk for depression.6 Although the findings regarding the role of IL-10 in patients with MD are currently inconsistent,1, 7, 8 there is strong evidence from animal research suggesting an inverse relationship between IL-10 and depression-like behavior.6, 9, 10, 11, 12 In addition, a genetic predisposition toward producing low levels of IL-10 has been related to depressive symptoms.13 On the basis of the functional relation between depressive symptoms and cardiovascular disease (CVD), peripheral inflammatory pathways are considered a possible link between both clinical conditions.14, 15, 16
Physical inactivity is associated with both inflammation and depression.17, 18, 19, 20 Meta-analyses suggest that exercise (that is, regular, planned and structured physical activity) has moderate to large antidepressant effects21, 22 and even reduces depressive symptom severity in patients who did not remit with pharmacological antidepressant treatment.23 In addition, exercise induces anti-inflammatory effects mainly via the release of anti-inflammatory cytokines, such as IL-10, and has thus been suggested as a beneficial treatment option for clinical conditions related to inflammation.24, 25, 26, 27, 28, 29, 30 Although several studies document the anti-inflammatory effects of exercise in healthy individuals and patients with medical illness,24 little research focuses on the impact of physical activity on immunity in patients with clinically-relevant depressive symptoms. One trial found no significant effects of exercise treatment on circulating pro-inflammatory cytokine levels in a subgroup of patients with MD who were currently taking selective serotonin reuptake inhibitors (SSRIs), but were not responding to this treatment.31 When considering this finding, it should be noted that physical activity may overlap, in part, with some of the potential mechanisms of SSRIs.22, 31, 32 Thus, the potential of exercise to affect immunological markers may be attenuated in pharmacological non-responders.
Cognitive-behavioral therapy (CBT) is a standard treatment for depression33 and can be modified to increase exercise in the course of behavioral activation.34, 35, 36, 37 This randomized controlled trial examined whether CBT that emphasizes exercise during behavioral activation affects immunological markers in patients with MD. Immunological measures included plasma levels of CRP, IL-6 and IL-10, as well as ex vivo lipopolysaccharide (LPS)-stimulated IL-6 production and circulating leukocyte subpopulations. Although we expected that CBT in conjunction with exercise might be associated with a decrease in systemic inflammation (that is, CRP) and an increase in anti-inflammatory IL-10, this trial was considered exploratory. This is because we assessed potential treatment effects on a wide range of immunological markers, including those with inconsistent findings in MD such as leukocytes38, 39 as well as mitogen-stimulated cytokines, which has been shown to be increased,40, 41, 42 decreased43, 44, 45, 46, 47, 48 or unaltered49, 50, 51 in MD.
Materials and Methods
Participants
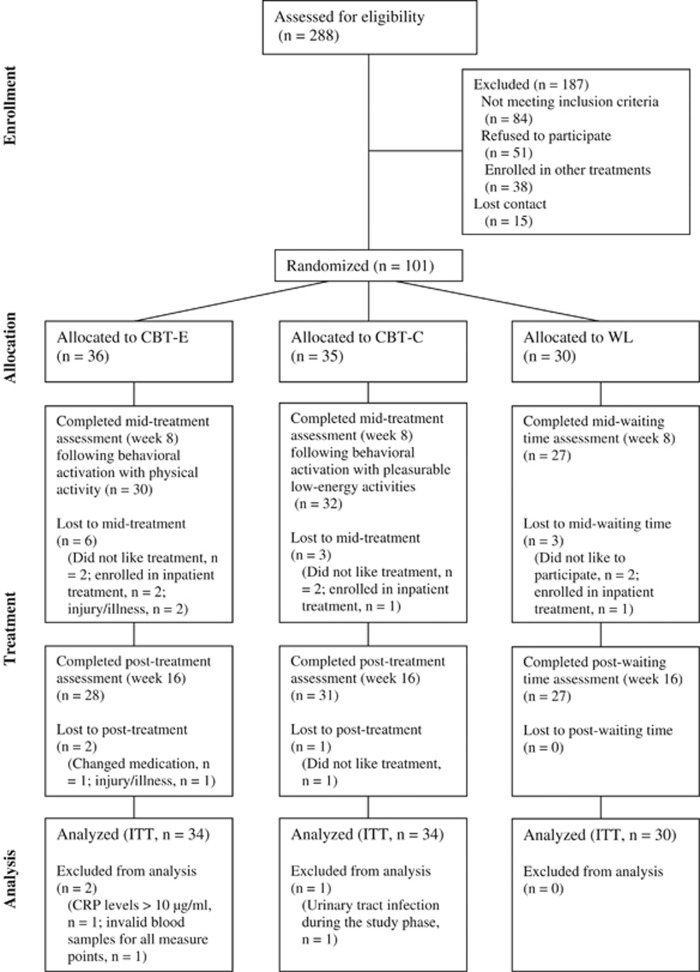
This randomized controlled trial was conducted from August 2011 to February 2015 with German Psychological Society Review Board approval. The study was part of the Outcome of Psychological Interventions in Depression (OPID) trial. OPID is an ongoing research project that aims to improve outcomes in treatment for MD. OPID involves four different arms: (i) CBT with exercise (CBT-E), (ii) an active control condition for CBT-E, including CBT with pleasurable low-energy activities (CBT-C), (iii) Cognitive Behavioral Analysis System of Psychotherapy and (iv) a passive waitlist control condition (WL). Arms (i–iii) also captured a comprehensive immunological evaluation and were funded as a separate subproject by the German Research Foundation from 2011–2015 (DFG RI 574/23-1/SCHE 341/20-1; ‘Effects of psychotherapy with physical activity on inflammatory markers in patients with major depression’). Power calculations52 to provide estimates for the necessary sample size for F-tests were conducted concerning CRP as a primary outcome for systemic inflammation. To detect a medium effect of group × time, with a statistical power of 1−β=0.80 and a level of significance of α<0.05, a sample size of at least 86 needs to be included when assuming that correlations between repeated measures are moderate.53 Considering lower stability as well as partially unknown and potentially smaller effect sizes for cytokines and immune cell counts, a total sample size of N=150 was targeted, but not reached due to the conclusion of funding and a slower than anticipated recruitment into the trial. Ninety-eight patients aged 18–65 who fulfilled criteria for MD in DSM-IV54 and who were randomly assigned using simple computerized randomization to either CBT-E, CBT-C or WL were analyzed (see Figure 1 for study flow). A sample of 30 age- and sex-matched healthy controls from the same community was studied to examine potential baseline alterations in immunological markers in MD. Patients were recruited via the Outpatient Clinic for Psychological Interventions of the University of Marburg, via advertisements, leaflets in pharmacies and waiting rooms of doctors, as well as press releases in local newspapers. Healthy controls were recruited via advertisements and press releases in local newspapers. After prescreening via phone, participants underwent a diagnostic session that included the German version of the structured clinical interview for DSM-IV55 and an interview that focused on exclusion criteria and socio-demographic variables. Exclusion criteria were neurological illness, psychotic symptoms, injuries and infections during the last 14 days, alcohol and/or drug abuse, antipsychotics, stimulants, current pregnancy and lactation in women, and any mental disorders according to DSM-IV for healthy controls. Patients who took antidepressants were considered for participation under the assumption that the dose had been stable for at least 2 weeks and would remain so during study participation. Informed written informed consent was obtained from all participants.
Figure 1.
Flow of participants through each stage of the trial. CBT-C, cognitive-behavioral therapy control condition; CBT-E, cognitive-behavioral therapy with physical activity; CRP, C-reactive protein; ITT, intention-to-treat; WL, waitlist control group.
Interventions
Both CBT treatments (that is, CBT-E and CBT-C) were based on a common CBT manual and structured through phases typically used in CBT.56 Patients participated in 50 min of individual manualized psychotherapy weekly for 16 weeks. All therapists were clinical psychologists with advanced or completed postgraduate clinical training in CBT. Patients and therapists were blinded to the purpose and study hypothesis. After an initial phase (weeks 1–4), patients received behavioral activation (weeks 5–9) with either exercise (CBT-E) or pleasant low-energy activities in the active control condition (CBT-C), followed by cognitive therapy (weeks 10–16).
For CBT-E, CBT was modified to increase physical activity according to the recommendations of the World Health Organization.57 During the initial phase, patients received psychoeducation on MD and on the relationship between thoughts, feelings and behavior, with a focus on physical activity as a health behavior potentially relevant for depressive symptoms.20, 58 Psychoeducation further addressed recommendations for being physically active.57, 58 Additional elements were case conceptualization (that is, assessment of individual risk factors for depression) and, if necessary, problem-solving strategies were applied to reduce barriers to physical activity (for example, coping with low social support, arranging options for exercise). Patients received a manual summarizing the content of psychoeducation and providing a list of potential physical activities (for example, walking, jogging, swimming, gyms), as well as physical activity dose recommendations based on the Ainsworth Compendium of Physical Activities.59 These issues were discussed within treatment sessions and used to prepare an individualized schedule with at least four 40-min homework exercise sessions per week, consisting of at least moderate physical activity. During the phase of behavioral activation, the schedule was applied and common behavioral activation techniques were used to assist patients (for example, reinforcement, activity and mood monitoring, problem solving). After the phase of behavioral activation, therapists prescribed patients to continue physical activity, but shifted their focus to cognitive aspects, such as modification of dysfunctional cognitions and beliefs, enhancement of cognitions that increase psychological well-being, as well as prevention of relapses.56
The active control condition (that is, CBT-C) involved CBT with behavioral activation emphasizing pleasurable experiences without a substantial increase in physical activity (that is, euthymic activities). Different from CBT-E, patients received psychoeducation on MD and on the relationship between thoughts, feelings and behavior with a focus on euthymic activities. The euthymic activities were based on a manual for euthymic therapy, an intervention for mental disorders that shares similarities with mindfulness therapy.60 Analogous to CBT-E, the activity schedule within behavioral activation involved at least four 40-min homework sessions per week including euthymic exercises that bring awareness to different senses such as hearing (for example, listening to music), tasting (for example, preparing and enjoying a meal), smelling (for example, taking a scented bath) or touching (for example, bringing attention to the sensations of skin contact with pleasant surfaces).60 After the phase of behavioral activation, therapists also prescribed patients to perform euthymic activities autonomously and shifted the focus to a cognitive therapy similar to CBT-E. Patients in the WL condition (that is, passive control condition) did not receive any treatment, but were involved in regular psychotherapy after their 16-week waiting time.
Depressive symptoms and physical activity measures
Self-reported outcomes included depressive symptoms as assessed by the German version of the Beck Depression Inventory-II61 and metabolic equivalent minutes per week (MET-min per week) for three domains of physical activity (walking, moderate-intensity and vigorous-intensity), as assessed by the long version of the International Physical Activity Questionnaire (IPAQ).62
Immunological measures
Before each blood sampling, participants were queried about acute infections during the last 14 days, chronic infections or illness, and a sample was considered missing if participants reported any one of these issues. Participants were instructed to avoid exercise and alcohol 24 h prior to blood withdrawal. Non-fasting blood samples were collected in EDTA-treated or heparinized tubes (S-Monovette, Sarstedt, Nümbrecht, Germany) between 0700 hours and 1000 hours. Plasma for CRP and cytokine measurements were separated by centrifugation at 2,000 g for 10 min at 4 °C, and plasma was stored at –80 °C (7 to 12 months) until analysis. CRP was measured using an enzyme-linked immunosorbent assay (CRP high-sensitive ELISA, IBL International, Hamburg, Germany) according to the manufacturer’s instructions. Plasma levels of IL-6 and IL-10 were analyzed by flow cytometry using bead-based assays (Bio-Plex Pro Human Cytokine Assays, Bio-Rad Laboratories, Hercules, CA, USA) as previously described.63 The sensitivity of the assays was 0.02 μg ml−1 for CRP, 0.45 pg ml−1 for IL-6 and 0.59 pg ml−1 for IL-10. Complete blood counts including the white blood cell differential were obtained using an automated hematology analyzer (XT-2000i, Sysmex, Horgen, Switzerland). Leukocyte subpopulations were determined by flow cytometry using a standard lyse/wash procedure and the following antibodies (all from BioLegend, San Diego, CA, USA): FITC-conjugated anti-human CD3 (clone SK7), Pacific Blue-conjugated anti-human CD4 (clone SK3), PE-Cy7-conjugated anti-human CD8 (clone SK1), APC-Cy7-conjugated anti-human CD14 (clone M5E2), PerCP-Cy5.5-conjugated anti-human CD19 (clone HIB19), PE-conjugated anti-human CD25 (clone BC96), PE-conjugated anti-human CD56 (clone MEM-188) and AF647-conjugated anti-human CD127 (clone A019D5). Samples were analyzed on a FACSCanto II flow cytometer (BD Biosciences, Heidelberg, Germany) using BD FACSDiva software (Version 8.0.1, BD Biosciences). For the assessment of ex vivo IL-6 production, heparinized blood was diluted 1:5 with cell culture medium (RPMI 1640, Invitrogen, Karlsruhe, Germany, containing 10% fetal calf serum, PAA, Cölbe, Germany, and Gentamicin 50 μg ml−1, Invitrogen, Karlsruhe, Germany) and stimulated with 5 μg ml−1 LPS from E. coli 0111:B4 (Sigma-Aldrich, Taufkirchen, Germany) in 24-well flat-bottom microtiter plates. After incubation (72 h, 37 °C, 5% CO2),64 culture supernatants were collected by centrifugation (300 g, 4 °C, 5 min) and stored at −80 °C (7 to 12 months) until analysis. IL-6 concentration in the supernatants was measured using a commercial ELISA (ELISA MAX Deluxe, BioLegend) according to the manufacturer’s instructions. The sensitivity of the assay was 4 pg ml−1.
Data analysis
Statistical analyses were carried out with SPSS version 20.0 for Windows (SPSS, Chicago, IL, USA). Baseline differences in group characteristics were calculated using pairwise comparisons with t-tests, if necessary with Welch’s correction, and χ2 tests. Intervention effects on outcomes were analyzed on an intention-to-treat basis using multilevel models (MLM).65 MLMs were tested with different covariance structures, and for each MLM, the covariance structure that provided the best fit was selected.66, 67 Missing values occurred, and extreme outliers in immunological variables were also considered missing values (that is, values more than three interquartile ranges above the 75th percentile).68 Among those who completed the interventions, the overall data were available as follows: BDI-II (87%), IPAQ (88%), CRP (89%), IL-6 (86%), IL-10 (77%), LPS-stimulated IL-6 (69%) and leukocyte counts and subsets (83%). Data from 98 randomized participants were analyzed in MLMs (see Figure 1 for study flow). Full information maximum likelihood estimation was used to handle missing data. Simulations suggest that multiple imputation does not contribute to the precision of MLMs and that full information maximum likelihood might be more appropriate to receive unbiased results.69, 70 Given the exploratory nature of this study, analyses were not corrected for multiple testing.71 However, in cases of significant (P<0.05) treatment effects only (that is, group × time interactions), post hoc contrasts were calculated to specify these effects by testing group differences (that is, CBT-E versus CBT-C versus WL) at week 8 (mid-treatment, post-behavioral activation) and at week 16 (post-treatment). Log transformation was applied if skewed data could theoretically be a concern.68, 72
An a priori subgroup analysis was defined for CRP. Unlike other inflammatory markers, longitudinal research on CRP has resulted in a position statement recommending cutoff levels of CRP <1, 1–3 and >3 μg ml−1 equating to low, intermediate and high risk for subsequent CVD.73 Accordingly, it has been suggested to reduce circulating CRP in case of values ⩾1 μg ml−1.74 Given the potential clinical relevance of this classification, we ran an a priori subgroup analysis for participants with elevated CRP (CRP⩾1 μg ml−1) to focus on patients who may have had at least intermediate CRP-associated cardiovascular risk and might thus have benefited from CRP reduction.73
Results
Baseline characteristics and psychometric outcomes
A total of 101 patients with MD underwent baseline assessment, and 86 (85%) completed the assigned interventions (see Figure 1 for study flow). Three participants were excluded because their CRP levels were above 10 μg ml−1 at all measure points (that is, indicating acute inflammation; n=1), invalid blood samples for almost all measures (n=1), or a retrospective report showed chronic urinary tract infection during the study phase (n=1). Descriptive statistics for all groups and comparisons between patients with MD and age- and sex-matched healthy controls are presented in Table 1. Compared to controls, patients with MD showed higher levels of CRP (t110.5=4.01, P<0.001), lower levels of circulating IL-10 (t30.8=−2.09; P=0.045), as well as a higher IL-6/IL-10 ratios (t84.1=2.71, P=0.008). Neutrophil counts (t39.5=2.15; P=0.038) and monocyte counts (t39.5= 2.15; P=0.006) were higher in MD than in healthy controls. The LPS-stimulated IL-6 production was lower in MD than in healthy controls (t86.3=−2.89; P=0.005). There were no significant differences between groups for any other immunological measures (P=0.061−0.924).
Table 1. Baseline characteristics of participants.
| Variable | MD, CBT-E (N=34) | MD, CBT-C (N=34) | MD, WL (N=30) | MD, total (N=98) | HC (N=30) | t or χ2 P-value |
|---|---|---|---|---|---|---|
| Age | 36.9 (10.8) | 37.2 (12.5) | 37.9 (13.5) | 37.3 (12.2) | 37.1 (12.2) | 0.08; 0.939 |
| Female, number (%) | 16 (47.1) | 19 (55.9) | 13 (43.3) | 48 (49) | 15 (50) | 0.01; 0.922 |
| Depressive symptom severity, BDI-II | 27 (9.1) | 27.3 (8.7) | 26.2 (9.9) | 26.9 (9.1) | 4.6 (5.5) | 18.26; <0.001 |
| DSM-IV Axis I comorbidity, number (%) | ||||||
| Anxiety disorders | 8 (23.5) | 7 (20.6) | 8 (26.7) | 23 (23.5) | 0 (0) | 8.58; 0.003 |
| Somatoform disorders | 2 (5.9) | 4 (11.8) | 6 (20) | 12 (12.2) | 0 (0) | 4.05; 0.044 |
| Body mass index, kg/m2 | 25.8 (4.1) | 26.2 (6.3) | 26.5 (5.7) | 26.1 (5.3) | 24 (4.2) | 1.96; 0.052 |
| Education (years) | 11.5 (1.7) | 11.3 (1.7) | 11 (1.7) | 11.3 (1.7) | 12.2 (1.5) | −2.83; 0.006 |
| Number of cigarettes per day | 3.5 (7.3) | 1.5 (4.8) | 4.2 (8.8) | 3 (7.1) | 0.5 (1.9) | 1.90; 0.060 |
| Antidepressant medication, number (%) | 14 (41.2) | 13 (38.2) | 10 (33.3) | 37 (37.8) | 0 (0) | 15.93; <0.001 |
| Physical activity, IPAQ, MET-minutes per week | ||||||
| Walking | 1405 (1646.6) | 1269.9 (1255.9) | 1425.1 (1266.5) | 1364.8 (1392.5) | 1919.5 (2020.6) | −1.61; 0.092 |
| Moderate-intensity activity | 1817.7 (2321) | 2441.9 (2321.3) | 1842.5 (1884.8) | 2040 (2227) | 2243.8 (2813.1) | −0.87; 0.388 |
| Vigorous-intensity activity | 917.5 (1561.1) | 898.2 (1574.4) | 760 (1921.1) | 861.1 (1670.6) | 1577.3 (1739.28) | −2.03; 0.045 |
| CRP, μg ml−1 | 1.4 (1.6) | 1.8 (1.9) | 1.8 (2) | 1.7 (1.8) | 0.8 (0.6) | 4.01; <0.001 |
| CRP ⩾1 μg ml−1, number (%) | 14 (41.2) | 15 (44.1) | 13 (43.3) | 42 (42.9) | 7 (23.3) | 4.39; 0.036 |
| IL-6, pg ml−1 | 4.6 (6.9) | 3.7 (4.3) | 3.6 (3.7) | 3.9 (5.1) | 5.9 (7.8) | −1.22; 0.230 |
| IL-10, pg ml−1 | 6.1 (9.6) | 6.6 (11.1) | 4.8 (6.2) | 5.8 (9.2) | 12.9 (16.3) | −2.09; 0.045 |
| IL-6/IL-10 ratio | 0.9 (0.8) | 0.9 (0.9) | 1.2 (1) | 1 (0.9) | 0.6 (0.4) | 2.71; 0.008 |
| LPS-stimulated IL-6 production, pg ml−1 | 161.3 (153.2) | 163 (116.2) | 217.9 (143) | 183.8 (138.5) | 249.8 (84.1) | −2.89; 0.005 |
| Immune cell counts per μl | ||||||
| Leukocytes | 7070 (2047) | 6013 (1525) | 7303 (1881) | 6780 (1889) | 6740 (2209) | 0.69; 0.924 |
| Lymphocytes | 2193 (764) | 2070 (531) | 2199 (717) | 2152 (669) | 2286 (679) | −0.82; 0.413 |
| Neutrophils | 4319 (1376) | 3479 (1174) | 4535 (1510) | 4099 (1420) | 3520 (989) | 2.15; 0.038 |
| Monocytes | 417 (143) | 373 (134) | 421 (122) | 403 (134) | 310 (153) | 2.78; 0.006 |
| Total T cells | 1510 (616) | 1368 (396) | 1492 (601) | 1454 (541) | 1494 (460) | −0.31; 0.761 |
| T helper cells | 962 (450) | 930 (435) | 954 (412) | 948 (427) | 920 (372) | 0.27; 0.789 |
| Cytotoxic T cells | 464 (247) | 402 (122) | 416 (182) | 426 (188) | 485 (147) | −1.34; 0.184 |
| Regulatory T cells | 83 (38) | 92 (49) | 86 (36) | 87 (41) | 73 (28) | 1.93; 0.061 |
| B cells | 213 (79) | 244 (131) | 248 (137) | 236 (147) | 197 (94) | 1.38; 0.169 |
| NK cells | 256 (152) | 261 (152) | 257 (143) | 258 (147) | 281 (128) | −0.65; 0.516 |
Abbreviations: BDI, Beck Depression Inventory; CBT-C, Cognitive-behavioral therapy control condition; CBT-E, Cognitive-behavioral therapy with exercise; CRP, C-reactive protein; DSM, Diagnostic and Statistical Manual of Mental Disorders; HC, healthy control group; IL, interleukin; IPAQ, International Physical Activity Questionnaire; LPS, lipopolysaccharide; MD, major depression; MET, metabolic equivalent; WL, waitlist control group.
Values are mean (s.d.) unless noted with percentage. Group differences were calculated using χ2 tests for categorical variables and analyses of variance or t-tests for continues variables.
Table 2 illustrates outcomes from baseline to week 8 (that is, mid-treatment, post-behavioral activation) and week 16 (that is, post-treatment) by treatment group. The profile of change over time between the three groups was statistically significant for depressive symptoms (group × time: F4,81.3=6.27; P=<0.001). As compared with WL, CBT-E was associated with significantly lower depressive symptoms at week 8 (t95.1=2.88; P=0.005) and week 16 (t86.9=2.90; P=0.005). As compared with WL, CBT-C was also related with lower depressive symptoms at week 8 (t94= 3.15; P=0.002) and 16 (t83=3.16; P=0.003). Depressive symptoms were not significantly different between CBT-E and CBT-C at week 8 (t74.7=0.23; P=0.816) and 16 (t86.5=−0.14; P=0.889).
Table 2. Outcomes from baseline to week 8 and week 16 by treatment group: descriptive statistics and group × time effect.
| Baseline | Week 8 post-behavioral activation | Week 16 post-treatment | MLM: group × time: F; P-value | |
|---|---|---|---|---|
| Depressive symptom severity, BDI-II | ||||
| CBT-E | 27 (9.1) | 18.4 (10.7) | 14.6 (13.5) | |
| CBT-C | 27.3 (8.7) | 19.1 (9.5) | 14.8 (11.4) | 6.27, <0.001 |
| WL | 26.2 (9.9) | 29.5 (12.1) | 23.5 (11) | |
| Walking, MET-minutes per week | ||||
| CBT-E | 1405 (1646.6) | 996 (897.5) | 1206.1 (1077) | |
| CBT-C | 1269.9 (1255.9) | 1030.5 (1002.4) | 1131.9 (1112.5) | 1.11, 0.358 |
| WL | 1425.1 (1266.5) | 1245.8 (1168.2) | 1923.5 (1566.9) | |
| Moderate-intensity activity, MET-minutes per week | ||||
| CBT-E | 1817.7 (2321) | 1981.5 (2667.4) | 2396.5 (3812.8) | |
| CBT-C | 2441.9 (2321.3) | 1437.3 (1997.6) | 1996.5 (2624.8) | 0.84, 0.499 |
| WL | 1842.5 (1884.8) | 1620 (1538.2) | 1712.3 (1751.8) | |
| Vigorous-intensity activity, MET-minutes per week | ||||
| CBT-E | 917.5 (1561.1) | 1640 (2137.9) | 1669.6 (2815.2) | |
| CBT-C | 898.2 (1574.4) | 549.7 (1153.3) | 714.6 (1287.9) | 3.25, 0.016 |
| WL | 760 (1921.1) | 565.7 (786) | 692.3 (1483.1) | |
| CRP, μg ml−1 | ||||
| CBT-E | 1.4 (1.6) | 1.3 (1.1) | 1.4 (1.1) | |
| CBT-C | 1.8 (1.9) | 1.3 (1.7) | 2.4 (2.7) | 1.78, 0.137 |
| WL | 1.8 (2) | 2 (2.5) | 2 (2) | |
| IL-6, pg ml−1 | ||||
| CBT-E | 4.6 (6.9) | 9.5 (15) | 8.1(11.5) | |
| CBT-C | 3.7 (4.3) | 4.5 (5.5) | 4.7 (5.2) | 2.12, 0.093 |
| WL | 3.6 (3.7) | 2.8 (2.7) | 2.8 (2.7) | |
| IL-10, pg ml−1 | ||||
| CBT-E | 6.1 (9.6) | 31.3 (52.4) | 20.6 (36.3) | |
| CBT-C | 6.6 (11.1) | 6.1 (9.6) | 5.2 (5.5) | 3.88, 0.005 |
| WL | 4.8 (6.2) | 4.2 (5.1) | 4.1 (5) | |
| IL-6/IL-10 ratio | ||||
| CBT-E | 0.9 (0.8) | 0.7 (0.6) | 0.7 (0.6) | |
| CBT-C | 0.9 (0.9) | 1 (0.8) | 1.3 (1.4) | 0.85, 0.500 |
| WL | 1.2 (1) | 1 (0.7) | 1.1 (0.8) | |
| LPS-stimulated IL-6 production, pg ml−1 | ||||
| CBT-E | 161.3 (153.2) | 194.5 (140.7) | 175.2 (156.4) | |
| CBT-C | 163 (116.2) | 159.2 (118.1) | 141.1 (885.1) | 1.78, 0.138 |
| WL | 217.9 (143) | 180.4 (115.5) | 175.2 (156.4) | |
| Leukocytes, cell counts per μl | ||||
| CBT-E | 7070 (2047) | 7195 (2159) | 7635 (1868) | |
| CBT-C | 6013 (1525) | 6289 (1959) | 6388 (1495) | 2.26, 0.068 |
| WL | 7303 (1881) | 6478 (1562) | 6630 (1612) | |
| Lymphocytes, cell counts per μl | ||||
| CBT-E | 2393 (764) | 2306 (797) | 2441 (756) | |
| CBT-C | 2070 (531) | 2051 (592) | 2168 (353) | 2.09, 0.086 |
| WL | 2199 (717) | 2065 (629) | 2101 (562) | |
| Neutrophils, cell counts per μl | ||||
| CBT-E | 4319 (1376) | 4408 (1514) | 4584 (1492) | |
| CBT-C | 3479 (1174) | 3605 (1127) | 3816 (1212) | 1.91, 0.114 |
| WL | 4535 (1510) | 3926 (1142) | 3893 (1299) | |
| Monocytes, cell counts per μl | ||||
| CBT-E | 417 (143) | 379 (156) | 410 (133) | |
| CBT-C | 373 (134) | 354 (126) | 358 (140) | 0.53, 0.711 |
| WL | 421 (122) | 369 (129) | 350 (132) | |
| Total T cells, cell counts per μl | ||||
| CBT-E | 1510 (616) | 1616 (674) | 1705 (607) | |
| CBT-C | 1368 (396) | 1349 (433) | 1433 (272) | 1.85, 0.123 |
| WL | 1492 (601) | 1416 (521) | 1466 (524) | |
| T helper cells, cell counts per μl | ||||
| CBT-E | 962 (450) | 952 (366) | 1155 (449) | |
| CBT-C | 930 (435) | 842 (273) | 931 (223) | 1.85, 0.123 |
| WL | 954 (412) | 944 (382) | 978 (398) | |
| Cytotoxic T cells, cell counts per μl | ||||
| CBT-E | 464 (247) | 449 (223) | 497 (249) | |
| CBT-C | 402 (122) | 407 (140) | 430 (147) | 1.29, 0.278 |
| WL | 416 (182) | 422 (228) | 395 (129) | |
| Regulatory T cells, cell counts per μl | ||||
| CBT-E | 83 (38) | 88 (33) | 105 (39) | 1.07, 0.372 |
| CBT-C | 92 (49) | 72 (31) | 85 (30) | |
| WL | 86 (36) | 82 (31) | 88 (32) | |
| B cells, cell counts per μl | ||||
| CBT-E | 213 (79) | 240 (107) | 230 (102) | |
| CBT-C | 244 (131) | 248 (97) | 260 (92) | 2.04, 0.099 |
| WL | 248 (137 | 230 (137 | 201 (114) | |
| NK cells, cell counts per μl | ||||
| CBT-E | 256 (152) | 204 (115) | 306 (180) | |
| CBT-C | 261 (152) | 255 (137) | 272 (122) | 0.64, 0.637 |
| WL | 257 (143) | 212 (100) | 262 (114) | |
Abbreviations: BDI, Beck Depression Inventory; CBT-C, cognitive-behavioral therapy control condition; CBT-E, cognitive-behavioral therapy with exercise; CRP, C-reactive protein; IL, Interleukin; LPS, lipopolysaccharide; MET, metabolic equivalent; MLM, multilevel models; WL, waitlist control group.
Values are mean (s.d.).
Change in vigorous-intensity activity in the three groups from baseline to week 8 and week 16 was statistically significant (group × time: F4,78.7=3.25; P=0.016). As compared to WL, CBT-E was associated with higher levels of vigorous-intensity activity at week 8 (t90.4=−2.00; P=0.049) with non-significant differences at week 16 (t78.3=−1.51; P=0.136). As compared to CBT-C, CBT-E was related with higher levels of vigorous-intensity activity at week 8 (t72.9=−2.74; P=0.008) and a trend for higher levels at week 16 (t78.4=−1.80; P=0.075). WL and CBT-C resulted in no statistically significant difference in vigorous-intensity activity at week 8 (t90=0.12; P=0.907) and 16 (t76.7=0.25; P=0.801). There were no statistically significant group × time interactions on either walking or moderate-intensity activity (P=0.358−0.499).
Immunological outcomes
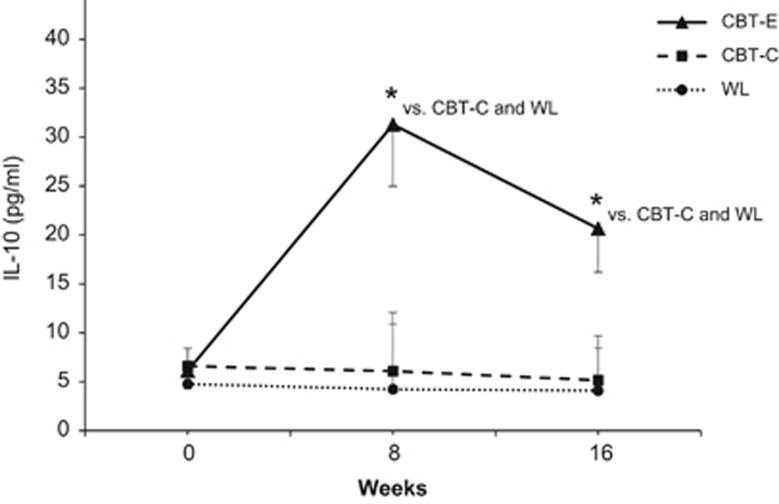
Changes in levels of CRP, pro-inflammatory cytokines and immune cell counts from baseline to week 8 and week 16 were not significantly different between groups (P=0.068−0.711) (Table 2). However, the profile of change over time between the three groups was statistically significant for anti-inflammatory IL-10 (group × time: F4,157.7=3.88; P=0.005) (Figure 2). Contrasts indicated that patients in the CBT-E group had higher levels of IL-10 at week 8 (t69=−2.96; P=0.004) and 16 (t66=−2.66; P=0.010) than patients in the WL condition. Compared to CBT-C, CBT-E was also related to higher levels of IL-10 at week 8 (t69=−2.88; P=0.005) and 16 (t66=−2.43; P=0.018). CBT-C and WL resulted in no statistically significant differences in IL-10 at week 8 (t69=−0.20; P=0.837) and 16 (t66=−0.17; P=0.866).
Figure 2.
Circulating levels of anti-inflammatory interleukin-10 (IL-10) from baseline to week 8 (post-behavioral activation), and to week 16 (post-treatment) by treatment group. Values are estimated marginal means (s.e.m.) from multilevel modeling. Pairwise contrasts for cognitive-behavioral therapy with exercise (CBT-E) versus cognitive-behavioral therapy with pleasurable low-energy activities (CBT-C, active control condition) versus waitlist (WL, passive control condition): *P<0.05.
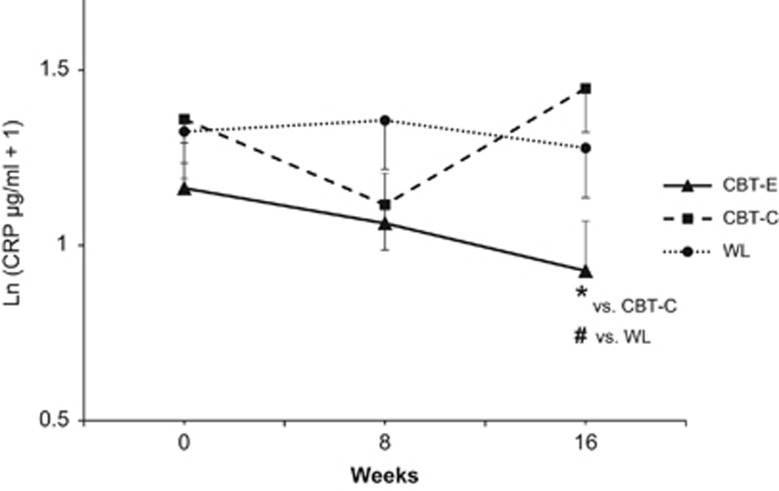
Forty-two patients with MD had elevated levels of CRP (CRP ⩾1 μg ml−1) at baseline and were thus included in the predefined subgroup analysis of participants with a potentially increased risk for CVD. Given the smaller sample size with respect to the total subgroup and groups for contrast calculations, the central limit theorem was not applied and log-transformation with a constant of 1 was used to reduce skewness.68 For this subgroup, the profile of change indicated that CBT-E reduces levels of CRP (group × time: F4,71.6=2.60; P=0.043) (Figure 3). As compared with WL, CBT-E resulted in a trend of lower CRP levels at week 16 (t83.2= 1.75; P=0.085), but not at week 8 (t88.3=1.47; P=0.145). As compared with CBT-C, CBT-E was associated with lower CRP levels at week 16 (t80= 2.75; P=0.007), but not at week 8 (t88.3=0.28; P=0.783). WL and CBT-C resulted in no statistically significant differences in CRP levels at week 8 (t79.3=−0.89; P=0.374) and 16 (t83.5=1.27; P=0.209).
Figure 3.
Subgroup analysis (N=42) of patients with potentially elevated cardiovascular risk (CRP ⩾1 μg ml−1). Circulating levels of C-reactive protein (CRP) from baseline to week 8 (post-behavioral activation), and to week 16 (post-treatment) by treatment group. Values are estimated marginal means (s.e.m.) from multilevel modeling. Pairwise contrasts for cognitive-behavioral therapy with exercise (CBT-E) versus cognitive-behavioral therapy with pleasurable low-energy activities (CBT-C, active control condition) versus waitlist (WL, passive control condition): *P<0.05, #P<0.1.
Discussion
Depression is associated with inflammation and increased cardiovascular risk.1, 2, 15 As there is evidence that physical activity can induce anti-inflammatory effects,27, 29, 75, 76 this randomized controlled trial examined the impact of CBT, emphasizing behavioral activation with exercise (that is, CBT-E), on immunological markers in patients with MD. At study entry, patients with MD showed decreased peripheral levels of anti-inflammatory IL-10 and increased levels of CRP compared to healthy controls, corroborating previous findings.2, 7, 8 CBT-E induced a significant increase in IL-10 plasma concentrations. This observation is novel in the context of depression and complements findings from other clinical conditions.27, 28, 30, 77 In addition, CBT-E reduced levels of CRP among those patients with potentially elevated cardiovascular risk (CRP⩾1 μg ml−1), confirming data from previous exercise trials.26, 27, 29, 76
For the total sample of patients in this study, there was no evidence for an effect of CBT with exercise on immunological markers with the exception of IL-10. One possible pathway of exercise-induced regulation of immunological mediators is a temporary increase of catecholamines resulting in activation of beta-adrenergic receptors on monocytes.78, 79, 80 Depressive symptoms are associated with reduced sensitivity of beta-adrenergic receptors.81 Thus, it is possible that the potential of exercise to affect immunological processes is reduced in patients with depression.39 However, IL-10 release is also mediated by beta-adrenergic pathways,82 and we found evidence for an effect of physical activity on CRP in a subsample with elevated CRP baseline levels. Thus, our findings may simply demonstrate that an anti-inflammatory effect of exercise is relevant for a subpopulation of patients with depression. As pointed out by Kiecolt-Glaser et al.,5 inflammation may neither be necessary nor sufficient to induce or sustain depression in general, but it is relevant for a substantial subpopulation of depressed individuals. Thus, observable reductions in inflammatory markers due to anti-inflammatory interventions may only be expected in this subpopulation, as observed in our subsample with increased baseline levels of CRP.
A limited number of studies so far analyzed the impact of CBT on immune functions. For example, a recent randomized controlled trial reported that CBT reduces levels of CRP in insomnia,83 a condition which is frequently associated with depression.84, 85 In contrast, two pre-post studies found no reduction in CRP levels during CBT in patients with depression, although one of these studies reported decreased expression of Toll-like receptors after CBT.86, 87 CBT mainly consists of two core components: behavioral activation and cognitive therapy.88 When applying behavioral activation, individuals may increase both physical activity and pleasurable experiences. Thus, it is important to note that this study also dismantles behavioral activation within CBT, in (1) behavioral activation with physical activity (that is, CBT-E), and (2) behavioral activation with pleasurable experiences without a substantial increase in physical activity (that is, CBT-C). In view of the observed pattern of changes in this study, a potential anti-inflammatory effect of CBT might be strengthened when explicitly focusing on physical activity75 during behavioral activation.
Patients with depression are at increased risk for CVD.14, 15, 16 Although the prospective value of CRP for CVD is well established,73 the clinical relevance of IL-10 is less clear,89, 90, 91 in particular for patients with depression. In vitro and in vivo studies in animals clearly demonstrate an atheroprotective role of IL-10.92 To the best of our knowledge, no longitudinal studies have examined the link between IL-10 and future health outcomes in patients with depression without ‘medical illness’. However, Parissis et al.93 studied the prognostic value of IL-10 and other biomarkers in patients with chronic heart failure (CHF) and comorbid clinically-relevant depressive symptoms. Similar to our findings, CHF patients with depressive symptoms had lower levels of circulating IL-10 than non-depressed CHF patients. Other than several pro-inflammatory markers, lower levels of IL-10 independently predicted major adverse cardiovascular events during a period of 1 year. Thus, longitudinal studies investigating the prognostic value of IL-10 in depressed patients without manifest baseline CVD are warranted to evaluate the clinical relevance of our findings.
MD patients showed significantly increased neutrophil and monocyte numbers, as well as significantly reduced IL-6 production after ex vivo LPS-stimulation at baseline. The clinical relevance in the context of MD is also unclear as data on numbers of circulating neutrophils and monocytes in MD,38, 94, 95 and findings from studies of mitogen-stimulated cytokine production are inconsistent and controversial.41, 42, 44, 45, 46, 47, 48 As LPS or mitogens-stimulated production of cytokines is not representative of systemic inflammation,2, 47, 96 a reduced ex vivo production of cytokines in MD patients may reflect an exhaustion of cell function subsequent to sustained systemic low-grade inflammation.46, 47
This study has several limitations. Given the exploratory nature of this trial, further research is necessary to confirm the observed findings. In addition, despite a sample size of 98 patients with MD, the number of subjects with elevated CRP was relatively small and thus our results need replication. Moreover, the total sample size was insufficient for detection of small effects. Our sample consisted of outpatients with MD who were eligible for psychological treatment. Thus, findings may not generalize to other samples of patients (for example, MD patients with psychotic features). Although the physical activity questionnaire used in this study has good reliability and validity,97, 98 response bias cannot be excluded and an additional use of objective measures would have been beneficial. Finally, despite the extensive data linking inflammation, in particular CRP, to cardiovascular risk, the clinical implications of the present and previous intervention studies are not clear. The most important question might be whether lowering CRP or increasing IL-10 in MD through interventions will translate into reduced risk for CVD. Given the lack of studies that focus on such long-term effects, it is not possible to answer this question. However, though clinical implications are speculative, our results indicate that an established risk marker for CVD (that is, CRP) and an anti-inflammatory cytokine with atheroprotective properties (that is, IL-10) can be influenced by behavioral treatments in MD.
This study demonstrates that CBT with exercise may have anti-inflammatory effects in patients with MD by increasing IL-10 and reducing CRP among those patients with increased levels of CRP and potentially elevated risk for CVD. Important strengths of this randomized controlled trial are the involvement of both an active and a passive control condition, a baseline comparison between patients with MD and healthy controls, as well as a broad assessment of immunological markers. The findings provide new insights into the immunological effects of behavioral treatments against depression. Given the links between depression, inflammation and CVD, this study introduces important implications regarding how behavioral treatments can be conceptualized to target inflammation and possibly promote cardiovascular health in MD.
Acknowledgments
This work was supported by Grants RI 574/23-1 and SCHE341/20-1 by the German Research Foundation to Winfried Rief and Manfred Schedlowski, respectively. The German Research Foundation had no role in the design and conduct of the study.
Footnotes
WR received honoraria for presentations and consultation about placebo mechanisms from Berlin Chemie, Bayer and Heel. ClinicalTrials.gov Identifier: NCT01464463 (The Impact of Psychological Interventions on Psychometric and Immunological Measures in Patients with Major Depression). The remaining authors declare no conflict of interest.
References
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK et al. A meta-analysis of cytokines in major depression. Biol Psychiatry 2010; 67: 446–457. [DOI] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 2009; 71: 171–186. [DOI] [PubMed] [Google Scholar]
- Stewart JC, Rand KL, Muldoon MF, Kamarck TW. A prospective evaluation of the directionality of the depression-inflammation relationship. Brain Behav Immun 2009; 23: 936–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 2015; 16: 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry 2015; 172: 1075–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9: 46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhabhar FS, Burke HM, Epel ES, Mellon SH, Rosser R, Reus VI et al. Low serum IL-10 concentrations and loss of regulatory association between IL-6 and IL-10 in adults with major depression. J Psychiatr Res 2009; 43: 962–969. [DOI] [PubMed] [Google Scholar]
- Song C, Halbreich U, Han C, Leonard BE, Luo H. Imbalance between pro- and anti-inflammatory cytokines, and between Th1 and Th2 cytokines in depressed patients: the effect of electroacupuncture or fluoxetine treatment. Pharmacopsychiatry 2009; 42: 182–188. [DOI] [PubMed] [Google Scholar]
- Voorhees JL, Tarr AJ, Wohleb ES, Godbout JP, Mo X, Sheridan JF et al. Prolonged restraint stress increases IL-6, reduces IL-10, and causes persistent depressive-like behavior that is reversed by recombinant IL-10. PLoS ONE 2013; 8: e58488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque S, Correia-Neves M, Mesquita AR, Palha JA, Sousa N. Interleukin-10: a key cytokine in depression? Cardiovasc Psychiatry Neurol 2009; 2009: 187894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y, Lin W, Wang W, Qi X, Wang D, Tang M. The effects of central pro-and anti-inflammatory immune challenges on depressive-like behavior induced by chronic forced swim stress in rats. Behav Brain Res 2013; 247: 232–240. [DOI] [PubMed] [Google Scholar]
- Mesquita AR, Correia-Neves M, Roque S, Castro AG, Vieira P, Pedrosa J et al. IL-10 modulates depressive-like behavior. J Psychiatr Res 2008; 43: 89–97. [DOI] [PubMed] [Google Scholar]
- Holtzman S, Abbey SE, Chan C, Bargman JM, Stewart DE. A genetic predisposition to produce low levels of IL-10 is related to depressive symptoms: a pilot study of patients with end stage renal disease. Psychosomatics 53: 155–161. [DOI] [PubMed] [Google Scholar]
- Williams ED, Steptoe A. The role of depression in the etiology of acute coronary syndrome. Curr Psychiatry Rep 2007; 9: 486–492. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch Gen Psychiatry 1998; 55: 580–592. [DOI] [PubMed] [Google Scholar]
- Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur Heart J 2006; 27: 2763–2774. [DOI] [PubMed] [Google Scholar]
- Colbert LH, Visser M, Simonsick EM, Tracy RP, Newman AB, Kritchevsky SB et al. Physical activity, exercise, and inflammatory markers in older adults: findings from the health, aging and body composition study. J Am Geriatr Soc 2004; 52: 1098–1104. [DOI] [PubMed] [Google Scholar]
- Reuben DB, Judd-Hamilton L, Harris TB, Seeman TE. The associations between physical activity and inflammatory markers in high-functioning older persons: macarthur studies of successful aging. J Am Geriatr Soc 2003; 51: 1125–1130. [DOI] [PubMed] [Google Scholar]
- Jerstad SJ, Boutelle KN, Ness KK, Stice E. Prospective reciprocal relations between physical activity and depression in female adolescents. J Consult Clin Psychol 2010; 78: 268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roshanaei-Moghaddam B, Katon WJ, Russo J. The longitudinal effects of depression on physical activity. Gen Hosp Psychiatry 2009; 31: 306–315. [DOI] [PubMed] [Google Scholar]
- Cooney GM, Dwan K, Greig CA, Lawlor DA, Rimer J, Waugh FR et al. Exercise for depression. Cochrane database Syst Rev 2013; 9: CD004366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuch FB, Vancampfort D, Richards J, Rosenbaum S, Ward PB, Stubbs B. Exercise as a treatment for depression: A meta-analysis adjusting for publication bias. J Psychiatr Res 2016; 77: 42–51. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Greer TL, Church TS, Carmody TJ, Grannemann BD, Galper DI et al. Exercise as an Augmentation Treatment for Nonremitted Major Depressive Disorder. J Clin Psychiatry 2011; 72: 677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen BK, Saltin B. Exercise as medicine—evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J Med Sci Sports 2015; 25: 1–72. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev 2008; 88: 1379–1406. [DOI] [PubMed] [Google Scholar]
- Di Raimondo D, Tuttolomondo A, Buttà C, Casuccio A, Giarrusso L, Miceli G et al. Metabolic and anti-inflammatory effects of a home-based programme of aerobic physical exercise. Int J Clin Pract 2013; 67: 1247–1253. [DOI] [PubMed] [Google Scholar]
- Goldhammer E, Tanchilevitch A, Maor I, Beniamini Y, Rosenschein U, Sagiv M. Exercise training modulates cytokines activity in coronary heart disease patients. Int J Cardiol 2005; 100: 93–99. [DOI] [PubMed] [Google Scholar]
- Kayambu G, Boots R, Paratz J. Early physical rehabilitation in intensive care patients with sepsis syndromes: a pilot randomised controlled trial. Intensive Care Med 2015; 41: 865–874. [DOI] [PubMed] [Google Scholar]
- Palmefors H, DuttaRoy S, Rundqvist B, Börjesson M. The effect of physical activity or exercise on key biomarkers in atherosclerosis—a systematic review. Atherosclerosis 2014; 235: 150–161. [DOI] [PubMed] [Google Scholar]
- Smith JK, Dykes R, Douglas JE, Krishnaswamy G, Berk S. Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischemic heart disease. JAMA 1999; 281: 1722–1727. [DOI] [PubMed] [Google Scholar]
- Rethorst CD, Toups MS, Greer TL, Nakonezny Pa, Carmody TJ, Grannemann BD et al. Pro-inflammatory cytokines as predictors of antidepressant effects of exercise in major depressive disorder. Mol Psychiatry 2012; 1–6. [DOI] [PMC free article] [PubMed]
- Ekkekakis P. Honey, I shrunk the pooled SMD! Guide to critical appraisal of systematic reviews and meta-analyses using the Cochrane review on exercise for depression as example. Ment Health Phys Act 2015; 8: 21–36. [Google Scholar]
- Cuijpers P, Berking M, Andersson G, Quigley L, Kleiboer A, Dobson KS. A meta-analysis of cognitive-behavioural therapy for adult depression, alone and in comparison with other treatments. Can J Psychiatry 2013; 58: 376–385. [DOI] [PubMed] [Google Scholar]
- Piette JD, Valenstein M, Himle J, Duffy S, Torres T, Vogel M et al. Clinical complexity and the effectiveness of an intervention for depressed diabetes patients. Chronic Illn 2011; 7: 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissner K, Parker SJ, Henderson CR, Pal A, Iannone L, Reid MC. A cognitive-behavioral plus exercise intervention for older adults with chronic back pain: race/ethnicity effect? J Aging Phys Act 2012; 20: 246–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudlitz K, Plag J, Dimeo F, Ströhle A. Aerobic exercise training facilitates the effectiveness of cognitive behavioral therapy in panic disorder. Depress Anxiety 2015; 32: 221–228. [DOI] [PubMed] [Google Scholar]
- Pentecost C, Farrand P, Greaves CJ, Taylor RS, Warren FC, Hillsdon M et al. Combining behavioural activation with physical activity promotion for adults with depression: findings of a parallel-group pilot randomised controlled trial (BAcPAc). Trials 2015; 16: 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun 2007; 21: 374–383. [DOI] [PubMed] [Google Scholar]
- Euteneuer F, Schwarz MJ, Schmidmaier R, Hennings A, Riemer S, Stapf TM et al. Blunted exercise-induced mobilization of monocytes in somatization syndromes and major depression. J Affect Disord 2014; 166: 156–164. [DOI] [PubMed] [Google Scholar]
- Schlatter J, Ortuño F, Cervera-Enguix S. Monocytic parameters in patients with dysthymia versus major depression. J Affect Disord 2004; 78: 243–247. [DOI] [PubMed] [Google Scholar]
- Kubera M, Kenis G, Bosmans E, Kajta M, Basta-Kaim A, Scharpe S et al. Stimulatory effect of antidepressants on the production of IL-6. Int Immunopharmacol 2004; 4: 185–192. [DOI] [PubMed] [Google Scholar]
- Seidel A, Arolt V, Hunstiger M, Rink L, Behnisch A, Kirchner H. Cytokine production and serum proteins in depression. Scand J Immunol 1995; 41: 534–538. [DOI] [PubMed] [Google Scholar]
- Krause DL, Riedel M, Müller N, Weidinger E, Schwarz MJ, Myint A-M. Effects of antidepressants and cyclooxygenase-2 inhibitor on cytokines and kynurenines in stimulated in vitro blood culture from depressed patients. Inflammopharmacology 2012; 20: 169–176. [DOI] [PubMed] [Google Scholar]
- Lisi L, Camardese G, Treglia M, Tringali G, Carrozza C, Janiri L et al. Monocytes from depressed patients display an altered pattern of response to endotoxin challenge. PLoS ONE 2013; 8: e52585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weizman R, Laor N, Podliszewski E, Notti I, Djaldetti M, Bessler H. Cytokine production in major depressed patients before and after clomipramine treatment. Biol Psychiatry 1994; 35: 42–47. [DOI] [PubMed] [Google Scholar]
- Humphreys D, Schlesinger L, Lopez M, Araya AV. Interleukin-6 production and deregulation of the hypothalamic-pituitary-adrenal axis in patients with major depressive disorders. Endocrine 2006; 30: 371–376. [DOI] [PubMed] [Google Scholar]
- Cyranowski JM, Marsland AL, Bromberger JT, Whiteside TL, Chang Y, Matthews KA. Depressive symptoms and production of proinflammatory cytokines by peripheral blood mononuclear cells stimulated in vitro. Brain Behav Immun 2007; 21: 229–237. [DOI] [PubMed] [Google Scholar]
- Guidi L, Bartoloni C, Frasca D, Antico L, Pili R, Cursi F et al. Impairment of lymphocyte activities in depressed aged subjects. Mech Ageing Dev 1991; 60: 13–24. [DOI] [PubMed] [Google Scholar]
- Miller GE, Rohleder N, Stetler C, Kirschbaum C. Clinical depression and regulation of the inflammatory response during acute stress. Psychosom Med 2005; 67: 679–687. [DOI] [PubMed] [Google Scholar]
- Anisman H, Ravindran AV, Griffiths J, Merali Z. Endocrine and cytokine correlates of major depression and dysthymia with typical or atypical features. Mol Psychiatry 1999; 4: 182–188. [DOI] [PubMed] [Google Scholar]
- Rothermundt M, Arolt V, Peters M, Gutbrodt H, Fenker J, Kersting A et al. Inflammatory markers in major depression and melancholia. J Affect Disord 2001; 63: 93–102. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang A-G, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007; 39: 175–191. [DOI] [PubMed] [Google Scholar]
- Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 2004; 350: 1387–1397. [DOI] [PubMed] [Google Scholar]
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders: DSM-IV. American Psychiatric Association: Washington, DC, USA, 1994. [Google Scholar]
- Wittchen H-U, Wunderlich U, Gruschitz S, Zaudig M. Strukturiertes Klinisches Interview für DSM-IV, Achse I (SKID-I). Hogrefe: Göttingen, 1997. [Google Scholar]
- Hautzinger M. Kognitive Verhaltenstherapie bei Depressionen. Psychologie Verlags Union: Weinheim, 2003. [Google Scholar]
- World Health OrganizationGlobal Recommendations on Physical Activity for Health. WHO Press: Geneva, Switzerland, 2010. [Google Scholar]
- Craft LL, Perna FM. The benefits of exercise for the clinically depressed. Prim Care Companion J Clin Psychiatry 2004; 6: 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Montoye HJ, Sallis JF et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 1993; 25: 71–80. [DOI] [PubMed] [Google Scholar]
- Lutz R. The therapeutic concept of euthymic treatment. The little school of pleasure. MMW Fortschr Med 2005; 147: 41–43. [PubMed] [Google Scholar]
- Hautzinger M, Kühner C, Keller F. Das Beck Depressionsinventar II. Deutsche Bearbeitung und Handbuch zum BDI II. Harcourt Test Services: Frankfurt, 2006. [Google Scholar]
- Craig CL, Marshall AL, SjÖStrÖM M, Bauman AE, Booth ML, Ainsworth BE et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 2003; 35: 1381–1395. [DOI] [PubMed] [Google Scholar]
- Grigoleit J, Kullmann JS, Wolf OT, Hammes F, Wegner A, Jablonowski S et al. Dose-dependent effects of endotoxin on neurobehavioral functions in humans. PLoS ONE 2011; 6: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groote D, Zangerle P, Gevaert Y, Fassotte M, Beguin Y, Noizat-Pirenne F et al. Direct stimulation of cytokines (IL-1 beta, TNF-alpha, IL-6, IL-2, IFN-gamma and GM-CSF) in whole blood. I. Comparison with isolated PBMC stimulation. Cytokine 1992; 4: 239–248. [DOI] [PubMed] [Google Scholar]
- SAGE. Hierarchical Linear Models. Available at: https://us.sagepub.com/en-us/nam/hierarchical-linear-models/book9230. Accessed on 12 March 2016.
- Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr 1974; 19: 716–723. [Google Scholar]
- Schwarz G. Estimating the dimension of a model. Ann Stat 1978; 6: 461–464. [Google Scholar]
- Tabachnick B, Fidell F. Using Multivariate Statistics. Pearson: Boston, MA, USA, 2014. [Google Scholar]
- Twisk J, de Boer M, de Vente W, Heymans M. Multiple imputation of missing values was not necessary before performing a longitudinal mixed-model analysis. J Clin Epidemiol 2013; 66: 1022–1028. [DOI] [PubMed] [Google Scholar]
- Peters SAE, Bots ML, den Ruijter HM, Palmer MK, Grobbee DE, Crouse JR et al. Multiple imputation of missing repeated outcome measurements did not add to linear mixed-effects models. J Clin Epidemiol 2012; 65: 686–695. [DOI] [PubMed] [Google Scholar]
- Bender R, Lange S. Adjusting for multiple testing—when and how? J Clin Epidemiol 2001; 54: 343–349. [DOI] [PubMed] [Google Scholar]
- Thomas E, Sokal Robert R, Jamesrohlf F. Introduction to Biostatistics, 2nd edn. W. H. Freeman and Co.: New York, NY, USA, 1987. [Google Scholar]
- Pearson TA. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003; 107: 499–511. [DOI] [PubMed] [Google Scholar]
- Ridker PM. Cardiology patient page. C-reactive protein: a simple test to help predict risk of heart attack and stroke. Circulation 2003; 108: e81–e85. [DOI] [PubMed] [Google Scholar]
- Petersen AMW, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol 2005; 98: 1154–1162. [DOI] [PubMed] [Google Scholar]
- Neefkes-Zonneveld CR, Bakkum AJ, Bishop NC, van Tulder MW, Janssen TW. Effect of long-term physical activity and acute exercise on markers of systemic inflammation in persons with chronic spinal cord injury: a systematic review. Arch Phys Med Rehabil 2015; 96: 30–42. [DOI] [PubMed] [Google Scholar]
- Ribeiro F, Alves AJ, Teixeira M, Miranda F, Azevedo C, Duarte JA et al. Exercise training increases interleukin-10 after an acute myocardial infarction: a randomised clinical trial. Int J Sports Med 2012; 33: 192–198. [DOI] [PubMed] [Google Scholar]
- Dimsdale JE, Moss J. Plasma catecholamines in stress and exercise. JAMA 1980; 243: 340–342. [PubMed] [Google Scholar]
- Hong S, Dimitrov S, Pruitt C, Shaikh F, Beg N. Benefit of physical fitness against inflammation in obesity: role of beta adrenergic receptors. Brain Behav Immun 2014; 39: 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Johnson TA, Farag NH, Guy HJ, Matthews SC, Ziegler MG et al. Attenuation of T-lymphocyte demargination and adhesion molecule expression in response to moderate exercise in physically fit individuals. J Appl Physiol 2004; 98: 1057–1063. [DOI] [PubMed] [Google Scholar]
- Euteneuer F, Ziegler MG, Mills PJ, Rief W, Dimsdale JE. In Vivo β-adrenergic receptor responsiveness: ethnic differences in the relationship with symptoms of depression and fatigue. Int J Behav Med 2013; 21: 843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenkov IJ, Papanicolaou DA, Wilder RL, Chrousos GP. Modulatory effects of glucocorticoids and catecholamines on human interleukin-12 and interleukin-10 production: clinical implications. Proc Assoc Am Physicians 1996; 108: 374–381. [PubMed] [Google Scholar]
- Irwin MR, Olmstead R, Breen EC, Witarama T, Carrillo C, Sadeghi N et al. Cognitive behavioral therapy and tai chi reverse cellular and genomic markers of inflammation in late-life insomnia: a randomized controlled trial. Biol Psychiatry 2015; 78: 721–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann D. Insomnia and comorbid psychiatric disorders. Sleep Med 2007; 8: S15–S20. [DOI] [PubMed] [Google Scholar]
- Sivertsen B, Salo P, Mykletun A, Hysing M, Pallesen S, Krokstad S et al. The bidirectional association between depression and insomnia: the HUNT study. Psychosom Med 2012; 74: 758–765. [DOI] [PubMed] [Google Scholar]
- Kéri S, Szabó C, Kelemen O. Expression of Toll-like receptors in peripheral blood mononuclear cells and response to cognitive-behavioral therapy in major depressive disorder. Brain Behav Immun 2014; 40: 235–243. [DOI] [PubMed] [Google Scholar]
- Taylor CB, Conrad A, Wilhelm FH, Strachowski D, Khaylis A, Neri E et al. Does improving mood in depressed patients alter factors that may affect cardiovascular disease risk? J Psychiatr Res 2009; 43: 1246–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimidjian S, Hollon SD, Dobson KS, Schmaling KB, Kohlenberg RJ, Addis ME et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol 2006; 74: 658–670. [DOI] [PubMed] [Google Scholar]
- Zhang D-F, Song X-T, Chen Y-D, Yuan F, Xu F, Zhang M et al. Prognostic performance of interleukin-10 in patients with chest pain and mild to moderate coronary artery lesions-an 8-year follow-up study. J Geriatr Cardiol 2016; 13: 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeschen C, Dimmeler S, Hamm CW, Fichtlscherer S, Boersma E, Simoons ML et al. Serum level of the antiinflammatory cytokine interleukin-10 is an important prognostic determinant in patients with acute coronary syndromes. Circulation 2003; 107: 2109–2114. [DOI] [PubMed] [Google Scholar]
- Cavusoglu E, Marmur JD, Hojjati MR, Chopra V, Butala M, Subnani R et al. Plasma interleukin-10 levels and adverse outcomes in acute coronary syndrome. Am J Med 2011; 124: 724–730. [DOI] [PubMed] [Google Scholar]
- Han X, Boisvert WA. Interleukin-10 protects against atherosclerosis by modulating multiple atherogenic macrophage function. Thromb Haemost 2014; 113: 505–512. [DOI] [PubMed] [Google Scholar]
- Parissis JT, Farmakis D, Nikolaou M, Birmpa D, Bistola V, Paraskevaidis I et al. Plasma B-type natriuretic peptide and anti-inflammatory cytokine interleukin-10 levels predict adverse clinical outcome in chronic heart failure patients with depressive symptoms: a 1-year follow-up study. Eur J Heart Fail 2009; 11: 967–972. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun 2001; 15: 199–226. [DOI] [PubMed] [Google Scholar]
- Herbert TB, Cohen S. Stress and immunity in humans: a meta-analytic review. Psychosom Med 1993; 55: 364–379. [DOI] [PubMed] [Google Scholar]
- Sjögren E, Leanderson P, Kristenson M, Ernerudh J. Interleukin-6 levels in relation to psychosocial factors: studies on serum, saliva, and in vitro production by blood mononuclear cells. Brain Behav Immun 2006; 20: 270–278. [DOI] [PubMed] [Google Scholar]
- Brühmann BA, Schmidt ME, Steindorf K. Assessment of physical activity in epidemiological studies: are questionnaires obsolete in the era of accelerometry? GMS Med Inform Biom Epidemiol 2014; 10; doi: 10.3205/mibe000155. [Google Scholar]
- Wanner M, Probst-Hensch N, Kriemler S, Meier F, Autenrieth C, Martin BW. Validation of the long international physical activity questionnaire: Influence of age and language region. Prev Med Rep 2016; 3: 250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]