Abstract
A subgroup of individuals with mood and psychotic disorders shows evidence of inflammation that leads to activation of the kynurenine pathway and the increased production of neuroactive kynurenine metabolites. Depression is hypothesized to be causally associated with an imbalance in the kynurenine pathway, with an increased metabolism down the 3-hydroxykynurenine (3HK) branch of the pathway leading to increased levels of the neurotoxic metabolite, quinolinic acid (QA), which is a putative N-methyl-d-aspartate (NMDA) receptor agonist. In contrast, schizophrenia and psychosis are hypothesized to arise from increased metabolism of the NMDA receptor antagonist, kynurenic acid (KynA), leading to hypofunction of GABAergic interneurons, the disinhibition of pyramidal neurons and striatal hyperdopaminergia. Here we present results that challenge the model of excess KynA production in affective psychosis. After rigorous control of potential confounders and multiple testing we find significant reductions in serum KynA and/or KynA/QA in acutely ill inpatients with major depressive disorder (N=35), bipolar disorder (N=53) and schizoaffective disorder (N=40) versus healthy controls (N=92). No significant difference was found between acutely ill inpatients with schizophrenia (n=21) and healthy controls. Further, a post hoc comparison of patients divided into the categories of non-psychotic affective disorder, affective psychosis and psychotic disorder (non-affective) showed that the greatest decrease in KynA was in the affective psychosis group relative to the other diagnostic groups. Our results are consistent with reports of elevations in proinflammatory cytokines in psychosis, and preclinical work showing that inflammation upregulates the enzyme, kynurenine mono-oxygenase (KMO), which converts kynurenine into 3-hydroxykynurenine and quinolinic acid.
Introduction
Elevations in circulating levels of proinflammatory mediators, a phenomenon usually associated with ‘systemic low-grade inflammation’ is observed across several psychiatric disorders including major depressive disorder (MDD),1, 2, 3 bipolar disorder (BD), schizophrenia and other psychotic disorders.4, 5, 6, 7, 8, 9 While this inflammatory profile can arise secondary to comorbid medical conditions, infections, stress, obesity or lifestyle factors, there is evidence that at least in some individuals, inflammation could be a causal mechanism underlying depression or psychosis. Notably, prospective studies have shown a positive association between circulating levels of C-reactive protein (CRP) or interleukin 6 (IL-6) concentrations at baseline and the development of de novo cases of MDD,10, 11 BD12 and psychosis.11 Furthermore, immunotherapy of hepatitis C or melanoma with interferon alpha or IL-2 induces a depressive episode in about 30–40% of patients.13, 14, 15, 16
The biological mechanisms by which inflammatory mediators cause depression and psychotic illness are only partially understood. Cytokines and other inflammatory molecules affect serotonergic, dopaminergic and glutamatergic neurotransmission.17, 18 At the circuit level, these alterations in neurotransmission can alter the function of the visceromotor network including the ventromedial prefrontal cortex (PFC), insula and hippocampus19, 20, 21 and additionally may induce hypoactivity of a ‘reward network’ centered on the ventral striatum.22, 23, 24 Activation of a key immunoregulatory network, the kynurenine pathway also could be crucial. Two landmark papers showed that lipopolysaccharide (LPS) does not cause depression-like behavior in rodents when the activation of the kynurenine pathway is genetically or pharmacologically blocked even when the levels of proinflammatory cytokines remain elevated.25, 26 Interferon gamma (IFNγ) and to a lesser extent other cytokines such as tumor necrosis factor alpha (TNF) activate the kynurenine pathway by increasing expression of the enzyme, indoleamine-2,3-dioxygenase (IDO) that converts tryptophan to kynurenine. Originally it was hypothesized that activation of the kynurenine pathway led to depression by depleting tryptophan and serotonin in the brain.27, 28, 29 However, LPS causes an increase in tryptophan and serotonin turnover in the brains of rodents,30 and the depressive effects of LPS can be blocked with the IDO inhibitor, 1-methyl-tryptophan, without affecting brain tryptophan and serotonin turnover.25
Myint and Kim proposed that it is the balance between neurotoxic and neuroprotective metabolites rather than the reduction in tryptophan that is central to the pathogenesis of depressive illness.31 This hypothesis has been supported by subsequent empirical studies.32, 33, 34, 35, 36 Kynurenine is metabolized along two mutually exclusive pathways to form either kynurenic acid (KynA) or alternatively, 3-hydroxykynurenine (3HK), 3-hydroxyanthrallic acid (3-HAA) and quinolinic acid (QA)37, 38, 39 (Supplementary Figure S1). Under physiological conditions approximately equal amounts of 3HK and KynA are produced from kynurenine.40 However, under inflammatory conditions, the production of 3HK and its metabolites are favored.32, 35, 41, 42 The metabolites in the ‘QA-pathway’, especially 3HK and QA are often described as ‘neurotoxic’ and ‘gliotoxic’. For instance, 3HK is capable of inducing oxidative stress, mitochondrial stress and cell death,43 while QA, which is produced by microglia and macrophages exerts neurotoxic effects through multiple different mechanisms, including activation of the N-methyl-d-aspartate (NMDA) receptor.44, 45 In contrast, KynA is thought to be an astrocyte-derived metabolite that among other roles, acts as an endogenous competitive antagonist of ionotropic excitatory amino-acid receptors including the NMDA receptor, and also acts as an antagonist of α7 nicotinic receptors.39, 44
In line with the original model by Myint and Kim,31 we and others have reported increases in neurotoxic metabolites and/or decreases in KynA in the blood36, 46, 47 and cerebrospinal fluid (CSF)48, 49 of MDD patients. However, the data are less consistent in the case of BD,50, 51, 52, 53, 54 and in schizophrenia, there is evidence for an increase in KynA levels in the blood,55 postmortem brain56 and the CSF.57, 58, 59
However, it is still unclear why a difference in the pattern of kynurenine pathway metabolism should exist between depression and psychosis/schizophrenia when inflammation appears common to both disorders. In the case of depression, excess production of 3HK and QA is thought to exert toxic effects via numerous mechanisms including free radical formation, impairment of mitochondrial function, induction of DNA damage, potentiation of neuronal glutamate release, inhibition of the astrocytic reuptake of glutamate, disruption of the blood–brain barrier and destabilization of the cellular cytoskeleton.43, 45 On the other hand, one mechanism underlying schizophrenia and psychosis more generally, is thought to involve an increased capacity for dopamine synthesis and presynaptic dopamine release, leading to an excess of striatal dopaminergic neurotransmission together with cortical hypodopaminergia.60, 61 Subsequently, this model was elaborated to account for the psychotomimetic effects of non-competitive NMDA receptor antagonists like phencyclidine (PCP) and ketamine, leading to the hypothesis that psychosis and/or schizophrenia is underpinned by NMDA receptor hypofunction acting on GABAergic interneurons and leading to the disinhibition of pyramidal neurons (including projections onto midbrain dopamine neurons), downstream excitotoxicity and striatal hyperdopaminergia.62, 63 In an elegant series of animal studies, Schwarcz, Erhardt and colleagues have demonstrated that similar to NMDA receptor antagonists such as phencyclidine and ketamine, increases in the endogenous NMDA antagonist, KynA, produces cognitive abnormalities that theoretically model clinical aspects of schizophrenia.64, 65, 66
In summary, although inflammatory processes are common to depression, affective psychosis and schizophrenia, the prevailing model of how activation of the kynurenine pathway contributes to these disorders is very different. Depression is believed to arise from the excess production of neurotoxic QA-pathway metabolites together with a reduction in KynA,32, 36, 37, 46 while in contrast, psychosis and schizophrenia are thought to be driven by increases in KynA.39, 54, 67 This ‘double dissociation’ is very interesting in light of the continued debate about the veracity of current diagnostic categories and the present trend towards the study of cross-disorder phenotypes (for example, Research Domain Criteria program).68
The central question that we address in this study is: where do patients with affective psychosis (that is, MDD with psychosis, BD with psychosis and schizoaffective disorder) fall on the kynurenine-pathway spectrum, that is, closer to MDD (without psychosis) or closer to schizophrenia? This is an important question because the existence of a reversed KynA/QA ratio in patients with affective psychosis versus patients with schizophrenia would not only have implications for understanding pathophysiology, but could potentially have utility as a non-invasive diagnostic, prognostic and treatment biomarker.
Materials and methods
Participants
The study was approved by the Western Institutional Review Board. All subjects were deemed capable of providing informed consent by the treating psychiatrist, and provided written consent prior to participation in the research.
Subjects were recruited from the adult acute inpatient unit at the Laureate Psychiatric Clinic & Hospital, in Tulsa, OK, and received a state-of-the-art psychiatric evaluation and DSM-IV diagnosis from a board-certified psychiatrist. Where possible, symptom severity was measured with the Hamilton Depression Scale (Ham-D), Young Mania Rating Scale (YMRS), Brief Psychiatric Rating Scale Expanded Version 4.0 (BPRS) and CORE Assessment of Psychomotor Change. In some cases (Table 1) the assessments could not be completed because the patients were too ill. Potential participants were excluded if their hospital admission was caused by substance abuse/dependence or if a comorbid medical illness was suspected to induce the mood or psychotic symptoms. Comorbid medical conditions by themselves were not considered exclusionary since their frequency is significantly elevated in severely-ill psychiatric populations.69, 70 No kynurenine data from these subjects have been previously published.
Table 1. Clinical ratings and kynurenine metabolite differences across the DSM-IV diagnostic groups.
| MDD | BD | SzA | SZ | HC | Statistic | Significant pairwise comparisons | |
|---|---|---|---|---|---|---|---|
| N | 35 | 53 | 40 | 21 | 92 | ||
| % F | 54 | 70 | 40 | 19 | 64 | χ2=22, P<0.001 | |
| Age | 38.8±13.8 | 40.2±11.0 | 39.0±13.0 | 38.9±12.9 | 32.3±10.4 | F4, 236=5.11, P=0.001 | BD>HC, SzA>HC |
| BMI (kg/m2) | 30±9 | 30±8 | 30±9 | 28±6 | 27±6 | F4, 236=1.95, P=0.103 | |
| BPRS | 55±9 (N=33) | 58±14 (N=38) | 66±17 (N=28) | 63±16 (N=13) | — | F3, 108=3.35, P=0.022 | MDD<SzA |
| CORE | 5±6 (N=33) | 5±6 (N=36) | 10±8 (N=27) | 9±8 (N=13) | — | F3, 105=4.09, P=0.009 | BD<SzA, MDD<SzA |
| HAM-D | 35±6 (N=33) | 28±9 (N=39) | 27±8 (N=28) | 26±10 (N=12) | 1±2 (N=81) | F4, 188=294, P<0.001 | All>HC, MDD>BD, MDD>SzA, MDD>SZ |
| YMRS | 6±5 (N=33) | 15±8 (N=33) | 13±9 (N=27) | 16±8 (N=12) | 0±0 (N=71) | F4, 177=58, P<0.001 | All>HC, MDD<BD, MDD<SZ, MDD<SzA |
| CRP (mg l−1) | 5.9±5.7 | 5.9±9.8 | 6.7±18.4 | 4.1±4.1 | 3.1±4.4 (N=78) | F4, 219=2.24, P=0.066 | |
| TRP (μm) | 60.6±14.2 | 56.2±14.3 | 57.6±16.0 | 60.6±11.3 | 62.8±16.7 | F4, 232=2.11, P=0.080 | |
| Kyn (nm) | 1.94±0.52 | 1.78±0.50 | 1.84±0.51 | 1.89±0.52 | 1.94±0.47 | F4, 232=2.58, P=0.038 | |
| KynA (nm) | 33.3±11.0 | 31.5±13.1 | 31.9±15.9 | 36.3±15.5 | 40.6±16.1 | F4, 232=7.17, P<0.001a | BD<HC, SzA<HC |
| 3HK (nm) | 30.1±10.7 | 28.1±9.4 | 30.8±12.1 | 28.5±11.1 | 32.7±13.8 | F4, 232=3.09, P=0.017 | |
| QA (nm) | 351±134 | 347±134 | 342±137 | 360±171 | 346±115 | F4, 232=0.46, P=0.769 | |
| Kyn/TRP | 0.033±0.009 | 0.033±0.011 | 0.033±0.010 | 0.032±0.010 | 0.032±0.008 | F4, 232=0.21, P=0.934 | |
| KynA/3HK | 1.17±0.42 | 1.16±0.45 | 1.08±0.43 | 1.30±0.43 | 1.31±0.43 | F4, 232=3.70, P=0.006a | SzA<HC |
| KynA/QA | 0.103±0.046 | 0.095±0.035 | 0.096±0.037 | 0.109±0.039 | 0.123±0.044 | F4, 232=5.81, P<0.001a | MDD<HC, BD<HC, SzA<HC |
Abbreviations: BD, bipolar disorder; BMI, body mass index; BPRS, Brief Psychiatric Rating Scale; CORE, CORE Assessment of Psychomotor Change; CRP, C-reactive protein; HAM-D, Hamilton rating scale for depression 25 items; HC, healthy control; Kyn, kynurenine; KynA, kynurenic acid; KynA/QA, ratio of kynurenic acid to quinolinic acid; Kyn/TRP, ratio of kynurenine to tryptophan; KynA/3HK, ratio of kynurenic acid to 3-hydroxykynurenine; MDD, major depressive disorder; QA, quinolinic acid; SzA, schizoaffective disorder; SZ, schizophrenia; TRP, tryptophan; YMRS, Young mania rating scale; 3HK, 3-hydroxykynurenine.
Statistically significant after Bonferroni correction for 9 tests: CRP, 5 kynurenine metabolites and 3 ratios (P⩽0.006).
Covariates: Sex, age, BMI and analysis batch.
Healthy controls were recruited from the surrounding community and were interviewed with the Structured Clinical Interview for DSM-IV-TR and the Family Interview for Genetic Studies. The following exclusion criteria applied to healthy controls: a personal history of a psychiatric illness; a first-degree relative with a mood or psychotic disorder; medical conditions or concomitant medications likely to influence central nervous system or immunological function including cardiovascular, respiratory, endocrine and neurological diseases and a history of drug or alcohol abuse within 6 months or a history of drug or alcohol dependence within 1 year (DSM-IV-TR criteria).
Morning fasting blood samples were obtained from inpatients and healthy controls and stored in BD Vacutainer serum tubes and processed according to the standard BD Vacutainer protocol. Samples for high-sensitivity C-reactive protein (hs-CRP) were measured immunoturbidimetrically with the Kamiya Biomedical K-Assay in the Saint Francis Hospital Clinical Laboratory. Serum samples for tryptophan (TRP), KYN, KynA, QA, and 3HK were stored at −80 °C and analyzed in two separate batches blind-to-diagnosis by Brains Online, LLC. The concentrations were determined by high-performance liquid chromatography (HPLC) followed by tandem mass spectrometry (MS/MS) using their standard protocols.
Statistical analysis
Statistical analysis was performed using SYSTAT 13. Deviations from normality were tested using Shapiro–Wilk and Anderson-Darling Statistics and non-normally distributed variables were log normalized. Between group differences in kynurenine pathway metabolites and CRP were tested with two separate multiple analysis of covariance (MANCOVAs), one with CRP and the individual kynurenine metabolites as dependent variables, and the other with Kyn/Trp, KynA/3HK and KynA/QA as dependent variables. The use of these ratios for assessing IDO activation and the neurotoxic imbalance has already been justified.34, 46, 50, 71, 72, 73 Two separate MANCOVAs had to be performed because the ratios are not independent of the individual metabolites and therefore cannot be run in the same model. For both MANCOVAs, age, sex, body mass index (BMI) and batch were used as covariates (Table 1). Where one or more of the dependent variables were significant in the omnibus test, univariate analysis of varinces (ANOVAs) and pairwise testing with Bonferroni correction for multiple testing were performed to identify which diagnostic groups differed from each other. Specifically, we adjusted the statistical threshold for 9 comparisons: CRP, 5 kynurenine metabolites and 3 ratios (P⩽0.006). Pearson correlation coefficient and linear least-square regressions were performed to assess the associations between the statistically significant kynurenine pathway metabolites and/or ratios and the psychiatric rating scales.
Results
All of the immune markers were non-normally distributed and were therefore log normalized prior to the use of parametric statistics.
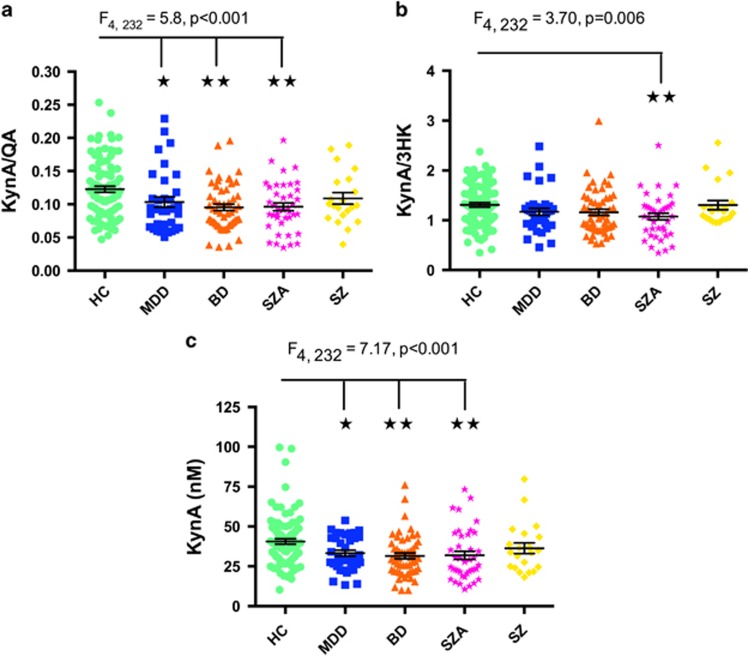
The MANCOVA for CRP, TRP, kynurenine, KynA, 3HK and QA revealed a significant group effect (Wilk’s λ=0.834, F20,757=2.13, P=0.003). Follow-up analysis of covariance with sex, age, BMI and batch as covariates, showed that KynA differed across groups (F4, 232=7.17, P<0.001) after correction for multiple comparisons, with significant reductions in MDD (P=0.042), BD (P=0.001) and schizoaffective disorder (SzA; P<0.001) compared with HCs (Table 1 and Figure 1). CRP concentrations were not significantly different across groups in the omnibus ANOVA (F4, 219=2.24, P=0.066) although when follow-up t-tests were performed there were either significantly lower or a trend towards significantly lower CRP concentrations in the healthy control group versus the MDD (t111=2.9, P=0.005), BD (t129=2.2, P=0.030), SzA (t118=1.8, P=0.071) but not schizophrenia (t95=0.2, P=0.818) groups.
Figure 1.
Scatterplots showing the difference in KynA/QA (a), KynA/3HK (b) and KynA (c) serum concentrations across the diagnostic groups. The error bars represent the s.e.m. BD, bipolar disorder; HC, healthy controls; Kyn, kynurenine; KynA, kynurenic acid; KynA/QA, ratio of kynurenic acid to quinolinic acid; KynA/3HK, ratio of kynurenic acid to 3-hydroxykynurenine; MDD, major depressive disorder; SZA, schizoaffective disorder; SZ, schizophrenia. *P<0.05. **P<0.01.
The MANCOVA analysis for KYN/TRP, KynA/3HK and KynA/QA also revealed a significant group effect (Wilk’s λ=0.872, F12, 608=2.69, P=0.002). Follow-up analysis of covariance with sex, age, BMI and batch as covariates, showed that KYN/TRP did not significantly differ across groups, however, KynA/3HK (F4, 232=3.70, P=0.006) was significantly reduced in schizoaffective disorder versus healthy controls (P=0.004) and KynA/QA (F4, 232=5.81, P<0.001) was significantly reduced in the MDD (P=0.029), BD (P=0.004) and the schizoaffective disorder (P=0.001) groups compared with healthy controls (Table 1 and Figure 1).
Neither KynA, KynA/3HK, nor KynA/QA correlated significantly with depression or mania rating scale scores in the schizoaffective disorder and BD groups.
The MANCOVA analyses for determining differences between the patient groups in KYN/TRP, KynA/3HK and KynA/QA and the individual kynurenine metabolites according to the potential confounds, urine drug screen (positive versus negative), comprehensive metabolic panel (abnormality versus no abnormality), tobacco use (yes versus no), concurrent anti-depressant drug use (yes versus no), concurrent anti-psychotic drug use (yes versus no), concurrent anti-convulsant drug use (yes versus no) and concurrent anxiolytic drug use (yes versus no) were all non-significant. Further, there was no significant difference in the concentrations of kynurenine pathway metabolites between the patients who presented with significant suicidal ideation versus those who did not present with significant suicidal ideation as determined by the treating psychiatrists. As an example, a visual representation of the data for KynA/QA is shown in Supplementary Figures S2 and S3.
Next we tested the relationship between inflammation (indexed by CRP) and the kynurenine metabolites. In the combined sample there were significant correlations between CRP and Kyn/Trp (rs=0.29, P<0.001), 3HK (rs= 0.25, P<0.001), QA (rs= 0.21, P=0.002), 3HK/Kyn (a potential surrogate marker of KMO activity, rs=0.14, P<0.034), as well as both KynA/3HK (rs= −0.18, P=0.006) and KynA/QA (rs= −0.15, P=0.026; Table 2). In contrast, there were no significant correlations between KynA and CRP (rs= 0.02, P=0.725) or KynA/Kyn (a potential surrogate marker of KAT enzyme activity, rs= −0.1, P=0.145).
Table 2. Spearman’s correlation coefficients between CRP and the kynurenine pathway metabolites.
| Metabolite | CRP (mg l−1) |
|---|---|
| TRP (μm) | rs= −0.18, P=0.008 |
| Kyn (nm) | rs= 0.17, P=0.011 |
| KynA (nm) | rs= 0.02, P=0.725 |
| 3HK (nm) | rs= 0.25, P<0.001 |
| QA (nm) | rs= 0.21, P=0.002 |
| Kyn/TRP | rs= 0.29, P<0.001 |
| KynA/3HK | rs= −0.18, P=0.006 |
| KynA/QA | rs= −0.15, P=0.026 |
| 3HK/Kyn | rs= 0.14, P=0.034 |
| KynA/Kyn | rs= −0.1, P=0.145 |
Abbreviations: CRP, C-reactive protein; Kyn, kynurenine; KynA, kynurenic acid; KynA/QA, ratio of kynurenic acid to quinolinic acid; Kyn/TRP, ratio of kynurenine to tryptophan; KynA/3HK, ratio of kynurenic acid to 3-hydroxykynurenine; TRP, tryptophan.
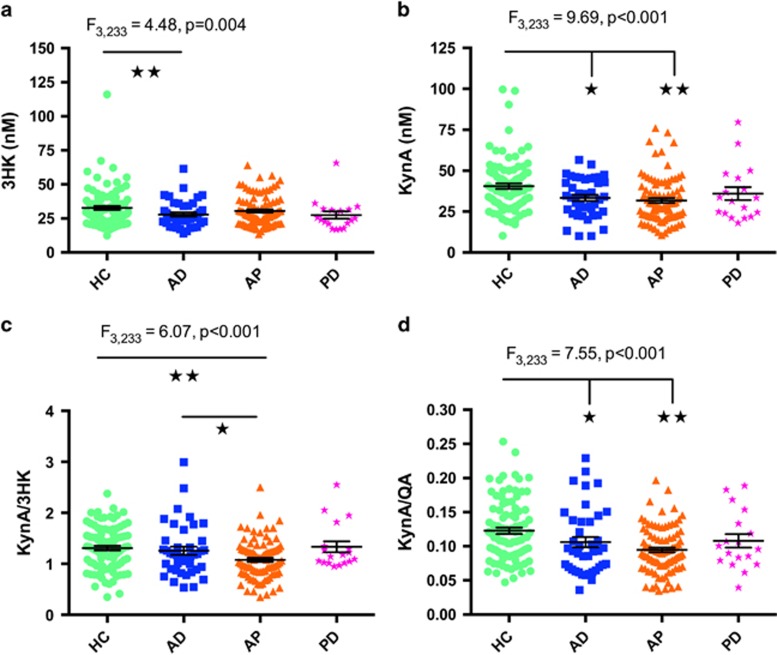
In exploratory analyses we categorized the patients into affective disorder (non-psychotic), affective psychosis and psychotic disorder (non-affective) groups in place of the standard DSM-IV categorizations (Supplementary Table S1 and Figure 2). First, the principal findings were that KynA differed between the groups (F3, 233=9.69, P<0.001) with both the affective disorder (P=0.017) and affective psychosis groups showing reduced levels of KynA compared with healthy controls (P<0.001). Second, KynA/3HK (F3,233=6.07, P<0.001) was significantly reduced in affective psychosis versus affective disorder (P=0.017) and affective psychosis versus healthy controls (P<0.001). Similarly, KynA/QA differed across groups (F3,233=7.55, P<0.001) and was significantly reduced in the affective disorder (P=0.035) and affective psychosis (P<0.001) groups compared with healthy controls (details are provided in Supplementary Table S1).
Figure 2.
Scatterplots showing the difference in 3HK (a), KynA (b) and KynA/3HK (c), and KynA/QA (d) serum concentrations across groups. The error bars represent the standard error of the mean. AD, affective disorder without psychosis; AP, affective psychosis; HC, healthy control; Kyn, kynurenine; KynA, kynurenic acid; KynA/QA, ratio of kynurenic acid to quinolinic acid; KynA/3HK, ratio of kynurenic acid to 3-hydroxykynurenine; PD, psychotic disorder (non-affective). *P<0.05. **P<0.01.
We also performed an additional secondary analysis to evaluate the effect of phase of illness in BD on CRP and kynurenine metabolite concentrations. No significant differences between BD patients with a current depressive (n=15), manic (n=25) or mixed episode (n=10) were found after controlling for age, sex and BMI (Wilk’s λ=0.850, F6,40=1.18, P=0.336). Similarly, there were no significant group differences in Kyn/Trp, KynA/3HK and KynA/QA (Wilk’s λ=0.862, F3,43=2.30, P=0.091).
Discussion
Here we report significantly lower KynA/3HK and KynA/QA ratios in acutely ill BD and/or schizoaffective disorder patients (and all affective psychosis patients combined). This effect was driven by a reduction in KynA concentration. These findings remained significant after applying a Bonferroni correction for multiple testing. The results also remained significant with and without statistically controlling for age, sex and BMI, and were not significantly affected by concurrent medication use, nicotine use, drugs of abuse and biochemical abnormalities assessed with a comprehensive metabolic panel. Further, we observed a reduction in KynA and KynA/QA in patients with MDD that replicated our previous results in independent samples of MDD outpatients.34, 46 Although CRP concentrations were not significantly different across groups in the omnibus ANOVA, the mean CRP score of the healthy control group was ~3 mg l−1 lower than both the MDD and BD groups, and 3.6 mg/l lower than the schizoaffective disorder group (Table 1). Follow-up t-tests indicated that the healthy controls had significantly lower concentrations of CRP or a statistical trend towards lower concentrations of CRP than the MDD, BD and SzA groups. However, there was no difference in CRP concentrations between the healthy controls and the schizophrenia group.
The significant positive correlation between CRP and the activation of the neurotoxic pathway, but not the concentration of KynA (Table 2), potentially is consistent with previous reports demonstrating that although during homeostasis approximately equal amounts of 3HK and KynA are produced from kynurenine,40 under conditions of inflammation the production of QA predominates over KynA, possibly because of the upregulation of kynurenine mono-oxygenase (KMO).32, 35, 41, 42 Our results thus are compatible with evidence suggesting that a proinflammatory drive on the kynurenine metabolic pathway exists in patients with mood disorders, and also indicate that this proinflammatory drive is greater in psychotic versus non-psychotic affective disease.
A question that remains is why Kyn/Trp concentrations do not differ significantly between patients and controls if the depression and schizoaffective disorder-associated decrease in KynA results from an inflammatory process? There are several possibilities. First, we cannot rule out the possibility that non-inflammatory factors such as genetic or epigenetic variants54 may have contributed to this neurotoxic shift. Second, much of the work that has drawn a link between increases in Kyn/Trp and depression is derived from either preclinical studies or treatment studies of medically-ill patients where inflammation is induced by LPS and immune-modifying drugs (for example, interferon α), respectively. LPS and interferon α cause a robust, systemic inflammatory response that is indexed by significant increases in CRP and proinflammatory cytokines including interferon γ, one of the most important activators of IDO.74 In contrast, the low-grade inflammation observed in non-medical illness-associated depression and psychosis may have different physiological effects. In fact, it is known that stress and major depression are associated with a shift away from Th1-type immunity, including a decrease in circulating concentrations of interferon γ.75, 76, 77, 78, 79, 80, 81 Consistent with these data, a recent meta-analysis reported that peripheral concentrations of interferon γ are reduced in MDD.82 Unfortunately, little is known about the inflammatory mediators that regulate the expression and/or activity of KMO and other kynurenine pathway enzymes but it is conceivable that depression-associated changes in immunological function alter the activity of KMO or the KAT enzymes via interferon γ-independent pathways.
Third, the Kyn/Trp ratio also is affected by the enzyme, tryptophan-2,3-dioxygenase (TDO), which converts tryptophan to N-formylkynurenine.83 TDO is induced by corticosteroids that may be either increased or decreased in depression and affective psychosis depending on illness subtype or whether cortisol readings are obtained with or without exposure to a laboratory stressor.84, 85, 86, 87 Thus, the absence of a significant difference in Kyn/Trp between patients and controls also may reflect HPA dysfunction such as blunted cortisol release.
While no previous study has measured kynurenine metabolites in patients with schizoaffective disorder, the results obtained in the psychotic BD group and the schizoaffective disorder group are strikingly consistent with the results of two studies of Myint, Kim, Leonard and colleagues. Myint et al. initially reported that plasma KynA concentrations in patients with bipolar mania were decreased by approximately 20% compared to healthy controls.51 Here we also observed a 20% decrease in the peripheral concentration of KynA in subjects with BD with psychosis. Subsequently, Myint et al. measured kynurenine metabolites in the plasma of medication-free patients with schizophrenia at admission and following 6 weeks of antipsychotic treatment.88 KynA was reduced and 3HK was elevated in the plasma of the patients with schizophrenia compared with healthy controls. Further, the KynA/3HK ratio also remained lower in the patients relative to controls but nevertheless had significantly increased relative to baseline after 6 weeks of treatment. Here we did not find a difference in kynurenine metabolite concentrations between patients with schizophrenia and controls. However, we report an approximate decrease of 18% in KynA/3HK in the schizoaffective disorder group versus the healthy control group.
In contrast, BD patients with psychosis have been reported to display elevations in KynA in the CSF and decreases in KMO gene expression postmortem that could theoretically increase KynA concentrations in the brain.54, 67 Here we find that the reductions in KynA in the serum were in fact most pronounced in BD patients with psychosis and patients with schizoaffective disorder, but not schizophrenia. Nevertheless, given the smaller sample of participants with schizophrenia, it is likely that our study was underpowered to detect small effect sizes in this group.
One explanation for the discrepant results in the affective psychosis literature may relate to stage of illness. In this study, the participants were acutely ill whereas in the studies that reported increased CSF KynA in BD, the participants were symptom-free outpatients with a history of psychosis.54, 67 Conceivably, the increase in KynA in these patients may be reflective of recovery from illness rather than a pathogenic marker, per se.
A limitation of our study design is that we measured kynurenine metabolites in the serum, and while kynurenine and 3HK readily cross the blood–brain barrier, other metabolites such as KynA and QA do not cross the blood–brain barrier and at least under normal conditions, do not contribute significantly to brain concentrations.89 Nevertheless, extant evidence suggests a significant correlation between peripheral and CSF levels of QA and KynA in the context of depression and inflammation. For instance, a highly significant association between the plasma and CSF concentrations of QA in IFNα-treated hepatitis C patients was reported by Raison, Miller and colleagues (r=0.72, P<0.001).33 Further, Brundin, Erhardt and colleagues have reported persistent reductions in KynA and increases in QA in the CSF of suicide attempters, consistent with our peripheral measures in subjects with mood disorders.48, 49 In addition, Steiner and colleagues observed an increased density of QA-positive microglial cells in the subgenual anterior cingulate cortex (sgACC) and the mid-anterior cingulate cortex in postmortem samples with either MDD or BD who died by suicide.90 Notably, psychosis is a significant risk factor for suicide attempts with up to 10% of first-episode psychosis patients making at least one suicide attempt.91, 92 Further, the presence of psychosis reportedly increases the risk of suicide in patients with MDD93 and self-harm and suicidality are highly prevalent in patients at ultra-high risk of psychosis.94
Further, the results of other studies that obtained central measures of ‘inflammation’ indirectly support our findings. First, most positron emission tomography (PET) studies of microglial translocator protein binding have reported increased labeling in non-psychotic patients with MDD versus healthy controls.95, 96 In addition, three PET studies using the PK 11195 ligand reported microglia activation in patients with schizophrenia or BD97, 98, 99 and these findings were recently replicated in a study of subjects at ultra-high risk of psychosis and patients with schizophrenia that made use of the second generation PBR28 ligand.100 Since QA is thought to be produced largely by microglia, conceivably, KMO activity may be elevated in the brain in both affective disorder and affective psychosis.
A second limitation was the imperfect control for medication effects. We grouped medication broadly by class, that is, anti-depressants, anti-psychotics, anti-convulsants and anxiolytics. However, this method cannot account for variation in the effects of types of medication within a class, differences in dose and the effects of multiple combinations of medications. Similarly, we measured the effects of tobacco use, metabolic abnormalities and suicidal behavior in a dichotomous manner and thus cannot account for amount, specificity and severity, respectively.
In summary, within the methodological limitations of the current design, our study questions the prevailing model of excess KynA production in affective psychosis, and indicates that further research is necessary to fully capture the biological role of the kynurenine pathway in both depression and affective psychosis.
Regarding the nosological boundaries of the psychiatric disorders studied here, the results indicate that schizoaffective disorder may be more closely related to affective psychosis than to schizophrenia with respect to manifesting a proinflammatory drive on kynurenine metabolism. Although most studies support the hypothesis that schizoaffective disorder resides on a continuum between BD and schizophrenia,101 the other data suggest that schizoaffective disorder should be subsumed under the schizophrenia category.102 Nevertheless, it also is conceivable that schizoaffective disorder is more heterogeneous, with some patients having a pathophysiology like that of BD and others having one like that of schizophrenia, and that advances in nosology will be needed to resolve such questions. It is also important to note that the mean differences in KynA between affective and non-affective psychosis were limited to the group level without directionality or linear associations. Thus at least in the short term, the measurement of kynurenine metabolites are unlikely to be useful for individual-level diagnosis.
Acknowledgments
The study was funded by the Laureate Institute of Brain Research and the National Institute of Mental Health (K01MH096077). The authors also thank all the research participants and wish to acknowledge the contributions of Brenda Davis, Debbie Neal, Chibing Tan and Ashlee Taylor from the laboratory of TKT towards the transport, processing and handling of all blood samples.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
WCD is an employee of Janssen Pharmaceuticals of Johnson & Johnson, Titusville, NJ, USA. JBS has received research funding from Janssen Pharmaceuticals for an independent study. The remaining authors declare no conflict of interest.
Supplementary Material
References
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 2009; 71: 171–186. [DOI] [PubMed] [Google Scholar]
- Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimaki M. Cumulative meta-analysis of interleukins 6 and 1beta, tumour necrosis factor alpha and C-reactive protein in patients with major depressive disorder. Brain Behav Immun 2015; 49: 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK et al. A meta-analysis of cytokines in major depression. Biol Psychiatry 2010; 67: 446–457. [DOI] [PubMed] [Google Scholar]
- Upthegrove R, Manzanares-Teson N, Barnes NM. Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophr Res 2014; 155: 101–108. [DOI] [PubMed] [Google Scholar]
- de Witte L, Tomasik J, Schwarz E, Guest PC, Rahmoune H, Kahn RS et al. Cytokine alterations in first-episode schizophrenia patients before and after antipsychotic treatment. Schizophr Res 2014; 154: 23–29. [DOI] [PubMed] [Google Scholar]
- Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry 2011; 70: 663–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargel AA, Godin O, Kapczinski F, Kupfer DJ, Leboyer M. C-reactive protein alterations in bipolar disorder: a meta-analysis. J Clin Psychiatry 2015; 76: 142–150. [DOI] [PubMed] [Google Scholar]
- Munkholm K, Brauner JV, Kessing LV, Vinberg M. Cytokines in bipolar disorder vs. healthy control subjects: a systematic review and meta-analysis. J Psychiatr Res 2013; 47: 1119–1133. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ. Immune System and Schizophrenia. Curr Immunol Rev 2010; 6: 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasco JA, Nicholson GC, Williams LJ, Jacka FN, Henry MJ, Kotowicz MA et al. Association of high-sensitivity C-reactive protein with de novo major depression. Br J Psychiatry 2010; 197: 372–377. [DOI] [PubMed] [Google Scholar]
- Khandaker GM, Pearson RM, Zammit S, Lewis G, Jones PB. Association of serum interleukin 6 and C-reactive protein in childhood with depression and psychosis in young adult life: a population-based longitudinal study. JAMA Psychiatry 2014; 71: 1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wium-Andersen MK, Orsted DD, Nordestgaard BG. Elevated C-reactive protein and late-onset bipolar disorder in 78 809 individuals from the general population. Br J Psychiatry 2015; 208: 138–145. [DOI] [PubMed] [Google Scholar]
- Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry 2003; 160: 1342–1345. [DOI] [PubMed] [Google Scholar]
- Capuron L, Hauser P, Hinze-Selch D, Miller AH, Neveu PJ. Treatment of cytokine-induced depression. Brain Behav Immunity 2002; 16: 575–580. [DOI] [PubMed] [Google Scholar]
- Wichers M, Maes M. The psychoneuroimmuno-pathophysiology of cytokine-induced depression in humans. Int J Neuropsychopharmacol 2002; 5: 375–388. [DOI] [PubMed] [Google Scholar]
- Bonaccorso S, Puzella A, Marino V, Pasquini M, Biondi M, Artini M et al. Immunotherapy with interferon-alpha in patients affected by chronic hepatitis C induces an intercorrelated stimulation of the cytokine network and an increase in depressive and anxiety symptoms. Psychiatry Res 2001; 105: 45–55. [DOI] [PubMed] [Google Scholar]
- Felger JC, Lotrich FE. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience 2013; 246: 199–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 2015; 16: 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biol Psychiatry 2009; 66: 407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison NA, Doeller CF, Voon V, Burgess N, Critchley HD. Peripheral inflammation acutely impairs human spatial memory via actions on medial temporal lobe glucose metabolism. Biol Psychiatry 2014; 76: 585–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannestad J, Subramanyam K, Dellagioia N, Planeta-Wilson B, Weinzimmer D, Pittman B et al. Glucose metabolism in the insula and cingulate is affected by systemic inflammation in humans. J Nucl Med 2012; 53: 601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biol Psychiatry 2010; 68: 748–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ et al. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alfa administration. Arch Gen Psychiatry 2012; 69: 1044–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li Z, Haroon E, Woolwine BJ, Jung MY, Hu X et al. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol Psychiatry 2015; 21: 1358–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry 2009; 14: 511–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson MA, Parrott JM, McCusker RH, Dantzer R, Kelley KW, O'Connor JC. Intracerebroventricular administration of lipopolysaccharide induces indoleamine-2,3-dioxygenase-dependent depression-like behaviors. J Neuroinflamm 2013; 10: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry 2002; 7: 468–473. [DOI] [PubMed] [Google Scholar]
- Widner B, Laich A, Sperner-Unterweger B, Ledochowski M, Fuchs D. Neopterin production, tryptophan degradation, and mental depression—what is the link? Brain Behav Immun 2002; 16: 590–595. [DOI] [PubMed] [Google Scholar]
- Lapin IP, Oxenkrug GF. Intensification of the central serotoninergic processes as a possible determinant of the thymoleptic effect. Lancet 1969; 1: 132–136. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Welch J. Stress- and endotoxin-induced increases in brain tryptophan and serotonin metabolism depend on sympathetic nervous system activity. J Neurochem 1991; 57: 1615–1622. [DOI] [PubMed] [Google Scholar]
- Myint AM, Kim YK. Cytokine-serotonin interaction through IDO: a neurodegeneration hypothesis of depression. Med Hypotheses 2003; 61: 519–525. [DOI] [PubMed] [Google Scholar]
- Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpe S, Maes M. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry 2005; 10: 538–544. [DOI] [PubMed] [Google Scholar]
- Raison CL, Dantzer R, Kelley KW, Lawson MA, Woolwine BJ, Vogt G et al. CSF concentrations of brain tryptophan and kynurenines during immune stimulation with IFN-alpha: relationship to CNS immune responses and depression. Mol Psychiatry 2010; 15: 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Drevets WC, Smith CM, Victor TA, Wurfel BE, Bellgowan PS et al. Putative neuroprotective and neurotoxic kynurenine pathway metabolites are associated with hippocampal and amygdalar volumes in subjects with major depressive disorder. Neuropsychopharmacology 2015; 40: 463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgin-Lavialle S, Moura DS, Salvador A, Chauvet-Gelinier JC, Launay JM, Damaj G et al. Mast cells' involvement in inflammation pathways linked to depression: evidence in mastocytosis. Mol Psychiatry 2016; 21: 1511–1516. [DOI] [PubMed] [Google Scholar]
- Myint AM, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B. Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord 2007; 98: 143–151. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O'Connor JC, Lawson MA, Kelley KW. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology 2011; 36: 426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Leonard BE, Myint AM, Kubera M, Verkerk R. The new '5-HT' hypothesis of depression: cell-mediated immune activation induces indoleamine 2,3-dioxygenase, which leads to lower plasma tryptophan and an increased synthesis of detrimental tryptophan catabolites (TRYCATs), both of which contribute to the onset of depression. Progress Neuropsychopharmacol Biol Psychiatry 2011; 35: 702–721. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci 2012; 13: 465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidetti P, Eastman CL, Schwarcz R. Metabolism of [5-3H]kynurenine in the rat brain in vivo: evidence for the existence of a functional kynurenine pathway. J Neurochem 1995; 65: 2621–2632. [DOI] [PubMed] [Google Scholar]
- Saito K, Markey SP, Heyes MP. Effects of immune activation on quinolinic acid and neuroactive kynurenines in the mouse. Neuroscience 1992; 51: 25–39. [DOI] [PubMed] [Google Scholar]
- Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B et al. NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6 J mice. Neuropsychopharmacology 2013; 38: 1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colin-Gonzalez AL, Maldonado PD, Santamaria A. 3-Hydroxykynurenine: an intriguing molecule exerting dual actions in the central nervous system. Neurotoxicology 2013; 34: 189–204. [DOI] [PubMed] [Google Scholar]
- Stone TW, Stoy N, Darlington LG. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol Sci 2013; 34: 136–143. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ. Quinolinic acid, the inescapable neurotoxin. FEBS J 2012; 279: 1356–1365. [DOI] [PubMed] [Google Scholar]
- Savitz J, Drevets WC, Wurfel BE, Ford BN, Bellgowan PS, Victor TA et al. Reduction of kynurenic acid to quinolinic acid ratio in both the depressed and remitted phases of major depressive disorder. Brain Behav Immun 2015; 46: 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Galecki P, Verkerk R, Rief W. Somatization, but not depression, is characterized by disorders in the tryptophan catabolite (TRYCAT) pathway, indicating increased indoleamine 2,3-dioxygenase and lowered kynurenine aminotransferase activity. Neuroendocrinol Lett 2011; 32: 264–273. [PubMed] [Google Scholar]
- Bay-Richter C, Linderholm KR, Lim CK, Samuelsson M, Traskman-Bendz L, Guillemin GJ et al. A role for inflammatory metabolites as modulators of the glutamate N-methyl-d-aspartate receptor in depression and suicidality. Brain Behav Immun 2015; 43: 110–117. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Lim CK, Linderholm KR, Janelidze S, Lindqvist D, Samuelsson M et al. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacology 2013; 38: 743–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz J, Dantzer R, Wurfel BE, Victor TA, Ford BN, Bodurka J et al. Neuroprotective kynurenine metabolite indices are abnormally reduced and positively associated with hippocampal and amygdalar volume in bipolar disorder. Psychoneuroendocrinology 2015; 52: 200–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myint AM, Kim YK, Verkerk R, Park SH, Scharpe S, Steinbusch HW et al. Tryptophan breakdown pathway in bipolar mania. J Affect Disord 2007; 102: 65–72. [DOI] [PubMed] [Google Scholar]
- Johansson AS, Owe-Larsson B, Asp L, Kocki T, Adler M, Hetta J et al. Activation of kynurenine pathway in ex vivo fibroblasts from patients with bipolar disorder or schizophrenia: cytokine challenge increases production of 3-hydroxykynurenine. J Psychiatr Res 2013; 47: 1815–1823. [DOI] [PubMed] [Google Scholar]
- Olsson SK, Samuelsson M, Saetre P, Lindstrom L, Jonsson EG, Nordin C et al. Elevated levels of kynurenic acid in the cerebrospinal fluid of patients with bipolar disorder. J Psychiatry Neurosci 2010; 35: 195–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavebratt C, Olsson S, Backlund L, Frisen L, Sellgren C, Priebe L et al. The KMO allele encoding Arg452 is associated with psychotic features in bipolar disorder type 1, and with increased CSF KYNA level and reduced KMO expression. Mol Psychiatry 2014; 19: 334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kegel ME, Bhat M, Skogh E, Samuelsson M, Lundberg K, Dahl ML et al. Imbalanced kynurenine pathway in schizophrenia. Int J Tryptophan Res 2014; 7: 15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry 2001; 50: 521–530. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, Engberg G. Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett 2001; 313: 96–98. [DOI] [PubMed] [Google Scholar]
- Linderholm KR, Skogh E, Olsson SK, Dahl ML, Holtze M, Engberg G et al. Increased levels of kynurenine and kynurenic acid in the CSF of patients with schizophrenia. Schizophr Bull 2012; 38: 426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson LK, Linderholm KR, Engberg G, Paulson L, Blennow K, Lindstrom LH et al. Elevated levels of kynurenic acid in the cerebrospinal fluid of male patients with schizophrenia. Schizophr Res 2005; 80: 315–322. [DOI] [PubMed] [Google Scholar]
- Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry 1991; 148: 1474–1486. [DOI] [PubMed] [Google Scholar]
- Howes O, McCutcheon R, Stone J. Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol 2015; 29: 97–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry 1995; 52: 998–1007. [DOI] [PubMed] [Google Scholar]
- Javitt DC. Glutamate and schizophrenia: phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiol 2007; 78: 69–108. [DOI] [PubMed] [Google Scholar]
- Pocivavsek A, Wu HQ, Potter MC, Elmer GI, Pellicciari R, Schwarcz R. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology 2011; 36: 2357–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linderholm KR, Andersson A, Olsson S, Olsson E, Snodgrass R, Engberg G et al. Activation of rat ventral tegmental area dopamine neurons by endogenous kynurenic acid: a pharmacological analysis. Neuropharmacology 2007; 53: 918–924. [DOI] [PubMed] [Google Scholar]
- Akagbosu CO, Evans GC, Gulick D, Suckow RF, Bucci DJ. Exposure to kynurenic acid during adolescence produces memory deficits in adulthood. Schizophr Bull 2012; 38: 769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson SK, Sellgren C, Engberg G, Landen M, Erhardt S. Cerebrospinal fluid kynurenic acid is associated with manic and psychotic features in patients with bipolar I disorder. Bipol Disord 2012; 14: 719–726. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry 2010; 167: 748–751. [DOI] [PubMed] [Google Scholar]
- Leboyer M, Soreca I, Scott J, Frye M, Henry C, Tamouza R et al. Can bipolar disorder be viewed as a multi-system inflammatory disease? J Affect Disord 2012; 141: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini A, Goracci A. The effects of undertreated chronic medical illnesses in patients with severe mental disorders. J Clin Psychiatry 2009; 70(Suppl 3): 22–29. [DOI] [PubMed] [Google Scholar]
- Young KD, Drevets WC, Dantzer R, Teague TK, Bodurka J, Savitz J. Kynurenine pathway metabolites are associated with hippocampal activity during autobiographical memory recall in patients with depression. Brain Behav Immun 2016; 56: 335–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocki T, Wnuk S, Kloc R, Kocki J, Owe-Larsson B, Urbanska EM. New insight into the antidepressants action: modulation of kynurenine pathway by increasing the kynurenic acid/3-hydroxykynurenine ratio. J Neur transmission 2012; 119: 235–243. [DOI] [PubMed] [Google Scholar]
- Schwieler L, Samuelsson M, Frye MA, Bhat M, Schuppe-Koistinen I, Jungholm O et al. Electroconvulsive therapy suppresses the neurotoxic branch of the kynurenine pathway in treatment-resistant depressed patients. J Neuroinflamm 2016; 13: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BM, Charych E, Lee AW, Moller T. Kynurenines in CNS disease: regulation by inflammatory cytokines. Front Neurosci 2014; 8: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun 2007; 21: 374–383. [DOI] [PubMed] [Google Scholar]
- Blume J, Douglas SD, Evans DL. Immune suppression and immune activation in depression. Brain Behav Immun 2011; 25: 221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun 2001; 15: 199–226. [DOI] [PubMed] [Google Scholar]
- Miller AH. Depression and immunity: a role for T cells? Brain Behav Immun 2010; 24: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leserman J. Role of depression, stress, and trauma in HIV disease progression. Psychosom Med 2008; 70: 539–545. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Levin MJ, Laudenslager ML, Olmstead R, Lucko A, Lang N et al. Varicella zoster virus-specific immune responses to a herpes zoster vaccine in elderly recipients with major depression and the impact of antidepressant medications. Clin Infec Dis 2013; 56: 1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-induced immune dysfunction: implications for health. Nat Rev Immunol 2005; 5: 243–251. [DOI] [PubMed] [Google Scholar]
- Kohler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS et al. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand 2017; 135: 373–387. [DOI] [PubMed] [Google Scholar]
- Yu CP, Pan ZZ, Luo DY. TDO as a therapeutic target in brain diseases. Metab Brain Dis 2016; 31: 737–747. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry 2002; 7: 254–275. [DOI] [PubMed] [Google Scholar]
- Gold PW. The organization of the stress system and its dysregulation in depressive illness. Mol Psychiatry 2015; 20: 32–47. [DOI] [PubMed] [Google Scholar]
- Berger M, Kraeuter AK, Romanik D, Malouf P, Amminger GP, Sarnyai Z. Cortisol awakening response in patients with psychosis: Systematic review and meta-analysis. Neurosci Biobehav Rev 2016; 68: 157–166. [DOI] [PubMed] [Google Scholar]
- Shah JL, Malla AK. Much ado about much: stress, dynamic biomarkers and HPA axis dysregulation along the trajectory to psychosis. Schizophr Res 2015; 162: 253–260. [DOI] [PubMed] [Google Scholar]
- Myint AM, Schwarz MJ, Verkerk R, Mueller HH, Zach J, Scharpe S et al. Reversal of imbalance between kynurenic acid and 3-hydroxykynurenine by antipsychotics in medication-naive and medication-free schizophrenic patients. Brain Behav Immunity 2011; 25: 1576–1581. [DOI] [PubMed] [Google Scholar]
- Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem 1991; 56: 2007–2017. [DOI] [PubMed] [Google Scholar]
- Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein HG, Sarnyai Z et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflamm 2011; 8: 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordentoft M, Jeppesen P, Abel M, Kassow P, Petersen L, Thorup A et al. OPUS study: suicidal behaviour, suicidal ideation and hopelessness among patients with first-episode psychosis. One-year follow-up of a randomised controlled trial. Br J Psychiatry Suppl 2002; 43: s98–106. [DOI] [PubMed] [Google Scholar]
- Clarke M, Whitty P, Browne S, Mc Tigue O, Kinsella A, Waddington JL et al. Suicidality in first episode psychosis. Schizophr Res 2006; 86: 221–225. [DOI] [PubMed] [Google Scholar]
- Zalpuri I, Rothschild AJ. Does psychosis increase the risk of suicide in patients with major depression? A systematic review. J Affect Disord 2016; 198: 23–31. [DOI] [PubMed] [Google Scholar]
- Taylor PJ, Hutton P, Wood L. Are people at risk of psychosis also at risk of suicide and self-harm? A systematic review and meta-analysis. Psychol Med 2015; 45: 911–926. [DOI] [PubMed] [Google Scholar]
- Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 2015; 72: 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards EM, Zanotti-Fregonara P, Fujita M, Walls T, Niciu MJ, Machado-Vieira R et al. Increased PET radioligand binding to translocator protein suggests that major depressive disorder is associated with neuroinflammation. Biol Psychiatry 2016; 77: S652. [Google Scholar]
- Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med 2009; 50: 1801–1807. [DOI] [PubMed] [Google Scholar]
- van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E et al. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry 2008; 64: 820–822. [DOI] [PubMed] [Google Scholar]
- Haarman BC, Riemersma-Van der Lek RF, de Groot JC, Ruhe HG, Klein HC, Zandstra TE et al. Neuroinflammation in bipolar disorder - A [(11)C]-(R)-PK11195 positron emission tomography study. Brain Behav Immun 2014; 40: 219–225. [DOI] [PubMed] [Google Scholar]
- Bloomfield PS, Selvaraj S, Veronese M, Rizzo G, Bertoldo A, Owen DR et al. Microglial activity in people at ultra high risk of psychosis and in schizophrenia: An [(11)C]PBR28 PET Brain Imaging Study. Am J Psychiatry 2016; 173: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta V, Cuesta MJ. Exploring the borders of the schizoaffective spectrum: a categorical and dimensional approach. J Affect Disord 2008; 108: 71–86. [DOI] [PubMed] [Google Scholar]
- Kotov R, Leong SH, Mojtabai R, Erlanger AC, Fochtmann LJ, Constantino E et al. Boundaries of schizoaffective disorder: revisiting Kraepelin. JAMA Psychiatry 2013; 70: 1276–1286. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.