Abstract
Depression is an accepted risk factor for dementia, but it is unclear if this relationship is causal. This study investigated whether dementia associated with depression decreases with antidepressant use and is independent of the time between exposure to depression and the onset of dementia. We completed a 14-year longitudinal study of 4922 cognitively healthy men aged 71–89 years, and collected information about history of past depression, current depression and severity of depressive symptoms. Other measures included use of antidepressants, age, education, smoking and history of diabetes, hypertension, coronary heart disease, and stroke. The onset of dementia and death during follow-up was ascertained via the Western Australian Data Linkage System. A total of 682 men had past (n=388) or current (n=294) depression. During 8.9 years follow-up, 903 (18.3%) developed dementia and 1884 (38.3%) died free of dementia. The sub-hazard ratios (SHRs) of dementia for men with past and current depression were 1.3 (95% confidence interval (CI)=1.0, 1.6) and 1.5 (95% CI=1.2, 2.0). The use of antidepressants did not decrease this risk. Compared to men with no symptoms, the SHRs of dementia associated with questionable, mild-to-moderate and severe depressive symptoms were 1.2 (95% CI=1.0, 1.4), 1.7 (95% CI=1.4, 2.2) and 2.1 (95% CI=1.4, 3.2), respectively. The association between depression and dementia was only apparent during the initial 5 years of follow-up. Older men with history of depression are at increased risk of developing dementia, but depression is more likely to be a marker of incipient dementia than a truly modifiable risk factor.
Introduction
Symptomatic treatments for dementia remain limited in scope and efficacy,1 and despite significant financial investment, there is no cure in sight.2 However, accumulating epidemiological data has revealed several potentially modifiable risk factors, offering hope that prevention is possible. Education, diabetes, hypertension, obesity, physical inactivity, smoking and depression account for ~30% of Alzheimer’s disease cases so that risk-modifying interventions could potentially reduce its prevalence worldwide.3 Emerging trial data indicate that this may be a strategy worth pursuing.4, 5, 6
Norton et al.3 estimated that 5–11% of Alzheimer’s disease cases could be attributed to depression. It means that the prevalence of dementia in the population would be reduced by the same amount if depression could be prevented or adequately treated. The personal and socioeconomic benefits of such a reduction would be substantial.7 This assumes, of course, that depression causes dementia. The most compelling way of demonstrating causality would be by means of a randomised controlled trial, but such a trial would not be ethical or feasible. Observational data consistently show that depression increases the risk of incident dementia,8 and there is tentative evidence for a dose-effect modulating this relationship,9, 10 particularly when the severity of symptoms increases over time.11, 12 In addition, older adults with depression show improved cognitive performance after successful treatment,13, 14 and there is some evidence that depression may lead to loss of hippocampal volume, particularly when symptoms are persistent.15 Finally, new observational data suggest that severe depression may contribute to accelerate cellular ageing, as measured by telomere length.16 Taken together, these findings imply that the association between depression and cognitive impairment is both consistent and plausible.
We used data from the Health In Men Study to investigate whether depression is likely to be causally related to incident dementia. On the basis of existing evidence, we hypothesised that the risk of incident dementia would be associated with: (1) past and current depression, (2) increasing severity of depressive symptoms and (3) lower exposure to antidepressant treatment. Finally, we hypothesised that the risk of dementia associated with depression would be independent of the duration of the follow-up period. This latter hypothesis is based on the premise that depression is not simply an early prodromal manifestation of dementia.
Materials and methods
Study design and setting
Health In Men Study is a community-based cohort study based in Western Australia.
Participants
We used the Australian Electoral Roll to recruit a sample of 12 203 men aged 65–84 years living in Perth in 1996—voting is compulsory in Australia.17 During a second wave of assessments between 2001 and 2004, 5514 participants provided information about depressive symptoms and past history of depression (details below)—592 of them had documented history of dementia (details below) or cognitive impairment (Mini-Mental State Examination score<24).18 Hence, the study sample consisted of 4922 men aged 71–89 years who were free of dementia/cognitive impairment. They were followed until the onset of dementia, death or up to 30 June 2015, whichever occurred first.
The study was conducted in accordance with the principles expressed in the Declaration of Helsinki and was approved by the Ethics Committees of the University of Western Australia and of the Department of Health of Western Australia. All men offered written informed consent.
Outcome of interest
Dementia was the primary endpoint of the study, and its onset was established through the Western Australian Data Linkage System. Western Australian Data Linkage System brings together data for all health contacts with the hospital system of Western Australia, including emergency departments, elective and non-elective admissions, mental health outpatient services, community aged care services, as well as cancer and death registries.19 Each occasion of service triggers the recording of the respective date and associated primary and up to 20 secondary diagnoses, which are coded according to the International Classification of Diseases 10th edition. The following codes indicated the diagnosis of dementia: F00, F01, F02, F03, G30, G31.0, G31.1 and G31.83. We did not examine individual types of dementia because ‘unspecified dementia’ was the most frequently recorded diagnosis. We used Western Australian Data Linkage System to exclude following up of men with a recorded diagnosis of dementia before 2001–2004, as described above. From 1 January 1979 to the 30 June 1999, the following International Classification of Diseases 9th edition codes were used for the diagnosis of dementia: 290, 294.1, 294.2, 331.0, 331.1, 331.2 and 331.82.
Other study measures
At the 2001–2004 assessment, we asked participants whether they had ‘ever been treated for an emotional or nervous illness such as depression’ (possible answers: yes/no), and considered that past depression was present when men answered in the affirmative. Participants also completed the short version of the Geriatric Depression Scale (GDS-15), and we considered that clinically significant symptoms of depression were present when the total score was 7 or greater.20 As previously described,21 participants were also grouped according to the severity of depressive symptoms: none (GDS-15=0), questionable (GDS-15 between 1 and 4), mild to moderate (GDS-15 between 5 and 9) and severe (GDS-15⩾10).
At this same point in time, participants were asked to provide a complete list of the medications they had been using on a regular basis. All medications were coded according to the Anatomical Therapeutic Chemical Classification System.22 Code N06A indicates the use of antidepressants. The codes N06AA, N06AB, N06AF and N06AX are the codes used for tricyclic, selective serotonin reuptake inhibitors, monoamine oxidase inhibitors (non-selective) and other antidepressants, respectively.
Participants provided information about their age (in years), date of birth, educational achievement (completed high school vs incomplete high school), smoking (never, past or current) and medical history. In addition, we asked them whether a doctor had ever told them that they had diabetes, hypertension, coronary heart disease or a stroke (yes/no).
Statistical analyses
We used the statistical package Stata 14.2 (StataCorp, College Station, TX, USA) to manage and analyse the data. We stratified the study population according to the history of depression (never, past only and current). We examined the distribution of study measures (counts and proportions) for the population and for depression groups, and calculated the odds ratio and respective 95% confidence interval (95% CI) relative to men who had never been depressed. As death was a competing risk of dementia, we used competing risk Cox regression models. The risk estimates are expressed as sub-hazard ratios (SHRs) and 95% CI. We repeated these same analyses by subsequently taking into account the interaction between the depression group and use of antidepressants. We then completed a series of planned analyses to investigate the effect of time of follow-up on the risk of dementia. We did this by limiting the analyses described above to a short (<5 years), medium (5 to up 10 years) and long (⩾10 years) duration of follow-up. We also investigated the SHR of dementia associated with individual groups of antidepressants: selective serotonin reuptake inhibitor, tricyclic, monoamine oxidase inhibitors (non-selective) and others. We calculated crude and adjusted associations—for the latter, we included in the models study measures associated with P<0.1. Finally, we calculated the annual rate of dementia per 1000 person-years for men with and without history of past or current depression. Alpha was set at 5% and all tests reported were two-tailed.
Computational codes used in the analysis of the data are available from the corresponding author upon request.
Sample size
The annual incidence proportion of dementia is about 53 new cases per 1000 persons.23 Using the Schoenfeld method, we estimated that the study would have 80% power to detect a hazard ratio of 1.3 or greater for a sample of 180 men with current depression and 180 men with past depression. As 38% of the sample died during follow-up, the number of participants with current and past depression would have to be at least 250 each to allow for censoring. The study included 294 men with current depression and 388 with past depression.
Results
This study included 4922 men free of dementia or clinically significant cognitive impairment. Their mean age was 77.2 years (s.d.=3.7), and their characteristics at the start of follow-up are summarised in Table 1.
Table 1. Sociodemographic and clinical characteristics of older men free of cognitive impairment at the start of the follow-up period according to their history of depression.
| Population (N=4922), n (%) | Ever depressed (N=682), n (%) | Ever depressed, OR (95% CI) | Past depression (N=388), n (%) | Past depression, OR (95% CI) | Current depression (N=294), n (%) | Current depression, OR (95% CI) | |
|---|---|---|---|---|---|---|---|
| Age group (years) | |||||||
| 70–74 | 1738 (35.3) | 222 (32.6) | 1 (Reference) | 148 (38.2) | 1 (Reference) | 74 (25.2) | 1 (Reference) |
| 75–79 | 2120 (43.1) | 292 (42.8) | 1.1 (0.9, 1.3) | 160 (41.3) | 0.9 (0.7, 1.1) | 132 (44.9) | 1.5 (1.1, 2.0) |
| 80–84 | 860 (17.5) | 131 (19.2) | 1.2 (1.0, 1.5) | 64 (16.3) | 0.9 (0.7, 1.2) | 67 (22.8) | 1.9 (1.3, 2.7) |
| ≥ 85 | 204 (4.1) | 37 (5.4) | 1.5 (1.0, 2.2) | 16 (4.1) | 1.0 (0.6, 1.7) | 21 (7.1) | 2.6 (1.5, 4.3) |
| High-school education | 2328 (47.3) | 306 (44.9) | 0.9 (0.8, 1.0) | 202 (52.1) | 1.2 (1.0, 1.5) | 104 (35.4) | 0.6 (0.5, 0.8) |
| Smoking | |||||||
| Never | 1605 (32.6) | 168 (24.6) | 1 (Reference) | 109 (28.1) | 1 (Reference) | 59 (20.1) | 1 (Reference) |
| Past | 3055 (62.1) | 467 (68.5) | 1.5 (1.3, 1.9) | 258 (66.5) | 1.3 (1.0, 1.7) | 209 (71.1) | 2.0 (1.5, 2.6) |
| Current | 258 (5.3) | 47 (7.0) | 1.9 (1.3, 2.7) | 21 (5.4) | 1.3 (0.8, 2.1) | 26 (8.8) | 3.0 (1.9, 4.9) |
| Diabetes | 740 (15.0) | 137 (20.1) | 1.5 (1.2, 1.9) | 65 (16.7) | 1.2 (0.9, 1.6) | 72 (24.5) | 2.0 (1.5, 2.6) |
| Hypertension | 2378 (48.3) | 379 (55.6) | 1.4 (1.2, 1.6) | 216 (55.7) | 1.4 (1.1, 1.7) | 163 (55.4) | 1.4 (1.1, 1.8) |
| Coronary heart disease | 1527 (31.0) | 255 (37.4) | 1.4 (1.2, 1.6) | 132 (34.0) | 1.2 (1.0, 1.5) | 123 (41.8) | 1.7 (1.3, 2.1) |
| Stroke | 636 (12.9) | 150 (22.0) | 2.2 (1.8, 2.7) | 67 (17.3) | 1.6 (1.2, 2.1) | 83 (28.2) | 3.0 (2.3, 4.0) |
| Using an antidepressant | 302 (6.1) | 201 (29.5) | 17.1 (13.2, 22.1) | 148 (38.1) | 25.3 (19.0, 33.6) | 53 (18.0) | 9.0 (6.3, 12.9) |
Abbreviations: 95% CI: 95% confidence interval of the odds ratio; OR, odds ratio.
Bold print used to highlight statistically significant associations.
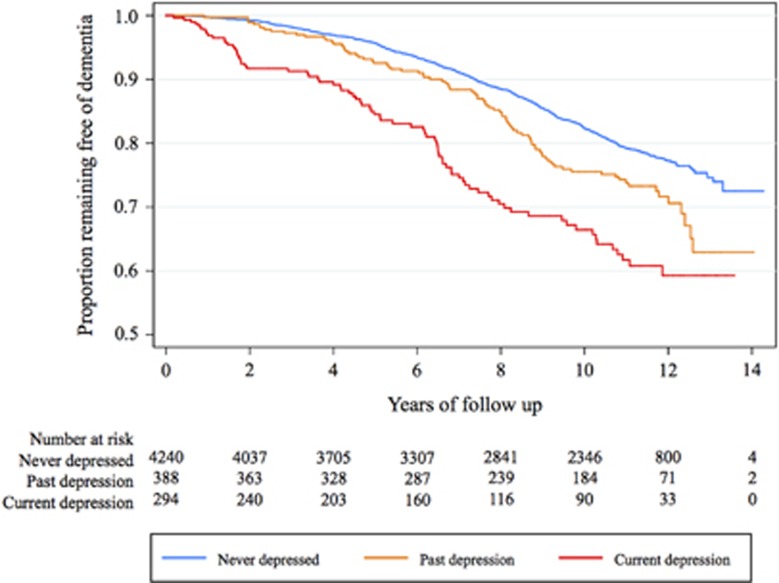
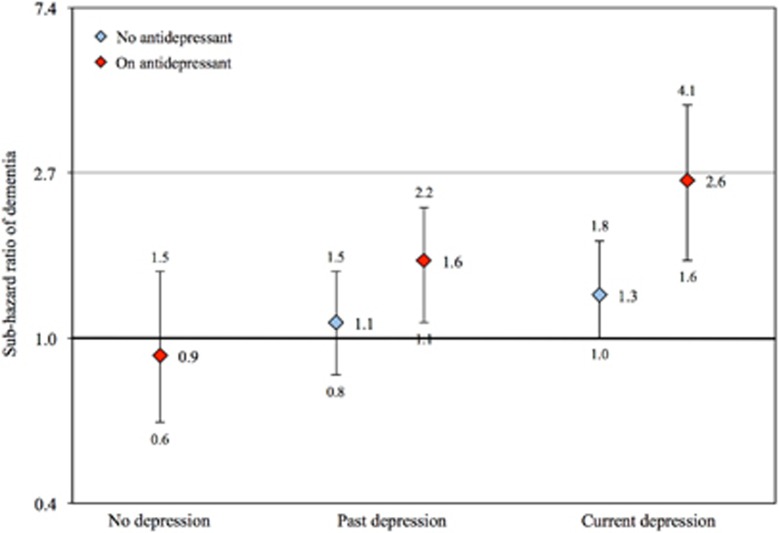
Participants were followed, on average, for 8.9 years (range: 0.02–14.3 years). During this time, 903 men received the diagnosis of dementia (18.3%) and 1884 died free of dementia (38.3%). The adjusted SHR of dementia among men who had ever been depressed (past or current) was 1.3 (95% CI=1.2, 1.7), and for men with a past history of depression only was 1.3 (95% CI=1.0, 1.6) and for men with current depression (with or without past depression) was 1.5 (95% CI=1.2, 2.0; Figure 1). 302 men (6.1%) were using antidepressants at the time of the assessment. Figure 2 shows the SHR of dementia (and respective 95% CI) according to history of depression and antidepressant use. The use of antidepressants did not decrease the risk of dementia (Figure 2). The interaction term between antidepressant use and history of depression did not reach statistical significance (P=0.120). All analyses were adjusted for age, and history of diabetes and stroke (other variables made no significant contribution to the model).
Figure 1.
The figure shows the proportion of men remaining free of dementia over 14 years according to history of depression at the start of the follow-up period. The sub-hazard ratios of dementia associated with past and with current depression were 1.4 (95% confidence interval (CI)=1.1, 1.7) and 1.8 (95% CI=1.4, 2.3).
Figure 2.
The figure shows the risk ratio of dementia over a follow-up period of up to 14 years according to history of depression and exposure to antidepressants at study entry (reference: men with no history of depression who were not using antidepressants). The diamonds show the sub-hazard ratio (SHR) of dementia and the whiskers the 95% confidence interval of the SHR. Blue and red diamonds show ratios for men not using and using antidepressants, respectively. The presentation was stratified into no, past and current depression.
We found that 1081 (22.0%) men had no depressive symptoms, 3171 (64.4%) had questionable symptoms, 509 (10.3%) had mild-to-moderate symptoms and 106 (2.1%) had severe symptoms. Fifty-five (1.1%) participants returned missing data for at least one GDS-15 item. Compared with men with no symptoms, the SHRs of dementia were 1.2 (95% CI=1.0, 1.4), 1.7 (95% CI=1.4, 2.2) and 2.1 (95% CI=1.4, 3.2) for participants with questionable, mild-to-moderate and severe depressive symptoms. Similarly, the respective adjusted SHRs were 0.2 (95% CI=0.0, 1.6) for men with no depressive symptoms on antidepressants, 1.1 (95% CI=0.9, 1.4) for men with questionable depressive symptoms not on antidepressants, 1.4 (95% CI=0.9, 2.0) for men with questionable depressive symptoms on antidepressants, 1.6 (95% CI=1.2, 2.1) for men with mild-to-moderate depressive symptoms who were not on antidepressants, 2.5 (95% CI=1.6, 3.8) for men with mild-to-moderate depressive symptoms on antidepressants, 1.5 (95% CI=0.9, 2.5) for men with severe depressive symptoms not on antidepressants and 4.8 (95% CI=2.3, 9.8) for men with severe depressive symptoms on antidepressants. The interaction between the severity of depressive symptoms and the use of antidepressants on dementia risk was statistically significant (P<0.05). All analyses were adjusted for age, and history of diabetes and stroke (all other measures made no obvious contribution to the model).
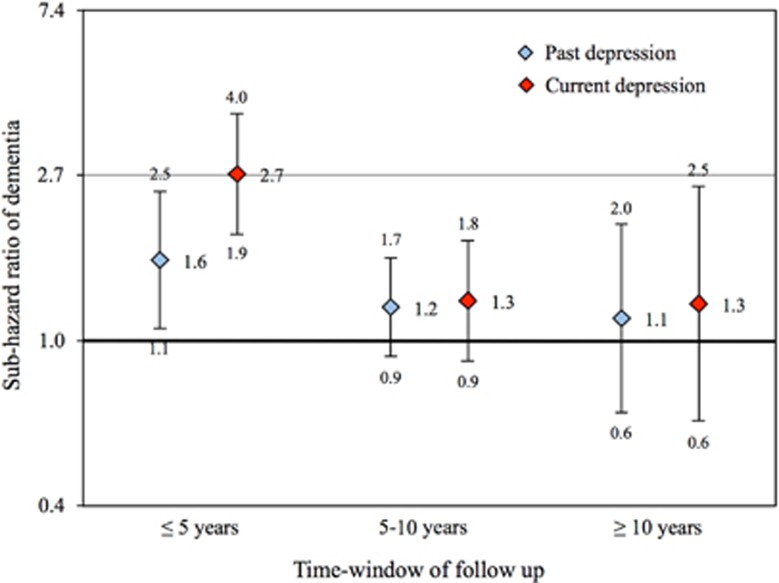
The number of men who developed dementia during the initial 5 years of follow-up was 234, from 5 to 10 years 516 and after 10 years 153. We stratified the data according to time of follow-up to investigate whether the risk of dementia associated with depression varied according to the duration of the follow-up period (Figure 3). All analyses were adjusted for age, and history of diabetes and stroke.
Figure 3.
The figure shows the risk ratio of dementia according to time of follow-up (⩽5, 5–10 and ⩾10). The diamonds show the sub-hazard ratio of dementia and the whiskers show the 95% confidence interval of the sub-hazard ratio. The blue and red diamonds depict men with past and current depression at study entry, respectively.
We then completed a series of post hoc analyses to investigate the contribution of individual antidepressant classes to dementia risk. The independent SHR of dementia associated with antidepressant use was 1.3 (95% CI=1.0, 1.7). The use of selective serotonin reuptake inhibitors, tricyclics, monoamine oxidase inhibitors (non-selective) and other antidepressants was reported by 127 (2.6%), 132 (2.7%), 8 (0.2%) and 50 (1.0%) men. The SHRs of dementia associated with the use of selective serotonin reuptake inhibitors, tricyclics, monoamine oxidase inhibitors (non-selective) and other antidepressants were 1.4 (95% CI=0.8, 2.1), 1.0 (95% CI=0.7, 1.5), 1.2 (95% CI=0.3, 5.0) and 2.2 (95% CI=1.3, 3.8). These analyses were adjusted for age, history of diabetes and stroke, and history of depression (past or current).
The annual rates of dementia per 1000 person-years for men without depression were 3.0 (95% CI=1.3, 6.5), 5.6 (95% CI=4.4, 7.2), 17.8 (95% CI=15.8, 20.1) and 41.1 (95% CI=37.3, 45.4) for ages 70–75, 75–80, 80–85 and >85 years. Similarly, for men with history of past or current depression, the respective rates were 3.1 (95% CI=0.4, 22.3), 19.2 (95% CI=13.5, 27.3), 34.2 (95% CI=27.0, 43.4) and 47.0 (95% CI=36.8, 60.1). There were 234 incidence cases of dementia during the first 5 years of follow-up (169 never depressed, 26 with history of past depression and 39 with current depression), 516 between 5 and 10 years (436 never depressed, 48 with history of past depression and 32 with current depression) and 153 after 10 years (133 never depressed, 12 with history of past depression and 8 with current depression).
Discussion
The results of this 14-year longitudinal study of older men confirmed that history of depression is associated with increased risk of incident dementia, and that this risk is particularly high among men with clinically significant symptoms of depression at the start of the follow-up period. We also found that there was a graded association between the severity of depressive symptoms and the risk of dementia, with the risk being more pronounced for men with severe depression. Contrary to our expectations, the use of antidepressants did not decrease the risk of dementia associated with depression. Finally, we found that the association between depression and incident dementia was largely due to cases of dementia accrued during the first 5 years of follow-up, after which the association between depression and incident dementia disappeared.
This project has the merit of having had access to a large and well-characterised cohort of older men and a suitably long follow-up period to ascertain the onset of dementia. Another important feature of a longitudinal study such as this is that follow-up data were available through Western Australian Data Linkage System for all participants, as the internal and external migratory movement of older Western Australians is negligible.24 The diagnosis of dementia itself, although not derived from structured clinical, neuropsychological and imaging investigations, included the best available information retrieved from hospital and community services. As the rates of dementia that we observed in our sample were largely consistent with those reported by others using different methods,25 we trust that our approach was appropriate to identify most cases of dementia. Nonetheless, we accept that some cases of dementia may have not been detected. If the distribution of these cases was random, some loss of power would have ensued, but not bias. It is conceivable, however, that the detection of cases may have occurred more frequently among men with depression because they come to medical attention more frequently than men without depression26—this would have increased the opportunity for the diagnosis of dementia to be established. Such bias could have inflated the number of cases of dementia associated with depression, so that the real association between depression and dementia could be less pronounced than our numbers suggest.
We also acknowledge that our assessment of depression was not based on a gold standard structured clinical interview, but on self-report and a depression scale. However, both approaches have good face-validity.20, 27 Perhaps, the most obvious limitation of our study design was the lack of information about the onset and recurrence of depressive symptoms and the use of antidepressants during follow-up. A considerable number of depression cases in later life are due to recurrence, but incident cases do occur particularly in people aged in their 80s.28 Such new false-negative cases of depression would have increased the chance of type II error and, potentially, would have led to an underestimation of the effect size of the association between depression and incident dementia. In addition, the lack of information about the use of medications during follow-up and their use as maintenance therapy creates uncertainty about the potential effect of antidepressants in modifying dementia risk. Confounding by indication is another issue that should be considered, as antidepressants may have been prescribed preferentially to the more severe cases of depression. However, it is helpful to note that only ~30% of antidepressant users suffer from depression (past or current),29 which together with our finding that the use of antidepressants by men without depression had no observable beneficial effect on the risk of dementia, suggest that the overall results of our study are likely to offer an acceptable, although tentative, estimate of risk associated with depression and antidepressant use.
Another potential caveat of the study is the lack of information about the timeline associated with history of past depression. It is conceivable that depression with onset in early or mid-life could contribute to modulate the risk of dementia later in life, whereas depression arising in older age may be more frequently an early manifestation of an underlying neurodegenerative process. As we did not have access to information about the age of onset of symptoms, we cannot be entirely certain that depression with early and late onset do not differ in their relationship with dementia risk.
It is also important to consider the issue of bias associated with competing risks. When assessing an event that occurs late in life (such as dementia),25 premature death will remove from the sample people who could have developed dementia later, if they had survived. This may be particularly important in this case, as people with depression die earlier than those without.21 The early censoring of older men with depression could have led to a biased lower risk of dementia among older men with history of depression. For this reason, and in contrast with previous studies,8 we used a competing risk Cox regression model that took into account the competing risk of death and the potential confounding associated with the overlapping risk factors for depression and dementia.3, 30 This approach to the analysis of the data enhances our confidence that our findings are most likely valid, although they are limited to men and cannot be generalised to women. Furthermore, we concede that some unmeasured factors, such as apolipoprotein E genotype and lipids, were not taken into account in our analyses, although findings from other relevant studies are consistent with ours.11, 12, 31
Like previous studies,8 we found that history of past or current depression increased the risk of dementia, but this relationship is unlikely to be causal. Treatment with antidepressants, despite less than ideal efficacy,32, 33 would have been expected to reduce the risk of dementia associated with depression—that did not occur. Moreover, the association between depression and dementia was limited to the first few years of follow-up, suggesting that depression may represent a prodromal manifestation of dementia rather than one of its causes. Data from the Rotterdam Study are consistent with this interpretation: 4393 older adults (mean age 73 years) were followed for up to 13.7 years for incident dementia, which was diagnosed according to the Diagnostic and Statistical Manual for Mental Disorders 3rd edition revised criteria.34 They found that 13% of them developed dementia and that depression increased the hazard of dementia by ~20% by 2 and 5 years, but not beyond that time point.34 In fact, depressive symptoms are common antecedents of several neurodegenerative disorders35, 36 and could be seen as an early non-specific behavioural manifestation of a brain under physiological stress. If that is the case, depression in later life may be better understood, at least in some cases, as an early sign of dementia rather than a factor that can be potentially modified to decrease future risk. There is evidence from longitudinal studies of depression that this may indeed be the case.13, 14 However, our data cannot dismiss the possibility that depressive disorder with onset in earlier life may be a potentially modifiable risk factor for dementia.
In conclusion, our findings indicate that older men with the history of depression are at increased risk of developing dementia, although depression in later life is more likely to be a marker of incipient dementia than a truly modifiable risk factor. Older people with depression may be better viewed as potential targets of indicated prevention strategies,37 rather like people with mild cognitive impairment.
Acknowledgments
We thank study participants for their generous contribution. The Health In Men Study has been funded by the following competitive project grants from the National Health and Medical Research Council of Australia: 279408, 379600, 403963, 513823, 540403, 540504, 540405, 634492, 1021416, 1045710 and 1060557.
Author contributions
OPA conceived and designed the experiments, which were performed by all authors. All authors contributed to obtain funding for this project. OPA analysed the data and drafted the manuscript. All authors edited the manuscript for important intellectual content and approved its submission to the journal. OPA acts as the guarantor of the data reported in this manuscript.
Disclaimer
The sponsors had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; or preparation, review or approval of the manuscript.
Footnotes
The authors declare no conflict of interest.
References
- Tan CC, Yu JT, Wang HF, Tan MS, Meng XF, Wang C et al. Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimers Dis 2014; 41: 615–631. [DOI] [PubMed] [Google Scholar]
- Salomone S, Caraci F, Leggio GM, Fedotova J, Drago F. New pharmacological strategies for treatment of Alzheimer’s disease: focus on disease modifying drugs. Br J Clin Pharmacol 2012; 73: 504–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol 2014; 13: 788–794. [DOI] [PubMed] [Google Scholar]
- Lautenschlager NT, Cox KL, Flicker L, Foster JK, van Bockxmeer FM, Xiao J et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA 2008; 300: 1027–1037. [DOI] [PubMed] [Google Scholar]
- Ngandu T, Lehtisalo J, Solomon A, Levalahti E, Ahtiluoto S, Antikainen R et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015; 385: 2255–2263. [DOI] [PubMed] [Google Scholar]
- Moll van Charante EP, Richard E, Eurelings LS, van Dalen JW, Ligthart SA, van Bussel EF et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet 2016; 388: 797–805. [DOI] [PubMed] [Google Scholar]
- Prince M, Wilmo A, Guerchet M, Ali G, Wu Y, Prina M World Alzheimer Report 2015: the Global Impact of Dementia: an Analysis of Prevalence, Incidence, Cost and Trends. Alzheimer’s Disease International: London, 2015. [Google Scholar]
- Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF 3rd. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry 2013; 202: 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracia-Garcia P, de-la-Camara C, Santabarbara J, Lopez-Anton R, Quintanilla MA, Ventura T et al. Depression and incident Alzheimer disease: the impact of disease severity. Am J Geriatr Psychiatry 2015; 23: 119–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeki Al Hazzouri A, Vittinghoff E, Byers A, Covinsky K, Blazer D, Diem S et al. Long-term cumulative depressive symptom burden and risk of cognitive decline and dementia among very old women. J Gerontol A Biol Sci Med Sci 2014; 69: 595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza SS, Wolters FJ, Swanson SA, Koudstaal PJ, Hofman A, Tiemeier H et al. 10-year trajectories of depressive symptoms and risk of dementia: a population-based study. Lancet Psychiatry 2016; 3: 628–635. [DOI] [PubMed] [Google Scholar]
- Kaup AR, Byers AL, Falvey C, Simonsick EM, Satterfield S, Ayonayon HN et al. Trajectories of depressive symptoms in older adults and risk of dementia. JAMA Psychiatry 2016; 73: 525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters MA, Becker JT, Nebes RD, Zmuda MD, Mulsant BH, Pollock BG et al. Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry 2000; 157: 1949–1954. [DOI] [PubMed] [Google Scholar]
- Beats BC, Sahakian BJ, Levy R. Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychol Med 1996; 26: 591–603. [DOI] [PubMed] [Google Scholar]
- Taylor WD, McQuoid DR, Payne ME, Zannas AS, MacFall JR, Steffens DC. Hippocampus atrophy and the longitudinal course of late-life depression. Am J Geriatr Psychiatry 2014; 22: 1504–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven JE, Revesz D, Epel ES, Lin J, Wolkowitz OM, Penninx BW. Major depressive disorder and accelerated cellular aging: results from a large psychiatric cohort study. Mol Psychiatry 2014; 19: 895–901. [DOI] [PubMed] [Google Scholar]
- Norman PE, Flicker L, Almeida OP, Hankey GJ, Hyde Z, Jamrozik K. Cohort Profile: The Health In Men Study (HIMS). Int J Epidemiol 2009; 38: 48–52. [DOI] [PubMed] [Google Scholar]
- Crum RM, Anthony JC, Bassett SS, Folstein MF. Population-based norms for the Mini-Mental State Examination by age and educational level. JAMA 1993; 269: 2386–2391. [PubMed] [Google Scholar]
- Holman CD, Bass AJ, Rosman DL, Smith MB, Semmens JB, Glasson EJ et al. A decade of data linkage in Western Australia: strategic design, applications and benefits of the WA data linkage system. Aust Health Rev 2008; 32: 766–777. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry 1999; 14: 858–865. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Alfonso H, Hankey GJ, Flicker L. Depression, antidepressant use and mortality in later life: the Health In Men Study. PLoS ONE 2010; 5: e11266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Collaborating Centre for Drug Statistics MethodologyGuidelines for ATC Classification and DDD Assignment 2013. WHO Collaborating Centre for Drug Statistics Methodology: Oslo, Norway, 2012. [Google Scholar]
- Fiest KM, Jette N, Roberts JI, Maxwell CJ, Smith EE, Black SE et al. The Prevalence and Incidence of Dementia: a Systematic Review and Meta-analysis. Can J Neurol Sci 2016; 43 Suppl 1: S3–S50. [DOI] [PubMed] [Google Scholar]
- ABS3412.0 - Migration, Australia, 2013-14. Australian Bureau of Statistics: Canberra, 2015. [Google Scholar]
- Matthews FE, Stephan BC, Robinson L, Jagger C, Barnes LE, Arthur A et al. A two decade dementia incidence comparison from the Cognitive Function and Ageing Studies I and II. Nat Commun 2016; 7: 11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prina AM, Huisman M, Yeap BB, Hankey GJ, Flicker L, Brayne C et al. Association between depression and hospital outcomes among older men. CMAJ 2013; 185: 117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart AL, Pasco JA, Jacka FN, Brennan SL, Berk M, Williams LJ. Comparison of self-report and structured clinical interview in the identification of depression. Compr Psychiatry 2014; 55: 866–869. [DOI] [PubMed] [Google Scholar]
- Blazer DG. Depression in late life: review and commentary. J Gerontol A Biol Sci Med Sci 2003; 58: 249–265. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Spira AP, Bienvenu OJ, Hock RS, Carras MC, Eaton WW et al. Antidepressant use and lifetime history of mental disorders in a community sample: results from the Baltimore Epidemiologic Catchment Area Study. J Clin Psychiatry 2015; 76: 40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida OP, Alfonso H, Pirkis J, Kerse N, Sim M, Flicker L et al. A practical approach to assess depression risk and to guide risk reduction strategies in later life. Int Psychogeriatr 2011; 23: 280–291. [DOI] [PubMed] [Google Scholar]
- Dal Forno G, Palermo MT, Donohue JE, Karagiozis H, Zonderman AB, Kawas CH. Depressive symptoms, sex, and risk for Alzheimer’s disease. Ann Neurol 2005; 57: 381–387. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Ford AH, Hirani V, Singh V, vanBockxmeer FM, McCaul K et al. B vitamins to enhance treatment response to antidepressants in middle-aged and older adults: results from the B-VITAGE randomised, double-blind, placebo-controlled trial. Br J Psychiatry 2014; 205: 450–457. [DOI] [PubMed] [Google Scholar]
- Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ, Johnson BT. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med 2008; 5: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza SS, de Bruijn RF, Direk N, Hofman A, Koudstaal PJ, Ikram MA et al. Depressive symptoms predict incident dementia during short- but not long-term follow-up period. Alzheimers Dement 2014; 10: S323–S329, e321. [DOI] [PubMed] [Google Scholar]
- van Duijn E, Craufurd D, Hubers AA, Giltay EJ, Bonelli R, Rickards H et al. Neuropsychiatric symptoms in a European Huntington’s disease cohort (REGISTRY). J Neurol Neurosurg Psychiatry 2014; 85: 1411–1418. [DOI] [PubMed] [Google Scholar]
- Leentjens AF, Van den Akker M, Metsemakers JF, Lousberg R, Verhey FR. Higher incidence of depression preceding the onset of Parkinson’s disease: a register study. Mov Disord 2003; 18: 414–418. [DOI] [PubMed] [Google Scholar]
- Almeida OP. Prevention of depression in older age. Maturitas 2014; 79: 136–141. [DOI] [PubMed] [Google Scholar]