Abstract
Schizophrenia (SZ) is considered to be a multifactorial brain disorder with defects involving many biochemical pathways. Patients with SZ show variable responses to current pharmacological treatments of SZ because of the heterogeneity of this disorder. Stress has a significant role in the pathophysiological pathways and therapeutic responses of SZ. Atypical antipsychotic drugs (AAPDs) can modulate the stress response of the hypothalamic–pituitary–adrenal (HPA) axis and exert therapeutic effects on stress by targeting the prefrontal cortex (PFC) and hippocampus. To evaluate the effects of AAPDs (such as clozapine, risperidone and aripiprazole) on stress, we compared neurochemical profile variations in the PFC and hippocampus between rat models of chronic unpredictable mild stress (CUMS) for HPA axis activation and of long-term dexamethasone exposure (LTDE) for HPA axis inhibition, using an ultraperformance liquid chromatography–mass spectrometry (UPLC–MS/MS)-based metabolomic approach and a multicriteria assessment. We identified a number of stress-induced biomarkers comprising creatine, choline, inosine, hypoxanthine, uric acid, allantoic acid, lysophosphatidylcholines (LysoPCs), phosphatidylethanolamines (PEs), corticosterone and progesterone. Specifically, pathway enrichment and correlation analyses suggested that stress induces oxidative damage by disturbing the creatine–phosphocreatine circuit and purine pathway, leading to excessive membrane breakdown. Moreover, our data suggested that the AAPDs tested partially restore stress-induced deficits by increasing the levels of creatine, progesterone and PEs. Thus, the present findings provide a theoretical basis for the hypothesis that a combined therapy using adenosine triphosphate fuel, antioxidants and omega-3 fatty acids as supplements may have synergistic effects on the therapeutic outcome following AAPD treatment.
Introduction
Stress has a powerful effect on the brain and body, and a significant role in the development and course of mental illness.1 Specifically, stress has been related to the development of schizophrenia (SZ) and its potential therapeutic targets.2, 3, 4, 5 The hypothalamic–pituitary–adrenal (HPA) axis is central to the stress response. Both hyper- and hypofunction of the HPA axis have been linked to presentations of SZ, such as first-episode psychosis, acute or clinically stable chronic psychosis, and so on.6
Despite great interest in this area of research, the mechanisms of the effects of antipsychotic medication, especially atypical or second-generation drugs, on stress and the relation to their pharmacological effects are not fully understood.6 To date, the data from available animal models cannot fully explain the manifestations of SZ.7 Nevertheless, endocrine and neuroimaging markers are often used to evaluate HPA axis activity in humans.6, 8 A previous study suggested that atypical antipsychotic drugs (AAPDs) can mediate a nonspecific inhibition of stress-induced activation of the HPA axis after achieving symptom relief in acute psychosis or a direct pharmacological effect on cortisol secretion.6 By contrast, such an effect is minimal in healthy subjects.9 Other biochemical and neuroimaging studies have also suggested that AAPDs increase the activity of the HPA axis following long-term treatment.10, 11, 12 In short, whether AAPDs are able to influence the function of the stress axis requires further investigation, especially because SZ is a heterogeneous brain disease involving abnormalities from multiple biochemical pathways, and none of the current treatments is fully beneficial to all SZ patients.
In the brain, the prefrontal cortex (PFC) and hippocampus have been shown to modulate the HPA axis to produce negative feedback regulating glucocorticoid release during the stress response.13 The PFC and hippocampus are also the two most-vulnerable brain regions in response to stress.13 Several stress-induced increases in adrenal glucocorticoid hormones have been shown to contribute to the pathophysiology of SZ.14, 15 Although the HPA axis is not a direct target of AAPDs, both the PFC and hippocampus regions are intimately involved in the action of AAPDs16 and are linked to HPA axis functioning.17, 18
Biomarkers are increasingly needed to predict therapeutic outcomes in the treatment of SZ and other psychotic disorders.19 Understanding the systemic metabolic effects of AAPDs on stress and identifying specific biomarkers will enhance our current knowledge of their pharmacological actions in SZ. We hypothesized that there are multiple targets for therapeutic efficacy in the stress-related metabolic pathways following AAPD treatment. Compared with that offered by biofluids, such as plasma and urine, which can reveal the overall metabolic state of a given organism, target tissue samples can offer a unique perspective on localized metabolic information, which will yield knowledge that is most relevant to the activity of the stress axis.20, 21 In the present study, using a metabolomic approach, AAPD effects on the stress-related regions of interest were investigated in the PFC and hippocampus from rats subchronically treated with the AAPDs clozapine (CLO), risperidone (RIS) and aripiprazole (ARI). In addition, the chronic unpredictable mild stress (CUMS)22 and long-term dexamethasone exposure (LTDE) rat models23 served as metabotypes of stress axis activation and inhibition, respectively. Using multivariate and univariate statistics to extract the shared and unique features of the metabolic signatures from rats exposed to AAPDs, CUMS and LTDE, we were able to identify potential biomarkers implicated in stress-related metabolic pathways.
Materials and methods
Animals
The study was approved by the Ethics Committee of the Central South University, and all of the experimental procedures conformed to the Declaration of Helsinki and local guidelines. A total of 42 male Sprague–Dawley rats (weighing 150–200 g) were used in this study and randomized into six groups: normal control (NC), CUMS, LTDE, CLO, RIS and ARI. The procedure for carrying out the animal experiments was in accordance with our previously published work24, 25 and is described in detail in the Supplementary Methods.
Sample preparation, UPLC–MS/MS assay, data acquisition and pretreatment
Both the left and right sides of the PFC and hippocampus in each rat were homogenized. The tissue samples were harvested and handled according to a previously published protocol21 and as indicated in Supplementary Figure S1 of the Supplementary Methods. The method used for metabolomic profiling was conducted as previously described,26 but with minor adjustments (details in the Supplementary UPLC–MS/MS assay).
Multivariate and univariate statistics
The three-dimensional data matrix compiled following pretreatment was subsequently analyzed using supervised multivariate statistics to extract useful information. A partial least square-discriminant analysis (PLS-DA) was performed using SIMCA-P v12.01 software (Umetrics, Umea, Sweden).
The variable selection procedure was based on a modified multicriteria assessment (MCA) strategy.20 Herein, the MCA was applied to narrow down and explore those variables that were most sensitive to the interventions, using a combination of the variable importance in the projection (VIP) statistic, the correlation coefficient (p(corr)) of the S-plot and the jack-knife-based confidence interval (CIJFjk). Finally, we selected those variables that satisfied the threefold criteria (that is, VIP>5.0, |p(corr)|>0.6, and the span of CIJFjk excluding zero) as the most significant and reliable variables that could serve as candidate biomarkers.
A nonparametric Kruskal–Wallis one-way analysis of variance followed by pairwise multiple comparisons were performed to estimate the difference in biomarker levels among the groups. The significance level was set at P<0.05. Given that the aim of this work was to find stress-induced biomarkers, only those with a distinct response were considered. That is, compared with that observed in the NC group, the stress-induced biomarkers should exhibit tendencies to change in an opposite direction in the LTDE versus the CUMS group. When the metabolites were compared across all groups, those that presented the same trend (that is, were either decreased or increased in all groups analyzed) or showed a nonsignificant trend after univariate analyses were further excluded. The final selected biomarkers were identified using previously established procedures.26
Two-tailed Spearman rank correlation analyses were performed to examine the relation between different categories of biomarkers with a significance level of P<0.05. Moreover, the total list of metabolites was also analyzed using the bioinformatic tool Metabolites Biological Role (MBRole; http://csbg.cnb.csic.es/mbrole/) to identify over-represented or enriched biological pathways that could be putatively active (P<0.05 was considered significant).27
All statistical analyses were performed blindly without knowledge of the origin of the samples. The schematic flowchart of the metabolic profiling and biomarker selection is illustrated in Supplementary Figure S3 of the Supplementary UPLC–MS/MS assay.
Results
Multivariate analysis of UPLC–MS/MS data
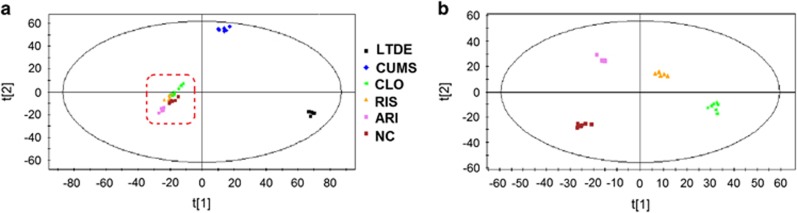
Metabolites from aqueous or organic extracts were identified by both positive and negative ion modes of mass spectrometry. For illustration, the score plots of related PLS-DA models projecting all six groups were initially obtained for the metabolic profile from the positive ion mode ultraperformance liquid chromatography–mass spectrometry (UPLC–MS/MS) data set derived from the aqueous extracts of the PFC tissue samples. As depicted by the PLS-DA model (Figure 1a), CUMS and LTDE metabolic profiles showed distinct separation from each other, whereas the AAPD and NC groups clustered in the lower left area. However, after amplifying the dotted region in Figure 1a, the inherent metabolic differences among the CLO, RIS, ARI and NC groups could be readily visualized (Figure 1b). The amplified score plot showed that the AAPD groups were clearly separate from the NC group, and CLO was the group that was the most remote (Figure 1b).
Figure 1.
Partial least square-discriminant analysis (PLS-DA) modeling of ultraperformance liquid chromatography–mass spectrometry (UPLC–MS/MS) spectral data derived from aqueous extracts of prefrontal cortex samples in the positive ion mode. (a) All seven groups; (b) atypical antipsychotic drug (AAPD) groups versus normal control (NC). ARI, aripiprazole; CLO, clozapine; CUMS, chronic unpredictable mild stress; LTDE, long-term dexamethasone exposure; RIS, risperidone.
Data mining for biomarker discovery
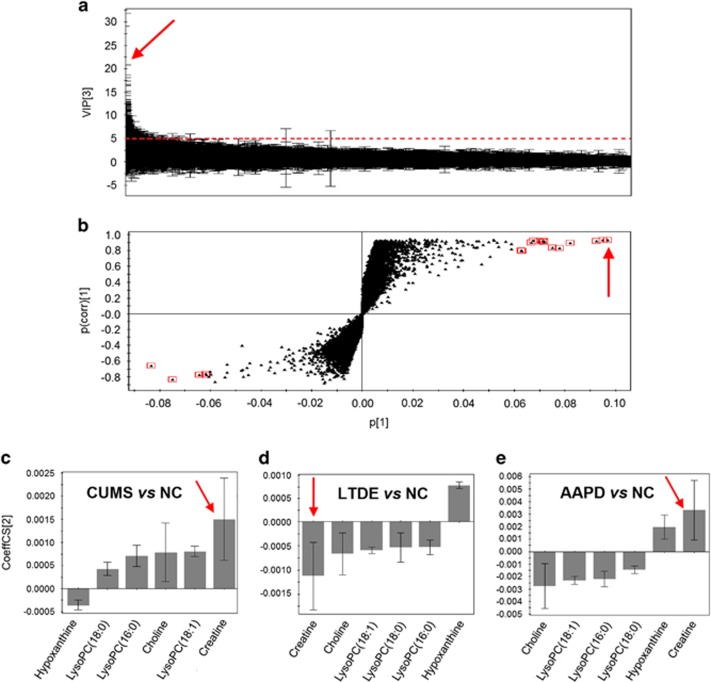
To identify which variables were accountable for the significant separation described above, an MCA strategy was initially used to preselect stress-induced biomarkers (Figure 2). According to the criterion for the VIP statistics (VIP>5.0), a total of 194 retention and m/z pairs were obtained for their contribution to discriminate the metabolic profiles among the CUMS, LTDE, AAPD and NC groups (Figure 2a). Subsequently, using the MCA strategy in which the variables met all three criteria, including VIP>5.0, |p(corr)|>0.6 (Figure 2b), and the span of CIJFjk excluding zero, we were able to reduce the 194 variables down to six representing individual metabolites (that is, creatine, choline, hypoxanthine, lysophosphatidylcholine (LysoPC; 16:0), LysoPC (18:1) and LysoPC (18:0)). These six metabolites could be considered as potential biomarkers. Specifically, creatine labeled with a red arrow in the VIP plot, S-plot and loading plot with CIJFjk (Figure 2c–e) exhibited the top VIP value of 20.7, the highest positive correlation coefficient (p(corr)=0.93), and a small confidence interval that did not cross zero. Thus, the biological significance of creatine deserves further investigation.
Figure 2.
Multicriteria strategy for the selection of stress-induced biomarkers. (a) Value of importance (VIP) plot, (b) S-plot and (c–e) loading plots with the jack-knife confidence interval (CIJFjk). Specifically, creatine is labeled with a red arrow in these plots. For further interpretation, please see the Results section. AAPD, atypical antipsychotic drugs; CUMS, chronic unpredictable mild stress; LTDE, long-term dexamethasone exposure.
Metabolic profiling further revealed the 13 most significant stress-induced biomarkers in the PFC after applying MCA strategies and univariate analyses (Table 1). Among them, creatine, progesterone and phosphatidylethanolamines (PE (16:0/22:6) and PE (18:0/22:6)) were also identified in the hippocampus and showed the same pattern of changes as that observed in the PFC (Supplementary Table S2 in Supplementary Data Analysis). The relative intensities of these biomarkers after normalization are provided in Supplementary Table S3 in the Supplementary Data Analysis.
Table 1. The stress-induced biomarkers identified by UPLC–MS/MS in prefrontal cortex among different groups and their change trends.
|
Retention (min) |
m/z |
Metabolitesa | P-valueb |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Aqueous | Organic | Positive | Negative | CUMS versus NC | LTDE versus NC | CLO versus NC | RIS versus NC | ARI versus NC | |
| 0.4 | — | 103.8 | — | Choline | 0.003↑ | 0.0001↓ | ns | ns | ns |
| 0.5 | — | 131.8 | — | Creatine | 0.033↑ | 0.020↓ | 0.001↑ | 0.001↑ | 0.003↑ |
| 0.7 | — | 136.9 | — | Hypoxanthine | 0.045↓ | 0.001↑ | ns | ns | ns |
| 0.6 | — | — | 167.1 | Uric acid | 0.004↓ | 0.045↑ | ns | ns | ns |
| 0.7 | — | — | 267.1 | Inosine | 0.023↓ | 0.010↑ | 0.006↑ | ns | ns |
| 0.8 | — | — | 174.9 | Allantoic acid | 0.002↑ | 0.043↓ | ns | ns | ns |
| 9.7 | — | 496.5 | 540.5 | LysoPC(16:0) | 0.033↑ | 0.001↓ | ns | ns | ns |
| 10.1 | — | 522.5 | 566.5 | LysoPC(18:1) | 0.045↑ | 0.001↓ | ns | ns | ns |
| 10.6 | — | 524.5 | 568.5 | LysoPC(18:0) | 0.031↑ | 0.001↓ | ns | ns | ns |
| — | 0.6 | 347.5 | — | Corticosterone | 0.025↑ | 0.001↓ | 0.023↓ | ns | ns |
| — | 0.7 | 315.4 | — | Progesterone | 0.027↓ | 0.0001↑ | 0.023↑ | ns | ns |
| — | 8.6 | — | 762.9 | PE(16:0/22:6) | 0.012↓ | 0.029↑ | 0.010↑ | 0.026↑ | ns |
| — | 10.9 | — | 790.7 | PE(18:0/22:6) | 0.001↓ | 0.014↑ | 0.005↑ | ns | ns |
Abbreviations: ↓, decreased; ↑, increased; ARI, aripiprazole; CLO, clozapine; CUMS, chronic unpredicatable mild stress; LTDE, long-term dexamethasone exposure; LysoPC, lysophosphatidylcholine; NC, normal control; ns, not significant; PE, phosphatidylethanolamine; RIS, risperidone; UPLC–MS/MS, ultraperformance liquid chromatography-tandem mass spectrometry.
Metabolites responding to atypical antipsychotics are bolded.
Nonparametric Kruskal–Wallis one-way analysis of variance followed by pairwise multiple comparisons.
Of these above-mentioned stress-induced biomarkers, six metabolites responded to AAPDs, especially to CLO (in bold in Table 1). In response to treatment with CLO, RIS or ARI, there was a common feature of increased levels of creatine in the PFC. Concomitantly, progesterone and PEs were also increased after treatment with CLO or RIS, but not with ARI. Similar effects of these AAPDs on stress-induced biomarkers were also found in the hippocampus (Supplementary Table S2 in the Supplementary Data Analysis).
Inter-relation of stress-induced biomarkers
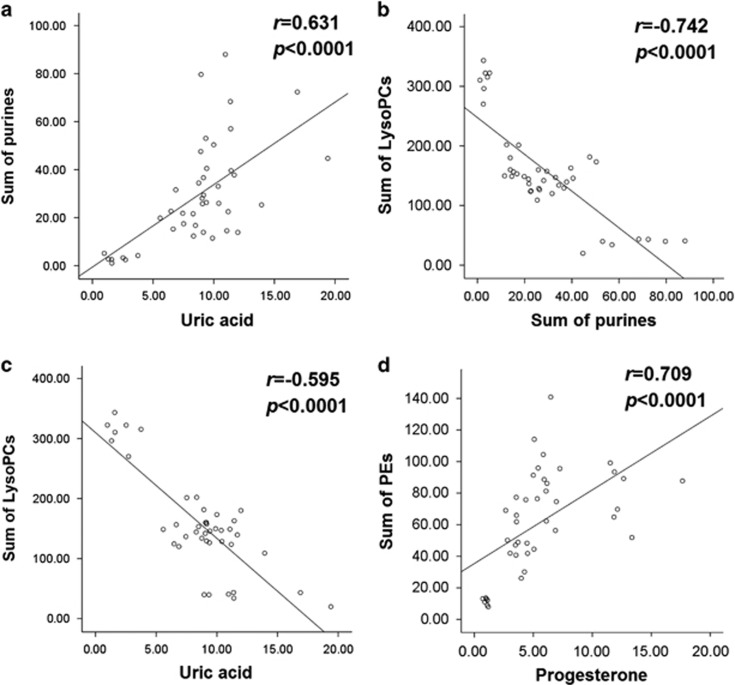
To assess whether there were links among the different metabolites from purine signaling, membrane phospholipids and neurosteroid catabolism, we performed correlation analyses among specific candidate biomarkers in the PFC (Figure 3). First, uric acid was positively correlated with the sum of inosine and hypoxanthine (Figure 3a) and negatively associated with the sum of LysoPCs (Figure 3c). Second, the sum of purine metabolites (inosine and hypoxanthine) was inversely correlated with the sum of LysoPCs (16:0, 18:0 and 18:1; Figure 3b). Third, progesterone was positively correlated with the sum of PEs (16:0/22:6 and 18:0/22:6; Figure 3d). The positive correlation between progesterone and the sum of PEs was also present in the hippocampus (Supplementary Figure S5 in the Supplementary Data Analysis).
Figure 3.
Spearman rank correlations between different categories of biomarkers in the prefrontal cortex (PFC). (a) The sum of purines (inosine and hypoxanthine) was positively correlated with uric acid in the PFC. (b) The sum of purines was negatively associated with the sum of lysophosphatidylcholines (LysoPCs 16:0, 18:0 and 18:1). (c) There was a negative correlation between uric acid and the sum of LysoPCs. (d) There was a positive association between progesterone and the sum of phosphatidylethanolamines (PEs).
Cellular pathway enrichment analysis was performed with MBRole. We summarized the disturbed stress-related metabolic pathways revealed by the Kyoto Encyclopedia of Genes and Genomes (KEGG; http://www.genome.jp/kegg/) and the Small Molecule Pathway Database (SMPDB; http://smpdb.ca/). As listed in Supplementary Table S4 of the Supplementary Data Analysis, the following six metabolites were associated with glycerophospholipid metabolism (P=0.000352): choline, LysoPC (16:0), LysoPC (18:1), LysoPC (18:0), PE (16:0/22:6) and PE (18:0/22:6). The following four metabolites were associated with purine metabolism (P=0.000138): inosine, hypoxanthine, uric acid and allantoic acid. Metabolites associated with steroid hormone biosynthesis (progesterone and corticosterone; P=0.0392) and glycine, serine and threonine metabolism (creatine and choline; P=0.0104) were also found. Purine metabolism (P=0.0103), phospholipid metabolism (P=0.0166) and steroidogenesis (P=0.0448) were also identified with the SMPDB.
Discussion
Previous studies have reported the roles of mitochondrial dysfunction,28 purine catabolism disturbances,29 membrane phospholipid abnormalities30 and neurosteroid biosynthesis dysregulation31 in the pathophysiology of SZ. Herein, by metabolomic mapping of stress-induced biomarkers, we provide multiple novel targets for a combined therapy for the treatment of SZ.
Initial energy deficiency: an imbalanced creatine–phosphocreatine circuit
The primary physiological function of creatine is to buffer the energy supply in tissues with significant and fluctuating energy demands, especially muscles and the brain. It has become increasingly evident that endogenous creatine has a pivotal role in a range of cognitive functions, including learning, memory, attention, speech and language, and possibly emotion.32 Recently, evidence of alterations in brain total creatine (creatine plus phosphocreatine) in psychiatric disorders has been provided by studies in various brain regions in vivo.33 However, previous studies in the brain have shown no consistent pattern of abnormalities in total creatine in SZ.34 Therefore, the dynamic transformation between creatine and phosphocreatine, rather than their total amount, might be more indicative of the pathogenesis of creatine abnormalities.
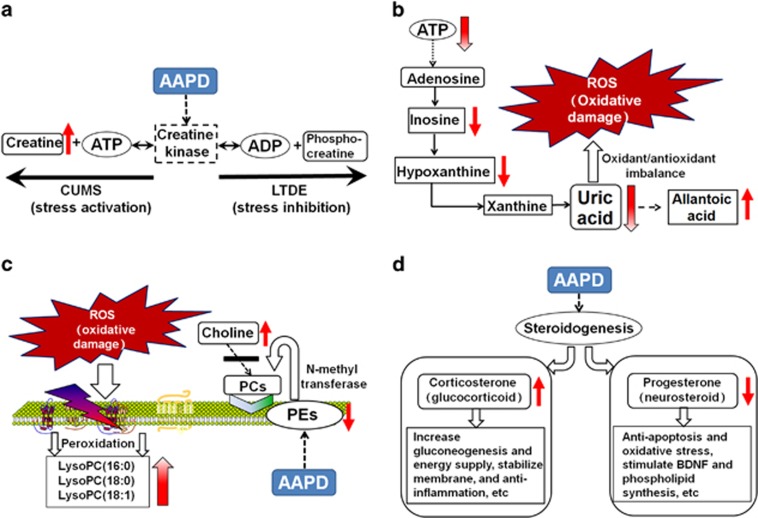
In the current study, we found significantly increased creatine levels in the PFC and hippocampus in response to CUMS, which suggests a compensatory mechanism. This mechanism implies that stress in SZ consumes more energy than usual in these brain regions and leads to the conversion of phosphocreatine to creatine to generate additional adenosine triphosphate (ATP; Figure 4a). Most ATP synthesis occurs during aerobic cellular respiration, which starts with glycolysis.32 Unfortunately, these complex, multistep metabolic pathways require time and energy. To this end, the creatine–phosphocreatine circuit could be considered to be a bioenergetics thermostat that quickly replenishes ATP in tissue to maintain stable levels when there are sudden and significant energy demands.32 Under long-term stress, mitochondrial dysfunction might arise when reserved phosphocreatine is repeatedly depleted and, thus, is no longer available to complement ATP. Instead, the cell has to shift back to the less-efficient glycolysis pathway to satisfy its energy needs, which could set the stage for a cascade of events causing related pathology (that is, energy shortage and altered phospholipid metabolism) in the brain.35
Figure 4.
The perturbed stress-related metabolic pathways and responses to AAPD treatment. (a) An imbalanced creatine–phosphocreatine circuit results in an inability to satisfy the demand for ATP; (b) insufficient ATP leads to abnormal purine metabolism and oxidative stress; (c) peroxidation and biosynthetic dysfunction of membrane phospholipids, and (d) altered steroidogenesis by stress and subsequent changes in multiple arrays of physiological function. AAPD, atypical antipsychotic drug; ADP, adenosine diphosphate; ATP, adenosine triphosphate; BDNF, brain-derived neurotrophic factor; CUMS, chronic unpredictable mild stress; LTDE, long-term dexamethasone exposure; LysoPC, lysophosphatidylcholine; PC, phosphatidylcholine; PE, phosphatidylethanolamine; ROS, reactive oxidative species.
Insufficient ATP leading to a homeostatic imbalance of purine catabolism and oxidative stress
Impaired brain energy metabolism and mitochondrial dysfunction are among the plausible hypotheses for the pathogenesis of SZ.28 Given that psychosocial stress and abnormalities in stress axis function occur at different clinical stages of SZ, they are frequently considered to be the precipitating factors.2 Similarly, the results of the current study also indicated that stress can deprive the energy supply of ATP by compromising creatine–phosphocreatine shuttling, which could provide a link between stress and SZ pathology. Interestingly, another set of aqueous metabolites affected by stress in the two brain regions studied of the CUMS and LTDE rats included, but were not limited to, inosine, hypoxanthine, uric acid and allantoic acid. This is the first report, to our knowledge, showing disturbed purine metabolism in brains affected by stress. These biomarkers are involved in the purine pathway, indicating that stress-induced ATP deficiency and the disturbance of purine metabolism are tightly integrated.
The precursors inosine and hypoxanthine are converted to uric acid, whereas allantoic acid is the end product of uric acid degradation (Figure 4b). In the present study, we found that significant reductions in inosine and hypoxanthine, together with an increase in allantoic acid, resulted in a decrease in levels of uric acid in the PFC. However, the enzyme (that is, uricase) required for the conversion of uric acid to allantoin and subsequently to allantoic acid is not present in humans.36 Therefore, uric acid is the end product of purine catabolism in humans.
Nevertheless, uric acid is a powerful antioxidant as well as a scavenger of singlet oxygen and radicals.37 It is about as effective an antioxidant as ascorbate but with considerably higher levels in plasma, making it one of the most abundant antioxidants in humans.37 In fact, plasma levels of uric acid have been shown to be significantly lower in clinically stable patients with chronic SZ38 and in first-episode antipsychotic-naive schizophrenia patients (FEAN-SZ)26, 29, 39 than in healthy control subjects. Moreover, plasma uric acid levels were inversely correlated with psychosis.38 A homeostatic imbalance of purine catabolism is likely to impair the antioxidant defense system, leading to oxidative damage in the PFC. This stress-induced deficit in the antioxidant defense system is also consistent with the notion of free radical-mediated neurotoxicity in SZ pathology.3, 4, 5
Degradation of membrane phospholipids and peroxidation of polyunsaturated fatty acids
Excess free radicals can cause cellular dysfunction, loss of membrane integrity and even cell death. The brain, which is rich in polyunsaturated fatty acids (PUFAs), is particularly susceptible to free radical-mediated damage. Thus, membrane pathology, which is secondary to a free radical-mediated insult, can contribute to specific aspects of SZ symptomatology and complications of its treatment.4 One of the best-described effects of free radicals on the cell is the oxidative modification of fatty acids within the membrane phospholipids.40 This lipid peroxidation predominantly occurs at the sn-2 position of phospholipids via the action of phospholipase A2, yielding oxidized fatty acid and LysoPCs.41 Our data showed that CUMS increased the release of LysoPCs (16:0), (18:0) and (18:1), which suggested increased membrane breakdown (Figure 4c). Furthermore, increased LysoPCs were also associated with decreased purine metabolites (Figure 3b and c).
In addition to PUFAs, choline is another product from the degradation of membrane phospholipids and is considered to be a marker of membrane turnover.42 Levels of choline were found to be significantly higher in the PFC of the CUMS group than in that of the NC group. The accumulation of choline suggests blocked biosynthesis of choline-containing phospholipids, especially phosphatidylcholine (PC), possibly because of ATP depletion under stressful conditions.43 Even if PC synthesis via the choline pathway is blocked in chronic stress, methylation of PE via phosphatidylethanolamine N-methyltransferase might be available to consume the contents of PE to compensate for the PCs that are degraded in the membrane.44 This could be one reason why we found that PEs were decreased concurrently with increased choline after stress stimulation (Table 1 and Figure 4c). Moreover, the decrease in PEs could indicate the early loss of myelin because these lipids are abundant in myelin.45
Steroidogenesis for coping with stress
As stated above, stress-induced energy depletion (Figure 4a), oxidative stress (Figure 4b) and membrane degradation (Figure 4c) are all metabolic signals that impair the structural and functional integrity of the brain. It appears that the human body also generates several feedback mechanisms for coping with stress. As expected, corticosterone, the major glucocorticoid in rodents, was also elevated in the CUMS group. Its increase is a classic endocrine response to stress (Figure 4d). The actions of glucocorticoid as a result of stress include a series of physiological consequences, such as an increase in gluconeogenesis and energy supply, stabilization of the membrane and anti-inflammation.46
Interestingly, our data revealed that chronic stress also decreased progesterone levels in the PFC and hippocampus. This could be related to a localized reduction in biosynthesis because circulating progesterone levels are not affected by stress.47 By contrast, stress axis inhibition by dexamethasone increased progesterone levels in these regions, which is consistent with previous findings.48 A range of actions of progesterone underlying its neuroprotective effects has been demonstrated, including a reduction in inflammation and oxidant capacity, preservation of mitochondrial functions, restoration of brain-derived neurotrophic factor and promotion of the survival of newborn neurons.49 Therefore, the decline in progesterone will inevitably contribute to the deterioration of the PFC and hippocampus after long-term stress axis hyperactivity.
Pathways modulated by AAPDs in the stress response
Both preclinical and clinical evidence suggest that atypical antipsychotics modulate the stress response and antagonize stress-induced deficits.50 Using the metabolomic approach, we investigated the emerging stress-modulatory profile of AAPDs to identify specific targets in the stress-related metabolic pathways for the therapeutic efficacy of these AAPDs.
Cerebral energy metabolism
One of the shared features among the AAPD-induced metabolic signatures is their regulatory effect on creatine levels, which is particularly strong in the PFC and hippocampus. CLO, RIS and ARI treatments have this feature in common. Creatine kinase (CK) catalyzes the reversible conversion of creatine and ATP to form phosphocreatine and adenosine diphosphate, respectively.51 As mentioned above, the CUMS-induced elevation of creatine could result from increased CK activity to satisfy the increased demand for ATP. The association between increased CK activity and behavior changes has also been identified in a SZ animal model.52 In fact, AAPDs, in contrast to typical antipsychotic drugs, have been shown to be able to regulate CK activity in the brain.53 Interestingly, our data suggested that AAPDs increased creatine levels in the PFC, but decreased them in the hippocampus. This differential regulatory function is associated with the efficacy of AAPDs in the pathology of altered CK activity in the schizophrenic brain.54, 55
Steroidogenesis and phospholipid metabolism
We have previously demonstrated the presence of a metabolic signature of increased progesterone after AAPD treatment and its correlation with symptomatology improvement in FEAN-SZ.26 Consistent with our previous findings, the present findings also identified increased progesterone levels in the two brain regions studied in response to AAPDs. Given the psychotropic-like properties of progesterone,56 its increment could account for the anxiolytic and neuroprotective effects of AAPDs through binding with intracellular and membrane progesterone receptors, as well as γ-aminobutyric acid type A receptors.49 Meanwhile, AAPD-induced upregulation of membrane PE in these brain regions could provide a novel mechanism for membrane regeneration.57
Recently, we demonstrated that progesterone could regulate lipid biosynthesis via a specific membrane-binding site, named progesterone receptor membrane component 1.25 Interestingly, the present data also showed that progesterone levels were positively associated with the sum of the PE concentrations in the CUMS, LTDE and AAPD treatment conditions. Given that PEs are rich in myelin, this association suggests a pivotal role for AAPD-induced progesterone elevation in myelination.58 However, the exact biological mechanism of this correlation is still unclear.
Potential synergistic effects with combined ATP fuel, antioxidant and PUFA supplementation
Pharmaceutical treatment for the many patients worldwide with SZ is limited to a handful of antipsychotics. Despite the proven efficacy of these drugs, the overall outcome for SZ remains suboptimal.59 Thus, alternative or adjunctive treatment options are urgently needed. As stated above, AAPDs could temporarily alleviate ATP shortage by regulating CK activity until the depletion of phosphocreatine. Thus, supplements of ATP fuel could help AAPDs to sustain mitochondrial function.60 In the present study, levels of purines, choline and LysoPCs were not significantly influenced by AAPD treatment, suggesting that oxidative stress is not the primary target of AAPDs. This also offers a plausible adjunctive therapeutic approach using AAPDs in the form of antioxidants.61 As illustrated in Figure 4, which depicts the altered stress-related metabolic pathways, ATP deficiency and membrane phospholipid breakdown are markedly linked. Moreover, Table 1 indicates that the stress-induced PUFA decreases mainly involved C22:6 (n-3; docosahexaenoic acid). This result implies that supplements of these essential PUFAs would facilitate PE synthesis under AAPD treatment in conditions of stress.62 Taken together, the addition of combined oral supplements of ATP fuel, antioxidants and essential PUFAs could provide synergistic effects with AAPD treatment.
Conclusion
To the best of our knowledge, this is the first metabolomic study that: (1) evaluates the metabolic profiles of different brain regions that are vulnerable to stress among CUMS, LTDE, AAPD and NC rats; (2) establishes a novel strategy for stress-induced biomarker screening; (3) identifies metabolic pathways specifically affected by stress that are responsive to AAPD treatment; and (4) validates stress-induced biomarkers as potential therapeutic targets for AAPD treatment. Taken together, these results show that stress can induce oxidative damage by disturbing the creatine–phosphocreatine circuit and purine pathway, leading to increased membrane lipid peroxidation. Moreover, the preliminary data suggest that AAPDs partially restore the stress-induced deficits by increasing the content of creatine, progesterone and PEs. These results provide a theoretical basis from which the development of novel therapeutic strategies, in combination with ATP fuel, antioxidant and omega-3 fatty acid supplementation, could occur.
Acknowledgments
This work was supported in part by the grants from the Nature Science Foundation of China (NSFC81401113 (HLC), NSFC81602846 (PJ), NSFC81101001 (PX) and NSFC81360665 (QYT)), the Specialized Research Fund for the Doctoral Program of Higher Education of China (SRFDP20130162120060 (HLC)), the Chinese Scholarship Council for an oversea study program (HLC), the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory R&D (Senior Research Career Scientist Award (JKY)) and the VA Pittsburgh Healthcare System (HLC and JKY).
Disclaimer
The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript. The contents of this article do not represent the views of the Second Xiangya Hospital of Central South University, Jining Medical University, Central South University (Changsha), the US Department of Veterans Affairs, or the US Government.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
The authors declare no conflict of interest.
Supplementary Material
References
- Herbert J. Fortnighly review. Stress, the brain, and mental illness. Br Med J 1997; 315: 530–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran C, Walker E, Huot R, Mittal V, Tessner K, Kestler L et al. The stress cascade and schizophrenia: etiology and onset. Schizophr Bull 2003; 29: 671–692. [DOI] [PubMed] [Google Scholar]
- Reddy RD, Yao JK. Free radical pathology in schizophrenia: a review. Prostaglandins Leukot Essent Fatty Acids 1996; 55: 33–43. [DOI] [PubMed] [Google Scholar]
- Yao JK, Reddy RD, van Kammen DP. Oxidative damage and schizophrenia: an overview of the evidence and its therapeutic implications. CNS Drugs 2001; 15: 287–310. [DOI] [PubMed] [Google Scholar]
- Yao JK, Keshavan MS. Antioxidants, redox signaling, and pathophysiology in schizophrenia: an integrative view. Antioxid Redox Signal 2011; 15: 2011–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley AJ, Dinan TG. A systematic review of hypothalamic-pituitary-adrenal axis function in schizophrenia: implications for mortality. J Psychopharmacol 2010; 24: 91–118. [DOI] [PubMed] [Google Scholar]
- Jones CA, Watson DJG. Fone KCF. Animal models of schizophrenia. Br J Pharmacol 2011; 164: 1162–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Pruessner M, Lord C, Buss C, Collins L et al. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations. Psychoneuroendocrinology 2010; 35: 179–191. [DOI] [PubMed] [Google Scholar]
- Cohrs S, Röher C, Jordan W, Meier A, Huether G, Wuttke W et al. The atypical antipsychotics olanzapine and quetiapine, but not haloperidol, reduce ACTH and cortisol secretion in healthy subjects. Psychopharmacology 2006; 185: 11–18. [DOI] [PubMed] [Google Scholar]
- Tulipano G, Rizzetti C, Bianchi I, Fanzani A, Spano P, Cocchi D. Clozapine-induced alteration of glucose homeostasis in the rat: the contribution of hypothalamic-pituitary-adrenal axis activation. Neuroendocrinology 2007; 85: 61–70. [DOI] [PubMed] [Google Scholar]
- Kaneda Y, Fujii A, Ohmori T. The hypothalamic-pituitary-adrenal axis in chronic schizophrenic patients long-term treated with neuroleptics. Prog Neuropsychopharmacol Biol Psychiatry 2002; 26: 935–938. [DOI] [PubMed] [Google Scholar]
- MacMaster FP, El-Sheikh R, Upadhyaya AR, Nutche J, Rosenberg DR, Keshavan M. Effect of antipsychotics on pituitary gland volume in treatment-naïve first-episode schizophrenia: a pilot study. Schizophr Res 2007; 92: 207–210. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Ishige A, Aburada M, Tabira T. Chronic stress attenuates glucocorticoid negative feedback: involvement of the prefrontal cortex and hippocampus. Neuroscience 2003; 119: 887–897. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Knable MB, O'Grady J, Orthmann J, Weickert CS. Regional specificity of brain glucocorticoid receptor mRNA alterations in subjects with schizophrenia and mood disorders. Mol Psychiatry 2002; 7: 985–994. [DOI] [PubMed] [Google Scholar]
- Jay TM, Rocher C, Hotte M, Naudon L, Gurden H, Spedding M. Plasticity at hippocampal to prefrontal cortex synapses is impaired by loss of dopamine and stress: importance for psychiatric diseases. Neurotox Res 2004; 6: 233–244. [DOI] [PubMed] [Google Scholar]
- Wang XD, Su YA, Guo CM, Yang Y, Si TM. Chronic antipsychotic drug administration alters the expression of neuregulin 1beta, ErbB2, ErbB3, and ErbB4 in the rat prefrontal cortex and hippocampus. Int J Neuropsychopharmacol 2008; 11: 553–561. [DOI] [PubMed] [Google Scholar]
- Park SW, Choi SM, Lee JG, Lee CH, Lee SJ, Kim NR et al. Differential effects of ziprasidone and haloperidol on immobilization-stress-induced CRF mRNA expression in the hypothalamic paraventricular nucleus of rats. Neuropsychobiology 2011; 63: 29–34. [DOI] [PubMed] [Google Scholar]
- Belda X, Armario A. Dopamine D1 and D2 dopamine receptors regulate immobilization stress-induced activation of the hypothalamus-pituitary-adrenal axis. Psychopharmacology 2009; 206: 355–365. [DOI] [PubMed] [Google Scholar]
- Banati R, Hickie IB. Therapeutic signposts: using biomarkers to guide better treatment of schizophrenia and other psychotic disorders. Med J Aust 2009; 190: S26–S32. [DOI] [PubMed] [Google Scholar]
- Ni Y, Su M, Lin J, Wang X, Qiu Y, Zhao A et al. Metabolic profiling reveals disorder of amino acid metabolism in four brain regions from a rat model of chronic unpredictable mild stress. FEBS Lett 2008; 582: 2627–2636. [DOI] [PubMed] [Google Scholar]
- Want EJ, Masson P, Michopoulos F, Wilson ID, Theodoridis G, Plumb RS et al. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat Protoc 2013; 8: 17–32. [DOI] [PubMed] [Google Scholar]
- Ayensu WK, Pucilowski O, Mason GA, Overstreet DH, Rezvani AH, Janowsky DS. Effects of chronic mild stress on serum complement activity, saccharin preference, and corticosterone levels in Flinders lines of rats. Physiol Behav 1995; 57: 165–169. [DOI] [PubMed] [Google Scholar]
- Gómez F, De Kloet ER, Armario A. Glucocorticoid negative feedback on the HPA axis in five inbred rat strains. Am J Physiol 1998; 274: R420–R427. [DOI] [PubMed] [Google Scholar]
- Jiang P, Zhang WY, Li HD, Cai HL, Liu YP, Chen LY. Stress and vitamin D: altered vitamin D metabolism in both the hippocampus and myocardium of chronic unpredictable mild stress exposed rats. Psychoneuroendocrinology 2013; 38: 2091–2098. [DOI] [PubMed] [Google Scholar]
- Cai HL, Tan QY, Jiang P, Dang RL, Xue Y, Tang MM et al. A potential mechanism underlying atypical antipsychotics-induced lipid disturbances. Transl Psychiatry 2015; 5: e661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai HL, Li HD, Yan XZ, Sun B, Zhang Q, Yan M et al. Metabolomic analysis of biochemical changes in the plasma and urine of first-episode neuroleptic-naïve schizophrenia patients after treatment with risperidone. J Proteome Res 2012; 11: 4338–4350. [DOI] [PubMed] [Google Scholar]
- Chagoyen M, Pazos F. MBRole: enrichment analysis of metabolomic data. Bioinformatics 2011; 27: 730–731. [DOI] [PubMed] [Google Scholar]
- Prabakaran S, Swatton JE, Ryan MM, Huffaker SJ, Huang JT, Griffin JL et al. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry 2004; 9: 684–697. [DOI] [PubMed] [Google Scholar]
- Yao JK, Dougherty GG, Reddy RD, Keshavan MS, Montrose DM, Matson WR et al. Homeostatic imbalance of purine catabolism in first-episode neuroleptic-naïve patients with schizophrenia. PLoS ONE 2010; 5: e9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JK, Leonard S, Reddy RD. Membrane phospholipid abnormalities in postmortem brains from schizophrenic patients. Schizophr Res 2000; 42: 7–17. [DOI] [PubMed] [Google Scholar]
- MacKenzie EM, Odontiadis J, Le Mellédo JM, Prior TI, Baker GB. The relevance of neuroactive steroids in schizophrenia, depression, and anxiety disorders. Cell Mol Neurobiol 2007; 27: 541–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallimann T, Tokarska-Schlattner M, Neumann D, Epand RM, Epand RF, Andres RH et alThe phosphocreatine circuit: molecular and cellular physiology of creatine kinases, sensitivity to free radicals, and enhancement by creatine supplementation. In: Saks V (ed). Molecular System Bioenergetics: Energy for Life. Wiley-VCH GmbH & Co., 2007, pp 195–264.
- Maddock RJ, Buonocore MH. MR spectroscopic studies of the brain in psychiatric disorders. Curr Top Behav Neurosci 2012; 11: 199–251. [DOI] [PubMed] [Google Scholar]
- Deicken RF, Johnson C, Pegues M. Proton magnetic resonance spectroscopy of the human brain in schizophrenia. Rev Neurosci 2000; 11: 147–158. [DOI] [PubMed] [Google Scholar]
- Stork C, Renshaw PF. Mitochondrial dysfunction in bipolar disorder: evidence from magnetic resonance spectroscopy research. Mol Psychiatry 2005; 10: 900–919. [DOI] [PubMed] [Google Scholar]
- Lee CC, Caskey CT, Wu XW, Muzny DM. Urate oxidase: primary structure and evolutionary implications. Proc Natl Acad Sci USA 1989; 86: 9412–9416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA 1981; 78: 6858–6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao JK, Reddy R, van Kammen DP. Reduced level of plasma antioxidant uric acid in schizophrenia. Psychiatry Res 1998; 80: 29–39. [DOI] [PubMed] [Google Scholar]
- Yao JK, Condray R, Dougherty GG, Keshavan MS, Montrose DM, Matson WR et al. Associations between purine metabolites and clinical symptoms in schizophrenia. PLoS ONE 2012; 7: e42165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalá A. Lipid peroxidation of membrane phospholipids generates hydroxy-alkenals and oxidized phospholipids active in physiological and/or pathological conditions. Chem Phys Lipids 2009; 157: 1–11. [DOI] [PubMed] [Google Scholar]
- Balboa MA, Balsinde J. Oxidative stress and arachidonic acid mobilization. Biochim Biophys Acta 2006; 1761: 385–391. [DOI] [PubMed] [Google Scholar]
- Bustillo JR, Rowland LM, Lauriello J, Petropoulos H, Hammond R, Hart B et al. High choline concentrations in the caudate nucleus in antipsychotic-naive patients with schizophrenia. Am J Psychiatry 2002; 159: 130–133. [DOI] [PubMed] [Google Scholar]
- Klein J. Membrane breakdown in acute and chronic neurodegeneration: focus on choline-containing phospholipids. J Neural Transm 2000; 107: 1027–1063. [DOI] [PubMed] [Google Scholar]
- Li Z, Agellon LB, Allen TM, Umeda M, Jewell L, Mason A et al. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab 2006; 3: 321–331. [DOI] [PubMed] [Google Scholar]
- Wilson R, Sargent JR. Lipid and fatty acid composition of brain tissue from adrenoleukodystrophy patients. J Neurochem 1993; 61: 290–297. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 2000; 21: 55–89. [DOI] [PubMed] [Google Scholar]
- Ward IL, Weisz J. Differential effects of maternal stress on circulating levels of corticosterone, progesterone, and testosterone in male and female rat fetuses and their mothers. Endocrinology 1984; 114: 1635–1644. [DOI] [PubMed] [Google Scholar]
- Yuan XH, Yang BQ, Hu Y, Fan YY, Zhang LX, Zhou JC et al. Dexamethasone altered steroidogenesis and changed redox status of granulosa cells. Endocrine 2014; 47: 639–647. [DOI] [PubMed] [Google Scholar]
- Guennoun R, Labombarda F, Gonzalez Deniselle MC, Liere P, De Nicola AF, Schumacher M. Progesterone and allopregnanolone in the central nervous system: response to injury and implication for neuroprotection. J Steroid Biochem Mol Biol 2015; 146: 48–61. [DOI] [PubMed] [Google Scholar]
- Marx CE, Grobin AC, Deutch AY, Lieberman JA. Chapter 5 Atypical antipsychotic drugs and stress. Tech Behav Neural Sci 2005; 15: 301–313. [Google Scholar]
- Allen PJ. Creatine metabolism and psychiatric disorders: does creatine supplementation have therapeutic value? Neurosci Biobehav Rev 2012; 36: 1442–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canever L, Oliveira L, D'Altoé de Luca R, Correa PT, de B Fraga D, Matos MP et al. A rodent model of schizophrenia reveals increase in creatine kinase activity with associated behavior changes. Oxid Med Cell Longev 2010; 3: 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assis LC, Scaini G, Di-Pietro PB, Castro AA, Comim CM, Streck EL et al. Effect of antipsychotics on creatine kinase activity in rat brain. Basic Clin Pharmacol Toxicol 2007; 101: 315–319. [DOI] [PubMed] [Google Scholar]
- Clark D, Dedova I, Cordwell S, Matsumoto I. A proteome analysis of the anterior cingulate cortex gray matter in schizophrenia. Mol Psychiatry 2006; 11: 459–470. [DOI] [PubMed] [Google Scholar]
- Burbaeva G, Savushkina OK, Boksha IS. Creatine kinase BB in brain in schizophrenia. World J Biol Psychiatry 2003; 4: 177–183. [DOI] [PubMed] [Google Scholar]
- Rupprecht R, Koch M, Montkowski A, Lancel M, Faulhaber J, Harting J et al. Assessment of neuroleptic-like properties of progesterone. Psychopharmacology 1999; 143: 29–38. [DOI] [PubMed] [Google Scholar]
- Fernø J, Raeder MB, Vik-Mo AO, Skrede S, Glambek M, Tronstad KJ et al. Antipsychotic drugs activate SREBP-regulated expression of lipid biosynthetic genes in cultured human glioma cells: a novel mechanism of action? Pharmacogenomics J 2005; 5: 298–304. [DOI] [PubMed] [Google Scholar]
- Baulieu E, Schumacher M. Progesterone as a neuroactive neurosteroid, with special reference to the effect of progesterone on myelination. Steroids 2000; 65: 605–612. [DOI] [PubMed] [Google Scholar]
- Meltzer HY. What’s atypical about atypical antipsychotic drugs? Curr Opin Pharmacol 2004; 4: 53–57. [DOI] [PubMed] [Google Scholar]
- Nicolson GL. Mitochondrial dysfunction and chronic disease: treatment with natural supplements. Integr Med 2014; 13: 35–43. [PMC free article] [PubMed] [Google Scholar]
- Reddy R, Reddy R. Antioxidant therapeutics for schizophrenia. Antioxid Redox Signal 2011; 15: 2047–2055. [DOI] [PubMed] [Google Scholar]
- Akter K, Gallo DA, Martin SA, Myronyuk N, Roberts RT, Stercula K et al. A review of the possible role of the essential fatty acids and fish oils in the aetiology, prevention or pharmacotherapy of schizophrenia. J Clin Pharm Ther 2012; 37: 132–139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.