Abstract
Brain-derived neurotrophic factor (BDNF) has a role in the pathophysiology of psychiatric disorders. The precursor proBDNF is converted to mature BDNF and BDNF pro-peptide, the N-terminal fragment of proBDNF; however, the precise function of these proteins in psychiatric disorders is unknown. We sought to determine whether expression of these proteins is altered in the brain and peripheral tissues from patients with psychiatric disorders. We measured protein expression of proBDNF, mature BDNF and BDNF pro-peptide in the parietal cortex, cerebellum, liver and spleen from control, major depressive disorder (MDD), schizophrenia (SZ) and bipolar disorder (BD) groups. The levels of mature BDNF in the parietal cortex from MDD, SZ and BD groups were significantly lower than the control group, whereas the levels of BDNF pro-peptide in this area were significantly higher than controls. In contrast, the levels of proBDNF and BDNF pro-peptide in the cerebellum of MDD, SZ and BD groups were significantly lower than controls. Moreover, the levels of mature BDNF from the livers of MDD, SZ and BD groups were significantly higher than the control group. The levels of mature BDNF in the spleen did not differ among the four groups. Interestingly, there was a negative correlation between mature BDNF in the parietal cortex and mature BDNF in the liver in all the subjects. These findings suggest that abnormalities in the production of mature BDNF and BDNF pro-peptide in the brain and liver might have a role in the pathophysiology of psychiatric disorders, indicating a brain–liver axis in psychiatric disorders.
Introduction
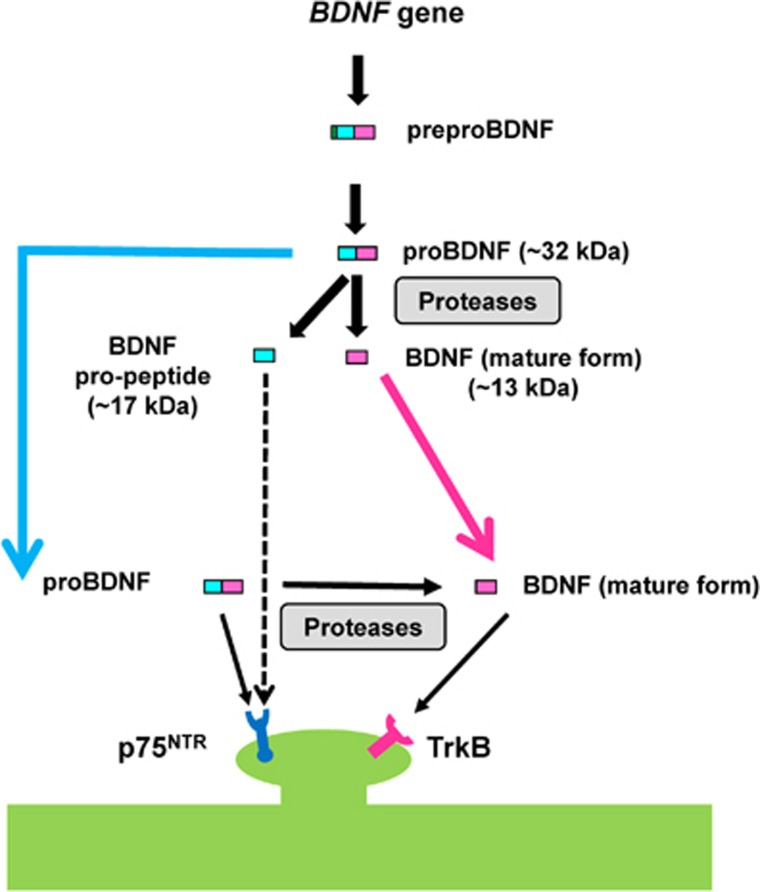
Multiple lines of evidence suggest that brain-derived neurotrophic factor (BDNF) is crucial in the pathophysiology of psychiatric disorders, such as major depressive disorder (MDD), schizophrenia (SZ) and bipolar disorder (BD).1, 2, 3, 4, 5, 6 Brain-derived neurotrophic factor (mature form) is a 13 kDa polypeptide, which is initially synthesized as the precursor protein, preproBDNF, in the endoplasmic reticulum. Following cleavage of the signal peptide, proBDNF (~32 kDa) is converted to mature BDNF and BDNF pro-peptide (~17 kDa), the N-terminal fragment of proBDNF (Figure 1). Both proBDNF and mature BDNF are active, eliciting opposing effects via the p75NTR and tropomyosin-related kinase B (TrkB) receptor, respectively, and both forms are important in several physiological functions.7, 8, 9, 10, 11 The expression levels of BDNF pro-peptide are increased during postnatal development and plateau in adult mice. In addition, BDNF pro-peptide is released from neurons in an activity-dependent manner.12 Interestingly, BDNF pro-peptide directly facilitates hippocampal long-term depression, requiring activation of the GluN2B subtype of N-methyl-d-aspartate receptors and p75NTR.13 A recent study showed that neuronal depolarization elicited a marked increase in extracellular BDNF pro-peptide, which, in turn, negatively regulated dendritic spines via caspase-3.14 Taken together, it is likely that proBDNF, BDNF pro-peptide and mature BDNF all share important biological functions.
Figure 1.
Production of BDNF and BDNF pro-peptide from its precursor. The human BDNF gene produces preproBDNF protein, which is processed to proBDNF (~32 kDa). Following cleavage of the signal peptide, proBDNF (~32 kDa) is converted to BDNF mature form (~13 kDa) and BDNF pro-peptide (~17 kDa) by intracellular and extracellular proteases. Mature BDNF preferentially binds the TrkB receptor, while proBDNF and BDNF pro-peptide bind to p75NTR. BDNF, brain-derived neurotrophic factor; TrkB, tropomyosin-related kinase B.
Increased levels of BDNF protein in the anterior cingulate cortex and hippocampus of patients with SZ have been reported.15, 16 However, Weickert et al.17 reported a significant reduction in BDNF messenger RNA (mRNA; 23%) and BDNF protein (40%) in the dorsolateral prefrontal cortex of SZ patients. Issag et al.18 also reported decreased BDNF in the prefrontal cortex of SZ patients. Furthermore, Karege et al.19 reported reduced BDNF levels in the hippocampus of suicide victims. Reduced gene expression of BDNF and its receptor TrkB was also detected in the prefrontal cortex of suicide subjects,20 and the anterior cingulate cortex of MDD patients21 and elderly depressed patients.22 Interestingly, in MDD subjects using antidepressant medication, increased hippocampal BDNF immunoreactivity was reported, suggesting that antidepressants could increase hippocampal BDNF levels.23 Other reports noted decreased expression of BDNF in the hippocampus24 and frontal cortex of BD patients.25 It is therefore likely that abnormalities in BDNF expression in the brain are pivotal to the pathophysiology of major psychiatric disorders. Our preclinical studies showed that regional differences in the expression of mature BDNF, its precursor proBDNF and BDNF pro-peptide confer resilience to inescapable stress.26, 27 However, there are no reports examining expression of BDNF isoforms in postmortem brain and peripheral tissues from patients with psychiatric disorders.
In addition to the brain, expression of BDNF and TrkB has been reported in the peripheral tissues including liver and spleen,28, 29, 30, 31 although the functional role of BDNF–TrkB signaling in liver and spleen is not fully understood. These two tissues (liver and spleen) from the same subjects are available from The Stanley Research Institute (Kensington, MD, USA). This study was, therefore, undertaken to examine whether expression levels of BDNF and its related peptides in postmortem tissues (parietal cortex, cerebellum, liver and spleen) taken from SZ, MDD and BD groups showed differences when compared with a healthy control group.
Materials and methods
Postmortem human samples
Human postmortem parietal cortex (Brodmann area 7), cerebellum, liver and spleen from normal controls (N=15), as well as patients with MDD (N=15), SZ (N=15) and BD (N=15) were obtained from the Stanley Foundation Brain Collection (Bethesda, MD, USA). The spleen samples from three SZ patients and one MDD patient were not included. The specimens are collected by medical examiners. Permission from the next of kin was obtained in all the cases. The demographic, clinical and storage information for cases has been previously published.32 Each diagnostic group was matched according to several parameters, including age at death, gender, postmortem interval, brain pH and brain weight (Table 1). This study was approved by the Research Ethics Committee of the Graduate School of Medicine, Chiba University (No. 442 on 16 September 2015 and No. 223 on 13 June 13 2016).
Table 1. Characteristics of the postmortem samples from Neuropathology Consortium of the Stanley Medical Research Institute.
| Characteristics | Control (n=15) | MDD (n=15) | SZ (n=15) | BD (n=15) | P-value |
|---|---|---|---|---|---|
| Age at death (years) | 48.1±10.7 (29–68) | 46.5±9.3 (30–65) | 44.5±13.1 (25–62) | 42.3±11.7 (25–61) | 0.540a |
| Gender (male/female) | 9/6 | 9/6 | 9/6 | 9/6 | |
| PMI (h) | 23.7±9.95 | 27.5±10.7 | 33.7±14.6 | 32.5±16.1 | 0.147a |
| Brain pH | 6.27±0.24 | 6.18±0.21 | 6.16±0.26 | 6.18±0.23 | 0.616a |
| Brain hemispheres (right/left) | 7/8 | 6/9 | 6/9 | 8/7 | 0.864b |
| Brain weight (g) | 1501.0±164.1 | 1462.0±142.1 | 1471.7±108.2 | 1441.2±171.5 | 0.740a |
| Storage days | 338.2±234.2 | 434.0±290.0 | 621.1±233.1 | 620.5±172.3 | 0.003a |
| Age of onset (years) | 33.9±13.3 | 23.2±8.0 | 21.5±8.4 | 0.003a | |
| Duration of disease (years) | 12.7±11.1 | 21.3±11.4 | 20.1±9.7 | 0.068a | |
| History of psychosis | 15 | 11 with (4 without) | 0.100c | ||
| Fluphenazine equivalent (mg) | 52267±62062 (1 never) | 20827±24016 (3 never) | 0.084d |
Abbreviations: BD, bipolar disorder; MDD, major depressive disorder; PMI, postmortem interval; SZ, schizophrenia.
One-way analysis of variance.
χ2 test for independence.
Fisher’s exact probability test.
Unpaired t-test.
The data are shown as the mean±s.d.
Western blot analysis
The western blot analysis was performed by one observer who was blinded to the four groups. Human samples were stored at −80 °C until biochemical analyses. The tissue samples were homogenized in Laemmli lysis buffer, then centrifuged at 3000 g at 4 °C, for 10 min to obtain the supernatants. The protein concentrations were determined using a BCA method assay kit (Bio-Rad, Hercules, CA, USA), then the samples were incubated for 5 min at 95 °C, with an equal volume of 125 mm Tris/HCl, pH 6.8, 20% glycerol, 0.1% bromophenol blue, 10% β-mercaptoethanol and 4% sodium dodecyl sulfate. The proteins were separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis, on 10% mini-gels (Mini-PROTEAN TGX Precast Gel; Bio-Rad). The separated proteins were then transferred onto polyvinylidene difluoride membranes using a Trans Blot Mini Cell (Bio-Rad). For immunodetection, the blots were blocked with 2% BSA in TBST (TBS+0.1% Tween-20) for 1 h at room temperature, then incubated with primary antibodies overnight, at 4 °C. The following primary antibodies were used: anti-human proBDNF (1:2000, Cat #: H10001G-MA, GeneCopoeia, Rockville, MD, USA), anti-BDNF (1:200, Cat #: H-117 (sc-20981), Santa Cruz Biotechnology, Santa Cruz, CA, USA) and β-actin (1:10 000, Sigma-Aldrich, St Louis, MO, USA). Anti-human proBNDF antibody (GeneCopoeia) and anti-BDNF (Santa Cruz Biotechnology) were used for the measurement of proBDNF, BDNF pro-peptide and mature BDNF, respectively.26, 27 The specificity of these BDNF antibodies was confirmed using brain samples from Bdnf knockout rats.27 The next day, the blots were washed three times in TBST and incubated with horseradish peroxidase conjugated anti-rabbit or anti-mouse antibody (1:5000) for 1 h, at room temperature. After three washes in TBST, the bands were detected using enhanced chemiluminescence, plus the western blotting detection system (GE Healthcare Bioscience, Tokyo, Japan). Finally, the blots were washed three times in TBST and incubated with a primary antibody directed against β-actin. The images were captured with a Fuji LAS3000-mini imaging system (Fujifilm, Tokyo, Japan), and immunoreactive bands were quantified.
Statistical analysis
The data were shown as the mean±s.d. The analysis was performed using PASW Statistics 20 (formerly SPSS statistics; SPSS, Tokyo, Japan). Analyses of covariance (ANCOVAs) were performed on normalized spot volumes, for each spot in each region, with brain pH, age of disease onset, gender, duration of disease, postmortem interval, frozen brain hemisphere side, lifetime neuroleptic drug use, severity of substance abuse, severity of alcohol abuse and/or frozen storage time. If ANCOVA reached significance, we performed the post hoc least significance difference test to identify group comparisons. Correlation was determined by Pearson or nonparametric Spearman correlations. A P-value <0.05 was considered to be statistically significant.
Results
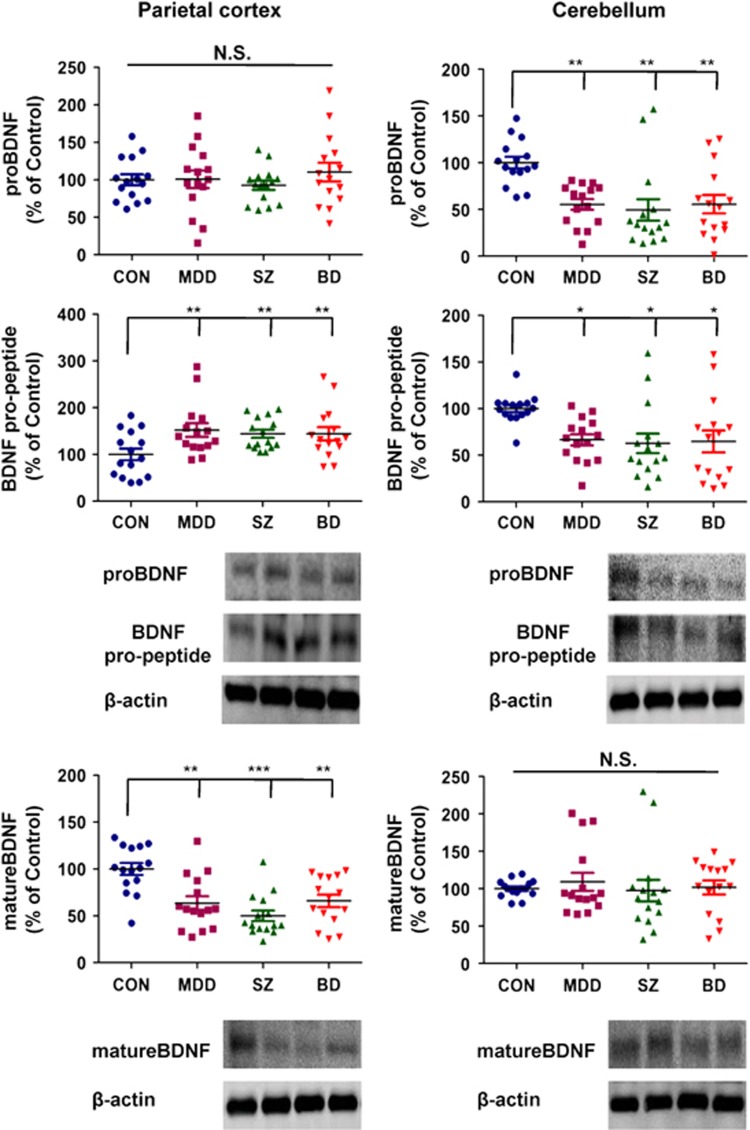
As the postmortem samples have many parameters, the ANCOVA has been used for statistical analyses of the data. The ANCOVA of the data from the parietal cortex showed the statistical results; [proBDNF: F3,56=0.500, P=0.684], [BDNF pro-peptide: F3,56=4.400, P=0.008], [mature BDNF: F3,56=6.087, P=0.001] from the four groups. A post hoc analysis showed that expression of BDNF pro-peptide in MDD (P=0.005), SZ (P=0.001) and BD (P=0.002) groups was significantly higher than in the control group (Figure 2). In contrast, expression of mature BDNF in the MDD (P=0.001), SZ (P<0.001) and BD (P=0.002) groups was significantly lower than in the control group (Figure 2).
Figure 2.
Levels of proBDNF, mature BDNF and BDNF pro-peptide in human brain regions derived from control, MDD, SZ and BD groups. Western blot analysis was performed on proBDNF, mature BDNF, BDNF pro-peptide and β-actin in the parietal cortex and cerebellum of control (n=15), MDD (n=15), SZ (n=15) and BD (n=15) groups. The data are expressed as a percentage of control group values. The data are shown as mean±s.d. *P<0.05, **P<0.01, ***P<0.001 compared with the control group. BD, bipolar disorder; BDNF, brain-derived neurotrophic factor; MDD, major depressive disorder; NS, not significant; SZ, schizophrenia.
Furthermore, the ANCOVA of the data from the cerebellum showed the statistical results; [proBDNF: F3,56=5.603, P=0.002], [BDNF pro-peptide: F3,56=3.044, P=0.039], [mature BDNF: F3,56=0.275, P=0.843] among the four groups. A post hoc analysis showed that expression of proBDNF, and BDNF pro-peptide in the MDD (proBDNF: P=0.001, BDNF pro-peptide: P=0.015), SZ (proBDNF: P=0.001, BDNF pro-peptide: P=0.011) and BD (proBDNF: P=0.002, BDNF pro-peptide: P=0.019) groups was significantly lower than in the control group (Figure 2).
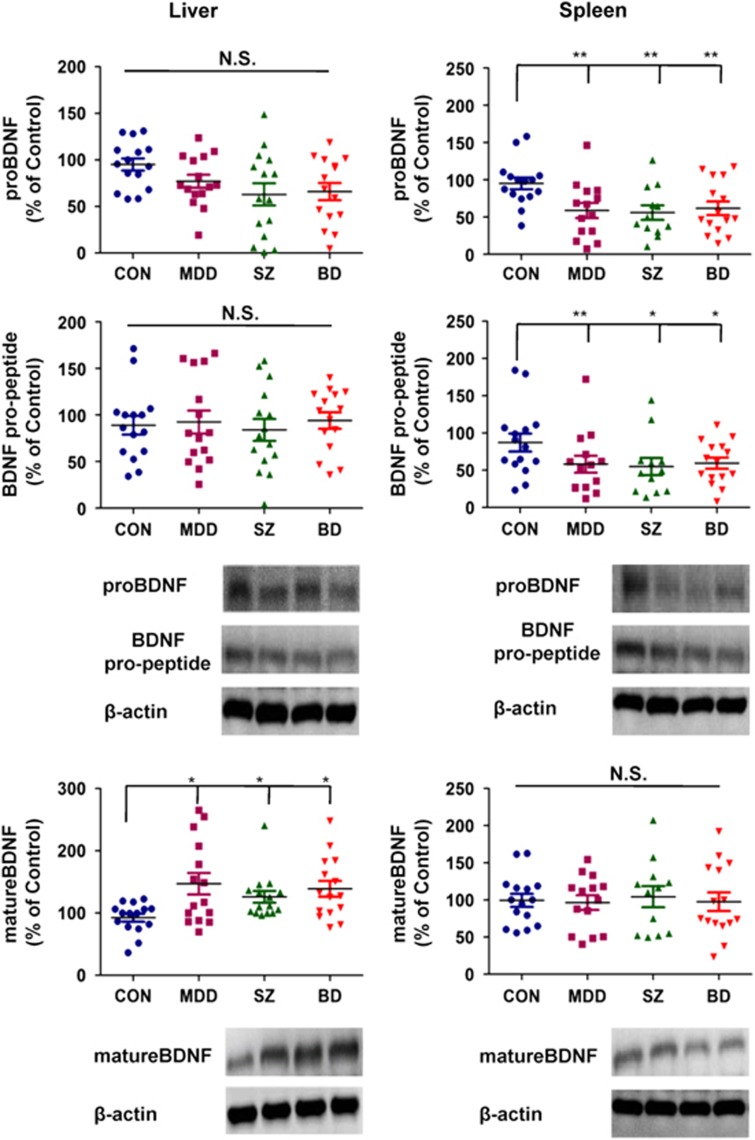
Moreover, the ANCOVA of the data from the liver showed the statistical results; [proBDNF: F3,56=1.594, P=0.202], [BDNF pro-peptide: F3,56=0.039, P=0.990], [mature BDNF: F3,56=3.010, P=0.044] among the four groups. A post hoc analysis showed that expression of mature BDNF in the MDD (P=0.021), SZ (P=0.016) and BD (P=0.023) groups was significantly higher than in the control group (Figure 3). The ANCOVA of the data from the spleen showed the statistical results; [proBDNF: F3,52=4.369, P=0.009], [BDNF pro-peptide: F3,52=3.352, P=0.034], [mature BDNF: F3,52=0.085, P=0.968] among the four groups. A post hoc analysis showed that the expressions of proBDNF and BDNF pro-peptide in the MDD (proBDNF: P=0.003, BDNF pro-peptide: P=0.008), SZ (proBDNF: P=0.001, BDNF pro-peptide: P=0.012) and BD (proBDNF: P=0.004, BDNF pro-peptide: P=0.021) groups were significantly lower than those of the control group (Figure 3).
Figure 3.
Levels of proBDNF, mature BDNF and BDNF pro-peptide in human liver and spleen from control, MDD, SZ and BD groups. Western blot analysis was performed on proBDNF, mature BDNF, BDNF pro-peptide and β-actin on the livers of control (n=15), MDD (n=15), SZ (n=15) and BD (n=15) groups. Western blot analysis of proBDNF, mature BDNF, BDNF pro-peptide and β-actin on the spleens of control (n=15), MDD (n=14), SZ (n=12) and BD (n=15) groups was also performed. The data are expressed as a percentage of control group values. The data are shown as mean±s.d. *P<0.05, **P<0.01 compared with the control group. BD, bipolar disorder; BDNF, brain-derived neurotrophic factor; MDD, major depressive disorder; SZ, schizophrenia.
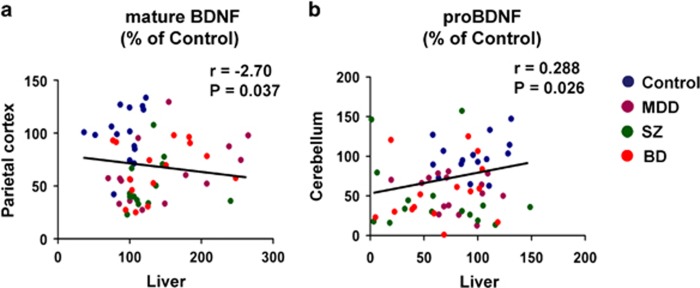
We analyzed the correlation between protein expression in the brain and liver. Interestingly, we found a negative correlation (r=−0.270, P=0.037) between mature BDNF in the liver and mature BDNF in the parietal cortex in all the subjects (Figure 4a). Furthermore, there was a positive correlation (r=0.288, P=0.026) between proBDNF in the liver and proBDNF in the cerebellum in all the subjects (Figure 4b).
Figure 4.
Correlation between mature BDNF (or proBDNF) in the brain and liver. (a) There was a negative correlation (r=−2.70, P=0.037) between mature BDNF in the parietal cortex and mature BDNF in the liver in all the subjects (N=60). (b) There was a positive correlation (r=0.288, P=0.026) between proBDNF in the cerebellum and proBDNF in the liver in all the subjects (N=60). BD, bipolar disorder; BDNF, brain-derived neurotrophic factor; MDD, major depressive disorder; SZ, schizophrenia.
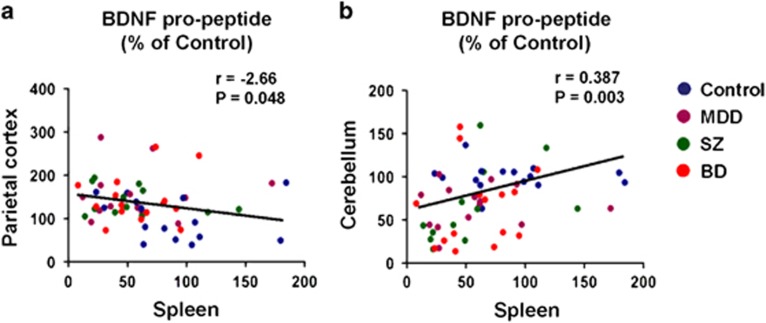
Next, we analyzed the correlation between protein expression in the brain and spleen. There was a negative correlation (r=−0.266, P=0.048) between BDNF pro-peptide in the parietal cortex and BDNF pro-peptide in the spleen in all the subjects (Figure 5a). Furthermore, there was also a positive correlation (r=0.387, P=0.003) between BDNF pro-peptide in the cerebellum and BDNF pro-peptide in the spleen in all the subjects (Figure 5b). However, there were no correlations between three BDNF isoforms in the brain and clinical variables in Table 1.
Figure 5.
Correlation between BDNF pro-peptide in the brain and spleen. (a) There was a negative correlation (r=−2.66, P=0.048) between BDNF pro-peptide in the parietal cortex and BDNF pro-peptide in the spleen in all the subjects (N=56). (b) There was a positive correlation (r=0.387, P=0.003) between BDNF pro-peptide in the cerebellum and BDNF pro-peptide in the spleen in all the subjects (N=56). BD, bipolar disorder; BDNF, brain-derived neurotrophic factor; MDD, major depressive disorder; SZ, schizophrenia.
Discussion
The major findings of this study are as follows: First, tissue levels of mature BDNF in the parietal cortex from MDD, SZ and BD groups were significantly lower than those of the control group. Interestingly, the tissue levels of BDNF pro-peptide in the parietal cortex from MDD, SZ and BD groups were significantly higher than in the control group. Second, the tissue levels of proBDNF and BDNF pro-peptide in the cerebellum from MDD, SZ and BD groups were significantly lower than those of the control group. Third, the tissue levels of mature BDNF in the liver from MDD, SZ and BD groups were significantly higher than controls. Fourth, the tissue levels of proBDNF and BDNF pro-peptide in the spleen from MDD, SZ and BD groups were significantly lower than in controls. Finally, there were correlations between BDNF isoforms in the brain and liver (or spleen) in all the subjects. Taken together, it is likely that abnormalities in the production of mature BDNF and BDNF pro-peptide from their precursor proBDNF in the brain and peripheral tissues may have a role in the pathophysiology of major psychiatric disorders.
The parietal cortex, one of four major lobes in the cerebral cortex of the human brain, has important roles in integrating sensory information from various parts of the body. In this study, we found decreased expression of mature BDNF and increased expression of BDNF pro-peptide in parietal cortices from the three major psychiatric disorders. It is therefore likely that decreased BDNF–TrkB signaling and increased BDNF pro-peptide–p75NTR signaling in the parietal cortex is crucial to the pathophysiology of these psychiatric disorders. Previous reports using western blot analysis showed reduced BDNF protein expression in the dorsolateral prefrontal cortex of SZ patients.17 A study using BDNF enzyme-linked immunosorbent assay (ELISA; Promega, Tokyo, Japan) kits showed increased BDNF in many brain regions, including parietal cortex from SZ compared with control groups.16 As this ELISA kit recognizes both mature and precursor proBDNF due to the limited specificity of its BDNF antibody, the obtained data represent total levels of mature BDNF and its precursors.33
Accumulating evidence suggests that the cerebellum is vital to many motor, cognitive and emotional processes, despite earlier beliefs that its role was limited to motor coordination.34 In this study, we found decreased expression of proBDNF and BDNF pro-peptide in the cerebellums of psychiatric disorder patients. Thus, it is likely that decreased proBDNF (or BDNF pro-peptide)–p75NTR signaling in the cerebellum may mediate the pathological processes underlying psychiatric disorders, although further investigations are needed. The reasons underlying the opposing findings in the parietal cortex and cerebellum are currently unknown.
A large-scale retrospective study reported a higher prevalence of liver disease and alcohol-related cirrhosis in veterans with SZ or BD.35 A subsequent population-based cohort study found that SZ and BD patients showed a significantly higher prevalence and incidence of chronic liver disease than the general population, and that even younger patients had a much higher prevalence and incidence of liver disorders than the general population.36, 37 Thus, there is a possible link between liver disease and SZ and BD. Furthermore, the high expression of BDNF and TrkB proteins in liver suggests neurotrophic support for autonomic innervation of this organ. It is reported that BDNF can normalize liver weights and the glycogen content of db/db mutant mice, and that these changes were not entirely associated with reduced food intake.38 Teillon et al.39 demonstrated that BDNF–TrkB signaling facilitated the development of metabolic disorders and liver damage, elicited by a high-fat diet. In contrast to the appetite-inhibiting effects of BDNF on the brain, it seems that BDNF in the liver might promote the detrimental effects of dietary stress, highlighting the complexity of BDNF signaling in these two organs. Here, we found increased levels of mature BDNF in livers from the three psychiatric disorders. Interestingly, we found a negative correlation between mature BDNF in the parietal cortex and mature BDNF in the liver, suggesting a role of brain–liver axis. Given the postulated role for the brain–liver axis,40 it is likely that increased levels of mature BDNF in liver tissue might contribute to the high incidence of liver disease in these psychiatric disorders. Nonetheless, further detailed studies of the underlying association between BDNF–TrkB signaling in the brain–liver axis and psychiatric disorders are needed.
It is reported that high-fat diet (21 weeks) caused a decrease in the BDNF (mature form) in the mouse brain (prefrontal cortex, hippocampus) as well as liver, suggesting that high-fat diet-induced reduction of BDNF–TrkB signaling in these tissues may have a role in the insulin resistance and the development of hepatic steatosis in mice.41, 42 As the dietary details of the cohort are not available, the role of dietary effects on BDNF isoforms in these tissues is unknown. Nonetheless, further study on role of diet on BDNF isoforms in the brain and peripheral tissues is needed.
Yamamoto et al.30 reported high expression of BDNF mRNA and low levels of TrkB mRNA in human spleen. A previous report showed that congenital absence of the thymus results in increased BDNF levels in the spleen, suggesting a role of BDNF in the immune system in spleen.43 Furthermore, a recent study reported that BDNF reduced NK cells, but increased NKT cell accumulation in the spleen of xenotransplanted mice, suggesting that BDNF may inhibit the rejection of peripheral nerve following xenotransplantation by regulating innate as well as adaptive immune system.44 Collectively, it is likely that BDNF might have a role in the immune system in the spleen. In this study, we found lower expression of proBDNF and BDNF pro-peptide in the spleen of patients with psychiatric disorders compared with controls. Interestingly, we found a negative correlation between BDNF pro-peptide in the parietal cortex and BDNF pro-peptide in the spleen in all the subjects. In addition, we also found a positive correlation between BDNF pro-peptide in the cerebellum and BDNF pro-peptide in the spleen in all the subjects. Thus, it is likely that, similar to brain–liver axis, the brain–spleen axis may have a role in the pathophysiology of these psychiatric disorders through the immune system. Considering the high levels of p75NTR in human spleen,30 it is likely that proBDNF (or BDNF pro-peptide)–p75NTR signaling may be integral to the physiological function of the spleen. Further detailed studies into the role of proBDNF (or BDNF pro-peptide)–p75NTR signaling in the spleen and the pathophysiology of psychiatric disorders are needed.
There is a high rate of misdiagnosis between MDD and BD in clinical practice. Recent studies using blood samples suggest that the measurement of mature BDNF and proBDNF in blood would be potential diagnostic biomarkers for MDD and BD.45, 46 Therefore, the measurement of three BDNF isoforms (proBDNF, mature BDNF, BDNF pro-peptide) in the blood will be of great interest for potential diagnostic biomarkers for MDD and BD.
In conclusion, this study suggests that abnormal metabolism of proBDNF into mature BDNF and BDNF pro-peptide in the brain and liver may be crucial to the development of psychiatric disorders. Further detailed investigations into this association between the brain–liver axis and psychiatric disorders are warranted.
Acknowledgments
We thank the Stanley Medical Research Institution (MD, USA) for providing the postmortem tissue samples. We also thank Dr Yasunori Sato (Department of Global Clinical Research, Chiba University Graduate School of Medicine) for valuable comments of statistical analysis. This study was in part supported by the Strategic Research Program for Brain Sciences from Japan Agency for Medical Research and Development, AMED (to KH), and a research fund from Otsuka Pharmaceutical (Tokyo, Japan; to KH). BY was supported by the China Scholarship Council. QR was supported by the Research Fellowship of the Japan Society for the Promotion of Science (JSPS; Tokyo, Japan).
Footnotes
The authors declare no conflict of interest.
References
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron 2002; 34: 13–25. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Shimizu E, Iyo M. Critical role of brain-derived neurotrophic factor in mood disorders. Brain Res Brain Res Rev 2004; 45: 104–114. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model of stress-related mood disorders. Biol Psychiatry 2006; 59: 1116–1127. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. Brain-derived neurotrophic factor as a biomarker for mood disorders: an historical overview and future directions. Psychiatry Clin Neurosci 2010; 64: 341–357. [DOI] [PubMed] [Google Scholar]
- Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev 2012; 64: 238–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JC, Yao W, Hashimoto K. Brain-derived neurotrophic factor (BDNF) - TrkB signaling in inflammation-related depression and potential therapeutic targets. Curr Neuropharmacol 2016; 14: 721–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B. Pro-region of neurotrophins: role in synaptic modulation. Neuron 2003; 39: 735–738. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. BDNF variant linked to anxiety-related behaviors. Bioessays 2007; 29: 116–119. [DOI] [PubMed] [Google Scholar]
- Martinowich K, Manji H, Lu B. New insights into BDNF function in depression and anxiety. Nat Neurosci 2007; 10: 1089–1093. [DOI] [PubMed] [Google Scholar]
- Deinhardt K, Chao MV. Shaping neurons: long and short range effects of mature and proBDNF signaling upon neuronal structure. Neuropharmacology 2014; 76: 603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltran RB, Diaz SL. BDNF isoforms: a round trip ticket between neurogenesis and serotonin? J Neurochem 2016; 138: 204–221. [DOI] [PubMed] [Google Scholar]
- Dieni S, Matsumoto T, Dekkers M, Rauskolb S, Ionescu MS, Deogracias R et al. BDNF and its pro-peptide are stored in presynaptic dense core vesicles in brain neurons. J Cell Biol 2012; 196: 775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizui T, Ishikawa Y, Kumanogoh H, Lume M, Matsumoto T, Hara T et al. BDNF pro-peptide actions facilitate hippocampal LTD and are altered by the common BDNF polymorphism Val66Met. Proc Natl Acad Sci USA 2015; 112: E3067–E3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Ji Y, Ding Y, Jiang W, Sun Y, Lu B et al. BDNF pro-peptide regulates dendritic spines via caspase-3. Cell Death Dis 2016; 7: e2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi M, Shirakawa O, Toyooka K, Kitamura N, Hashimoto T, Maeda K et al. Abnormal expression of brain-derived neurotrophic factor and its receptor in the corticolimbic system of schizophrenic patients. Mol Psychiatry 2000; 5: 293–300. [DOI] [PubMed] [Google Scholar]
- Durany N, Michel T, Zöchling R, Boissl KW, Cruz-Sánchez FF, Riederer P et al. Brain-derived neurotrophic factor and neurotorophin 3 in schizophrenic psychoses. Schizophr Res 2001; 52: 79–86. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Hyder TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry 2003; 8: 592–610. [DOI] [PubMed] [Google Scholar]
- Issa G, Wilson C, Terry AV Jr, Pillai A. An inverse relationship between cortisol and BDNF levels in schizophrenia: data from human postmortem and animal studies. Neurobiol Dis 2010; 39: 327–333. [DOI] [PubMed] [Google Scholar]
- Karege F, Vaudan G, Schwald M, Perroud N, La Harpe R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res Mol Brain Res 2005; 136: 29–37. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Conley RR, Roberts RC, Tamminga CA, Pandey GN. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch Gen Psychiatry 2003; 60: 804–815. [DOI] [PubMed] [Google Scholar]
- Tripp A, Oh H, Guilloux JP, Martinowich K, Lewis DA, Sibille E. Brain-derived neurotrophic signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry 2012; 169: 1194–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi XR, Zhao J, Liu J, Fang H, Swaab DF, Zhou JN. Abnormal retinoid and TrkB signaling in the prefrontal cortex in mood disorders. Cereb Cortex 2015; 25: 75–83. [DOI] [PubMed] [Google Scholar]
- Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry 2001; 50: 260–265. [DOI] [PubMed] [Google Scholar]
- Thompson Ray M, Weickert CS, Wyatt E, Webster MJ. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J Psychiatry Neurosci 2011; 36: 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HW, Rapoport SI, Rao JS. Altered expression of apoptotic factors and synaptic markers in postmortem brain from bipolar disorder patients. Neurobiol Dis 2010; 37: 596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Shirayama Y, Zhang JC, Ren Q, Hashimoto K. Regional differences in brain-derived neurotrophic factor levels and dendritic spine density confer resilience to inescapable stress. Int J Neuropsychopharmacol 2015; 18: pyu121. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yang B, Yang C, Ren Q, Zhang JC, Chen QX, Shirayama Y et al. Regional differences in the expression of brain-derived neurotrophic factor (BDNF) pro-peptide, proBDNF and preproBDNF in the brain confer stress resilience. Eur Arch Psychiatry Clin Neurosci 2016; 266: 765–769. [DOI] [PubMed] [Google Scholar]
- Cassiman D, Denef C, Desmer VJ, Roskams T. Human and rat hepatic stellate cells express neurotrophins and neurotrophin receptors. Hepatology 2001; 33: 148–158. [DOI] [PubMed] [Google Scholar]
- Yang ZF, Ho DW, Lam CT, Luk JM, Lum CT, Yu WC et al. Identification of brain-derived neurotrophic factor as a novel functional protein in hepatocellular carcinoma. Cancer Res 2005; 65: 219–225. [PubMed] [Google Scholar]
- Yamamoto M, Sobue G, Yamamoto K, Terao S, Mitsuma T. Expression of mRNAs for neurotrophic factors (NGF, BDNF, NT-3, and GDNF) and their receptors (p75NGFR, trkA, trkB, and trkC) in the adult human peripheral nervous system and nonneural tissues. Neurochem Res 1996; 21: 929–938. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Jarskog LF, Vadiamudi S. Maternal infection regulates BDNF and NGF expression in fetal and neonatal brain and maternal-fetal unit of the rat. J Neuroimmunol 2003; 138: 49–55. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Webster M, Knable M, Johnston N, Yolken RH. The Stanley Foundation brain collection and neuropathology consortium. Schizophr Res 2000; 44: 151–155. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Ishikawa M, Iyo M, Hashimoto K. Serum levels of mature brain-derived neurotrophic factor (BDNF) and its precursor proBDNF in healthy subjects. Open Clin Chem J 2012; 5: 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JR, Hewedi DH, Eissa AM, Moustafa AA. The cerebellum and psychiatric disorders. Front Public Health 2015; 3: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller BE, Rodriguez VL, Linke A, Sikirica M, Dirani R, Hauser P. Prevalence of liver disease in veterans with bipolar disorder or schizophrenia. Gen Hosp Psychiatry 2011; 33: 232–237. [DOI] [PubMed] [Google Scholar]
- Hsu JH, Chien IC, Lin CH, Chou YJ, Chou P. Increased risk of chronic liver disease in patients with schizophrenia: a population-based cohort study. Psychosomatics 2014; 55: 163–171. [DOI] [PubMed] [Google Scholar]
- Hsu JH, Chien IC, Lin CH. Increased risk of chronic liver disease in patients with bipolar disorder: a population-based study. Gen Hosp Psychiatry 2016; 42: 54–59. [DOI] [PubMed] [Google Scholar]
- Tonra JR, Ono M, Liu X, Garcia K, Jackson C, Yancopoulos GD et al. Brain-derived neurotrophic factor improves blood glucose control and alleviates fasting hyperglycemia in C57BLKS-Lepr(db)/lepr(db) mice. Diabetes 1999; 48: 588–594. [DOI] [PubMed] [Google Scholar]
- Teillon S, Calderon GA, Rios M. Deminished diet-induced hyperglycemia and dyslipidemia and enhanced expression of PPARα and FGF21 in mice with hepatic ablation of brain-derived neurotrophic factor. J Endocrinol 2010; 205: 37–47. [DOI] [PubMed] [Google Scholar]
- Mighiu PI, Filippi BM, Lam TK. Linking inflammation to the brain-liver axis. Diabetes 2012; 61: 1350–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camer D, Yu Y, Szabo A, Fernandez F, Dinh CH, Huang XF. Bardoxolone methyl prevents high-fat diet-induced alterations in prefrontal cortex signalling molecules involved in recognition memory. Prog Neuropsychopharmacol Biol Psychiatry 2015; 59: 68–75. [DOI] [PubMed] [Google Scholar]
- Camer D, Yu Y, Szabo A, Dinh CH, Wang H, Cheng L et al. Bardoxolone methyl prevents insulin resistance and the development of hepatic steatosis in mice fed a high-fat diet. Mol Cell Endocrinol 2015; 412: 36–43. [DOI] [PubMed] [Google Scholar]
- Jouda J, Wildmann J, Schäfer M, Roggero E, Besedovsky HO, del Rey A. T cells affect central and peripheral noradrenergic mechanisms and neurotrophin concentration in the spleen and hypothalamus. Ann N Y Acad Sci 2012; 1261: 18–25. [DOI] [PubMed] [Google Scholar]
- Yu X, Lu L, Liu Z, Yang T, Gong X, Ning Y et al. Brain-derived neurotrophic factor modulates immune reaction in mice with peripheral nerve xenotransplantation. Neuropsychiatr Dis Treat 2016; 12: 685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K. Brain-derived neurotrophic factor (BDNF) and its precursor proBDNF as diagnostic biomarkers for major depressive disorder and bipolar disorder. Eur Arch Psychiatry Clin Neurosci 2015; 265: 83–84. [DOI] [PubMed] [Google Scholar]
- Zhao G, Zhang C, Chen J, Su Y, Zhou R, Wang F et al. Ratio of mBDNF to proBDNF for differential diagnosis of major depressive disorder and bipolar depression. Mol Neurobiol; e-pub ahead of print 9 September 2016; doi: 10.1007/s12035-016-0098-6. [DOI] [PubMed]