Abstract
Cancer is frequently associated with a hypercoagulable state. Almost 15% of patients with cancer will suffer a thromboembolic event during their clinical course. The aetiology of this hypercoagulable state is multifactorial and includes procoagulant factors associated with malignancy as well as the host's inflammatory response. Cancer-associated thrombophilia can present as venous thromboembolism, migratory superficial thrombophlebitis, arterial thrombosis, disseminated intravascular coagulation, thrombotic microangiopathy and rarely non-bacterial thrombotic endocarditis (NBTE). In this paper, we will describe an uncommon presentation of lung cancer on a non-smoker middle-aged woman, with recent diagnosis of pulmonary embolism, who develops malignant recurrent pleural effusion, NBTE with cutaneous and neurological manifestations, with a rapid evolution into shock, culminating in death. Diagnosis of NBTE requires a high degree of clinical suspicion. The mainstay of treatment is systemic anticoagulation to prevent further embolisation and underlying cancer control whenever is possible.
Keywords: venous thromboembolism, lung cancer (oncology), pulmonary embolism, stroke, valvular diseases
Background
Paraneoplastic pulmonary embolism (PE) was diagnosed 1 month before clinical deterioration. Recurrent malignant pleural effusion and aortic non-bacterial endocarditis occurred prior to lung cancer diagnosis. It was a complex case and most educative, therefore I believe it should be shared in the interest of scientific and medical community.
Case presentation
A 67-year-old woman, non-smoker, with a personal history of hypertension and dyslipidaemia, was admitted to the hospital with a 1-month history of progressive pleuritic chest pain. One week prior to admission, she also presented with productive cough, fever and haemoptysis. She had already been treated with antibiotics and systemic steroids, with no clinical response. Family history was unremarkable for heart or lung diseases as well as for cancer.
On admission, she presented hypotensive (99/67 mm Hg), heart rate of 75 bpm and left lower limb oedema. Laboratorial evaluation showed leucocytosis (13 900×106/L), elevated C-reactive protein (CRP, 3.9 mg/dL) and elevated D-dimer level (48.23 mcg/mL). CT pulmonary angiography (CTPA) revealed bilateral and multisegmental PE with lung infarction (LI). Deep vein thrombosis (DVT) of left popliteal vein was also detected on Doppler ultrasound. She was admitted to the hospital, and anticoagulant treatment with low-molecular weight heparin (LMWH) was started. Clinical improvement was noted and she was discharged on oral anticoagulation with rivaroxaban.
Two weeks later, the patient was readmitted to the hospital with fever and confusion. Since the previous discharge, she started having daily mild fever and sputum-productive cough, despite being on amoxicillin-clavulanic acid and clarithromycin treatment.
On admission, she was confused and disorientated, afebrile, normotensive and polypneic. Laboratory testing showed an increase of acute phase reactants (neutrophilic leucocytosis and CRP), D-dimers (6.17 mg/dL) and lactic dehydrogenase (786 UI/L), with acute kidney injury (blood urea nitrogen (BUN) 20 and serum creatinine 1.8 mg/dL). Arterial blood gas analysis showed respiratory alkalosis with alkalaemia. Head CT scan and chest radiography were unremarkable.
Investigations
Blood, urine and cerebrospinal fluid cultures were performed. Empirical treatment with ceftriaxone and clarithromycin (later changed to metronidazole) was started, and all microbiological cultures were unremarkable. Whole body CT scan revealed LI worsening (without new PE), large right-side pleural effusion (figure 1), unspecified pleural nodules and pyeloureteral junction stenosis causing left hydronephrosis.
Figure 1.
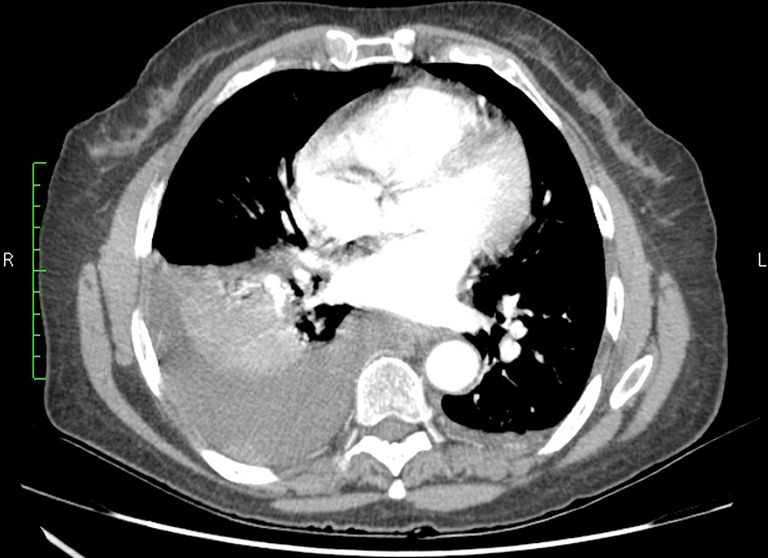
Right-side pleural effusion on chest CT scan.
Thoracentesis was performed with drainage of 1000 mL of sterile haemorrhagic exudate (haematocrit <10%, lactate dehidrogenase (LDH) 1323 UI/L, total protein 4.7 g/dL, 7000 cells being 50% mesothelial cells, 9% cells with increased nucleus/cytoplasm relation), according to Light’s Criteria (pleural/serum LDH >0.6). Pleural biopsy was also performed at that time.
On the 11th day of hospital admission, the patient had sudden global aphasia with left hemiparesis, and the head CT scan revealed bilateral ischaemic cerebral infarction of the parietal lobes. Over the next 3 days, clinical deterioration was noted and splinter haemorrhage appeared (figure 2). Transoesophageal echocardiography showed aortic valve vegetation with moderate to severe regurgitation (figures 3 and 4).
Figure 2.
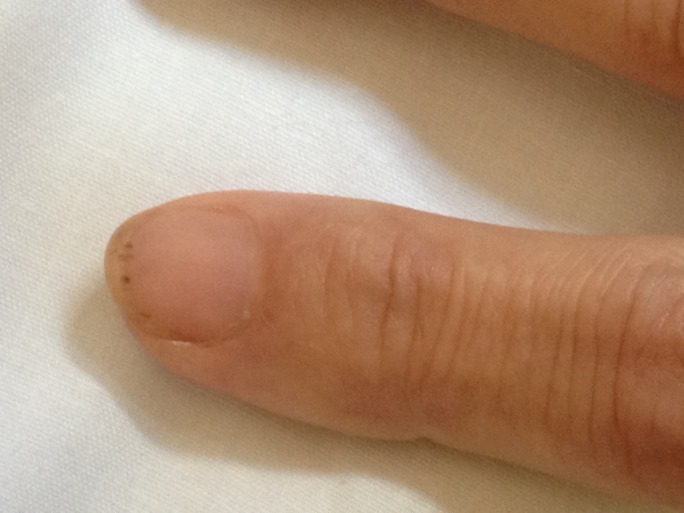
Splinter haemorrhage under the nail.
Figure 3.
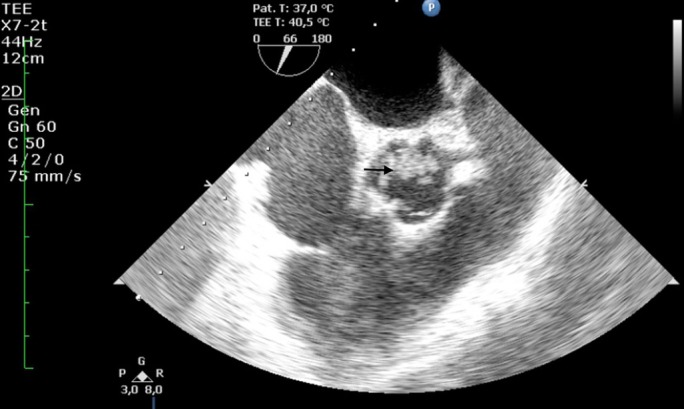
Aortic valve vegetation on transoesophageal echocardiography (TEE) (indicated with arrow).
Figure 4.
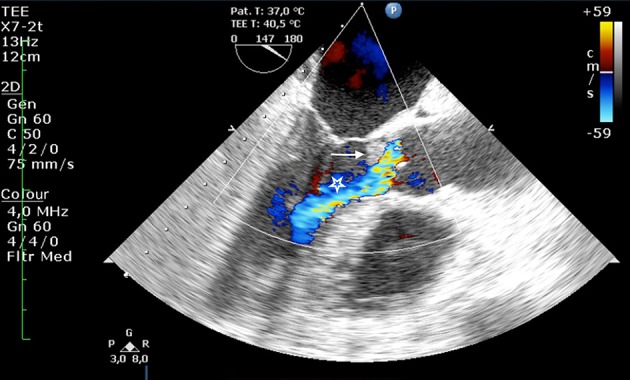
Aortic valve vegetation with moderate to severe regurgitation on transoesophageal echocardiography (TEE) (indicated with arrow and star, respectively).
Differential diagnosis
Further investigation for infectious and autoimmune aetiologies was unremarkable.
Treatment
Antibiotics: ceftriaxone, clarithromycin and metronidazole
Fluids: crystalloids
Vasopressors: norepinephrine
Anticoagulation: LMWH in therapeutic dosage, interrupted for thoracic procedures and restarted after; also, switched to prophylactic dosage after stroke diagnosis
Outcome and follow-up
Progressive respiratory and haemodynamic instability was observed. She was then transferred to the intermediate care unit, where death occurred on the 18th day of admission. Family refused autopsy. Pleural biopsy results available after death revealed lung cancer (papillary adenocarcinoma positive for thyroid transcription factor). Owing to the fact that the diagnosis was only established postmortem, despite high clinical suspicion, no specific treatment to cancer was started.
Discussion
Venous thromboembolism is the most frequent thromboembolic event (TE) in patients with cancer and can precede its diagnosis in years.1 The risk factors are immobilisation, surgery, chemotherapy, adjuvant hormone therapy and the presence of indwelling central venous catheters.2 3 The diagnosis is usually made by limb venous Doppler ultrasound in case of DVT or by CTPA/lung scintigraphy when PE is suspected.
Non-bacterial thrombotic endocarditis (NBTE) is a rare TE that can affect patients with advanced cancer and may occur in 4% of all patients with end-stage cancer. However, the exact prevalence is unknown because it is usually diagnosed postmortem. In a large autopsy study, NBTE was found in 1.6% of the adult population. In this study, 51 of 65 cases of NBTE were associated with malignancies. When featuring signs of embolisation, NBTE's postmortem prevalence rises to 32% in patients with cancer and cerebral ischaemia.4 5
Lung, pancreas, gastric, ovarian and adenocarcinoma of unknown origin are the most common cancers associated with NBTE. Adenocarcinoma represents the most frequent histological type. At the time of diagnosis of NBTE, nearly all of the patients have already disseminated disease.2 5 6
The pathogenesis involves macrophage interaction with malignant cells releasing cytokines, which damage endothelium, promoting platelet deposition, and thrombus formation. Overactivated coagulation cascade enhances the hypercoagulable state, promoting thrombus growth. In this condition, vegetations are sterile, composed of small platelet-fibrin aggregates. There is no evidence of inflammatory reaction at the site of vegetation attachment, which makes them easier to detach and embolise.2 6
The major clinical features of NBTE result from systemic emboli associated with ischaemic events, occurring in 40%–50% of patients. Valve dysfunction can be present, but rarely is symptomatic or severe. Embolisation to central nervous system, kidneys, spleen, limb extremities and coronary arteries are the most common regions.4 6–9 Ischaemic stroke is characterised by multiple areas of infarction of varying sizes and can be responsible for important morbidity.9 Embolisation signs are related with organ’s involvement and include focal neurological deficits, painful or cyanotic extremities, acute abdomen, flank pain and haematuria.2 4
NBTE diagnosis is difficult and requires a high level of suspicion. A clinical triad of a known underlying disease associated to NBTE, new-acquired heart murmur and signs of multiple systemic embolisation strongly suggests NBTE, therefore this condition should always be excluded.10 An echocardiography is required to the diagnosis, being transoesophageal the gold standard, due to higher sensitivity and specificity.11 Patients with cancer with sudden neurological deficit should be submitted ideally to an MRI to differentiate brain metastases from ischaemic lesions.2
In the differential diagnosis between infective endocarditis and NBTE, a global approach should focus on clinical history, physical findings, modified Duke's criteria, echocardiography images and stroke patterns on MRI.12 13
Management of paraneoplastic TE should be directed on treating the underlying cancer and systemic anticoagulation. Long-term anticoagulation should be considered, since recurrent events can occur after medication withdrawal. Heparin is the most effective anticoagulant, especially unfractionated (but also low molecular weight). Oral anticoagulation with vitamin K antagonists is complex and generally not recommended due to drug interactions, malnutrition and being less effective compared with heparin.14–16 Management of the underlying cancer is also important. However, frequently the disease is already disseminated, with indication only for palliative care. Cardiac surgery is rarely necessary and should be reserved for patients with severe valve dysfunction, large vegetations or recurrent embolism despite adequate anticoagulation.6 17
Prognosis is poor and is related to the underlying cancer and other patient’s comorbidities.
Our case illustrates an uncommon presentation of lung cancer gathering some particularities: non-smoker middle-aged woman with no relevant family history, recent paraneoplastic PE before clinical deterioration with malignant recurrent pleural effusion, NBTE with cutaneous and neurological manifestations, and rapid evolution into shock culminating in death. The final diagnosis of lung cancer was established with postmortem studies.
Learning points.
Uncommon presentation of lung cancer on a non-smoker middle-aged woman.
Clinical presentation with recurrent pleural effusion and pleural biopsy was essential for diagnosis.
The finding of new heart valve vegetations, unexplained by other causes, should raise the suspicion of non-bacterial thrombotic endocarditis, particularly in the context of cancer background.
Anticoagulation with heparin and treatment of underlying cancer are essential to prevent recurrent embolisation.
Footnotes
Contributors: TLF conceived the work, and was responsible for drafting of the article. RA was also responsible for drafting of the article. TJ was responsible for critical revision of the article. MFD was supervisor of all the work. All authors contributed to refinement of the drafting and approved the final manuscript.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Deitcher SR. Cancer and thrombosis: mechanisms and treatment. J Thromb Thrombolysis 2003;16:21–31. 10.1023/B:THRO.0000014589.17314.24 [DOI] [PubMed] [Google Scholar]
- 2.el-Shami K, Griffiths E, Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist 2007;12:518–23. 10.1634/theoncologist.12-5-518 [DOI] [PubMed] [Google Scholar]
- 3.Prandoni P, Falanga A, Piccioli A. Cancer and venous thromboembolism. Lancet Oncol 2005;6:401–10. 10.1016/S1470-2045(05)70207-2 [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Frishman WH. Nonbacterial Thrombotic Endocarditis: Pathogenesis, Diagnosis, and Management. Cardiol Rev 2016;24:244–7. 10.1097/CRD.0000000000000106 [DOI] [PubMed] [Google Scholar]
- 5.Biller J, Challa VR, Toole JF, et al. Nonbacterial thrombotic endocarditis. A neurologic perspective of clinicopathologic correlations of 99 patients. Arch Neurol 1982;39:95–8. [DOI] [PubMed] [Google Scholar]
- 6.Wigger O, Windecker S, Bloechlinger S. Nonbacterial thrombotic endocarditis presenting as intracerebral hemorrhage. Wien Klin Wochenschr 2016;128:922–4. 10.1007/s00508-016-1020-y [DOI] [PubMed] [Google Scholar]
- 7.Graus F, Rogers LR, Posner JB. Cerebrovascular complications in patients with cancer. Medicine 1985;64:16–35. 10.1097/00005792-198501000-00002 [DOI] [PubMed] [Google Scholar]
- 8.Joshi SB, Richards MJ, Holt DQ, et al. Marantic endocarditis presenting as recurrent arterial embolisation. Int J Cardiol 2009;132:e14–e16. 10.1016/j.ijcard.2007.07.106 [DOI] [PubMed] [Google Scholar]
- 9.Rogers LR, Cho ES, Kempin S, et al. Cerebral infarction from non-bacterial thrombotic endocarditis. Clinical and pathological study including the effects of anticoagulation. Am J Med 1987;83:746–56. [DOI] [PubMed] [Google Scholar]
- 10.Gundersen H, Moynihan B. An Uncommon Cause of Stroke: Non-bacterial Thrombotic Endocarditis. J Stroke Cerebrovasc Dis 2016;25:e163–e164. 10.1016/j.jstrokecerebrovasdis.2015.11.009 [DOI] [PubMed] [Google Scholar]
- 11.Roldan CA, Qualls CR, Sopko KS, et al. Transthoracic versus transesophageal echocardiography for detection of Libman-Sacks endocarditis: a randomized controlled study. J Rheumatol 2008;35:224–9. [PubMed] [Google Scholar]
- 12.Ménard GE. Establishing the diagnosis of Libman-Sacks endocarditis in systemic lupus erythematosus. J Gen Intern Med 2008;23:883–6. 10.1007/s11606-008-0627-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singhal AB, Topcuoglu MA, Buonanno FS. Acute ischemic stroke patterns in infective and nonbacterial thrombotic endocarditis: a diffusion-weighted magnetic resonance imaging study. Stroke 2002;33:1267–73. 10.1161/01.STR.0000015029.91577.36 [DOI] [PubMed] [Google Scholar]
- 14.Salem DN, Stein PD, Al-Ahmad A, et al. Antithrombotic therapy in valvular heart disease--native and prosthetic: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest 2004;126::457S–-482S. 10.1378/chest.126.3_suppl.457S [DOI] [PubMed] [Google Scholar]
- 15.Liu J, Frishman WH. Nonbacterial Thrombotic Endocarditis. Cardiol Rev 2016;24:244–7. 10.1097/CRD.0000000000000106 [DOI] [PubMed] [Google Scholar]
- 16.Mandalà M, Falanga A, Roila F. Clinical practice guidelines management of venous thromboembolism (VTE) in Cancer patients: esmo clinical Practice guidelines on behalf of the ESMO guidelines Working Group. Ann Oncol 2011;22:85–92. [DOI] [PubMed] [Google Scholar]
- 17.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for themanagement of patients with valvular heart disease: executive summary: a report of the AmericanCollege of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014:129. [DOI] [PubMed] [Google Scholar]


