Abstract
Fabry’s disease (FD) is a recognised mimic of multiple sclerosis (MS). It is an X-linked storage lysosomal disorder with deficiency of α-galactosidase A and enzyme replacement therapy is available. Patients with FD may satisfy modified McDonald criteria if the diagnosis of FD has not been pursued. We present a case of FD in a 65-year-old woman masquerading as benign MS for 40 years. She has recurrent posterior circulation stroke-like symptoms, hearing loss and acroparaesthesia, but typical radiological features of MS on MRI brain. Later she developed an ischaemic stroke, infiltrative cardiomyopathy and chronic renal failure. There was a missense mutation at p.R342Q in the galactodisdase alpha (GLA) gene. Neurologists need to consider FD and look for red flags in atypical MS cases and should not be over-reliant on MRI findings. Missed diagnosis of FD could lead to unnecessary immunosuppression, inappropriate disease counselling and missed treatment opportunity.
Keywords: Neurology, Multiple sclerosis, Neurogenetics
Background
Fabry’s disease (FD) is a recognised mimic of multiple sclerosis (MS). Patients with FD may satisfy modified McDonald criteria if the diagnosis of FD has not been pursued.1 However, there are often red flags to alert clinicians to alternative diagnoses. We report a case of FD in a woman masquerading as benign MS for 40 years.
Case presentation
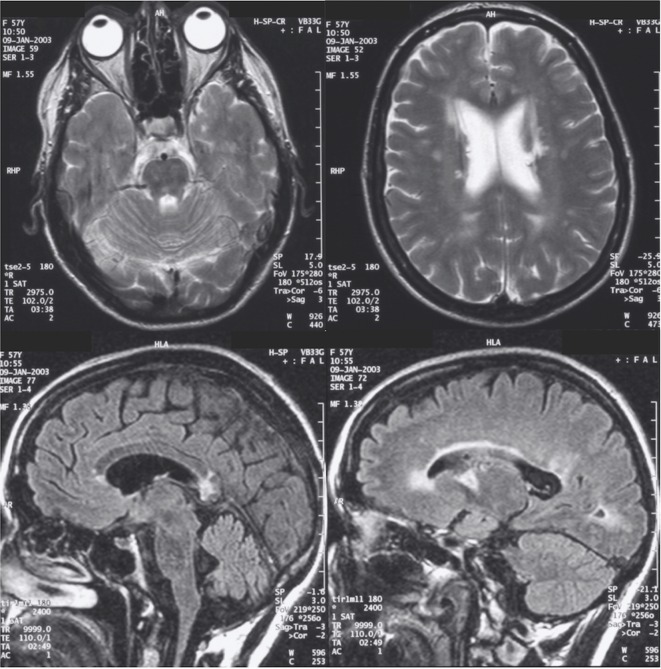
A 65-year-old woman had a diagnosis of MS since young adulthood. She developed transient left facial weakness at 16 years old and transient diplopia at 20 years old. Both of these episodes lasted 1 week and resolved without treatment. Her neurologist started her on interferon beta after she experienced another transient episode of diplopia at 52 years old. Her interferon treatment was complicated by seropositive cutaneous lupus and Sjogren’s syndrome. We reviewed her in our MS clinic and stopped the interferon due to stability of her disease over 30 years. The MR scan of her head at age 56 years showed asymmetrical confluent white matter signal abnormalities with significant involvement of the corpus callosum and deep white matter cavitatory lesions. Periaqueductal grey, pontine and cerebellar peduncle also had subtle T2/fluid-attenuated inversion recovery (FLAIR) hyperintensities. There were no gadolinium-enhancing lesions or major cerebral artery abnormalities (figure 1). On the repeated MRI head 2 years later, the changes were stable. Her MRI spine was normal. Cerebrospinal fluid (CSF)did not show unmatched oligoclonal bands. Afterwards, she received bilateral cochlear implants due to bilateral progressive hearing loss of unknown cause from childhood. At the age of 64 years, the patient suffered from a left middle cerebral artery ischaemic stroke with the presenting symptom of mild dysphasia. She developed progressive dyspnoea and exertional angina after her stroke. On systems review, she reported that she suffered from intermittent widespread arthralgia, burning feet, urinary urgency and colonic hypomobility.
Figure 1.
The top panel shows T2 axial subtle pontine hyperintensity (left) and confluent periventricular hyperintensity (right). The bottom panel shows T2 sagittal corpus callosum involvement (left) and periventricular hyperintensity (right).
Her prior medical history also included hypertension, migraines, asthma, depression, osteoporosis and fibromyalgia. Her medications consisted of Plaquenil, indomethacin, diazepam, aspirin, ranitidine and an oestradiol patch. She has two healthy daughters and both of her parents died in their 80s with cardiovascular disease. The remainder of the family history was not contributory.
Her examination showed mild left facial droop and ataxic paraparesis. She had hyperreflexia and hyperaesthesia in lower limbs. Plantar responses were downgoing. There was no evidence of skin angiokeratoma or corneal opacities.
Investigations
Echocardiography showed severe left ventricular hypertrophy consistent with infiltrative cardiomyopathy. Her estimated glomerular filtration rate was 41 mL/min. Renal ultrasound revealed multiple simple renal cysts. Urinary protein creatine ratio was 18 mg/mmol. Nerve conduction studies did not reveal any large fibre neuropathy or active denervation. Cochlear implants precluded further MR scan. Genetic mutation at p.R342Q in the galactodisdase alpha (GLA) gene was detected. Cascade genetic screening did not identify any further affected family members.
Treatment
She was commenced on agalsidase-alfa and ramipril to prevent further deterioration of cardiac and renal function. She was also started on clopidogrel and atorvastatin for secondary stroke prevention; pregabalin and tapentadol for neuropathic pain.
Outcome and follow-up
At 2 years of follow-up, her cardiac and renal function remains stable on enzyme replacement therapy and she has not developed any further stroke-like episodes.
Discussion
FD is an X-linked lysosomal storage disorder with mutations in GLA gene and deficiency of enzyme α-galactosidase A.2 Its incidence ranges from 1:40 000 to 1:11 700. Glycosphingolipids, predominantly globotriaosylceramide, accumulate in lysosomes of cells throughout the body, but preferentially affect the skin, eyes, kidney, heart, brain and nerve. Acroparaesthesia, hypohidrosis, angiokeratoma and corneal opacities occur early in hemizygous men. Ischaemic strokes, infiltrative cardiomyopathy, myocardial infarction and renal failure occur in the third to fifth decades. Heterozygous women with FD are not just carriers but have variable penetrance due to variable X-inactivation with different tissues.3 A recent case series suggests that FB heterozygous D313Y mutation can manifest as central nervous system symptoms alone.4 Clinicians can use serum α-galactosidase A level as a screening test but GLA gene mutation testing is required for confirmation of FD, especially in heterozygous women. Most importantly, enzyme replacement therapies can prevent progression of lethal cardiac and renal complications of FD.5
Our patient has sporadic-onset FD presented with recurrent posterior circulation stroke-like symptoms, hearing loss and acroparaesthesia. Her diagnosis of MS was made in the pre-MRI era based on the original criteria. Her MRI head showed typical radiological features of MS. In retrospect, she did not satisfy the caveat of ‘no better explanation.’1 Absence of unmatched CSF oligoclonal bands and normal MRI spine argue against the diagnosis of MS. One unique feature of our patient is that profound hearing loss in heterozygous women has not been documented in the literature to the best of our knowledge.6 Several reviews have proposed potential red flags for FD: (1) neurological features: prominent ischaemic stroke or recurrent stroke-like episodes, hearing loss; (2) extraneurological features: renal disease, cutaneous angiokeratomas, cardiomyopathy, cornea/lenticular opacity, recurrent abdominal pain; (3) family history of unspecified renal, cardiac or cerebrovascular disease; (4) ancillary test: absence of unmatched CSF oligoclonal bands; (5) radiological features: pulvinar T1 hyperintensity, vertebrobasilar dolichoectasia, normal MRI spine.7 8
FD poses a diagnostic challenge with its overlapping features with MS and variable presentations and radiological features, especially in heterozygous women. Neurologists should consider FD in atypical MS cases and should not be over-reliant on MRI findings. Missed diagnosis of FD could lead to unnecessary immunosuppression, inappropriate disease counselling and missed treatment opportunity.
Learning points.
Heterozygote women with Fabry’s disease (FD) could present with brainstem symptoms and neuroimaging findings compatible with the diagnosis of multiple sclerosis.
Normal MRI spine, absence of unmatched CSF oligoclonal bands, small fibre neuropathy and other organ involvement should prompt clinicians to revisit the diagnosis of multiple sclerosis.
Enzyme replacement therapy can prevent progression of lethal complications of FD.
Footnotes
Contributors: WYY, MJF-P and AK contributed equally to the conception and planning of the case report; the drafting and revisions of the manuscript; approval of the final version of the manuscript; and ensured that all questions regarding the accuracy and integrity of the manuscript have been investigated and resolved.
Competing interests: None declared.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fellgiebel A, Müller MJ, Ginsberg L. CNS manifestations of Fabry's disease. Lancet Neurol 2006;5:791–5. 10.1016/S1474-4422(06)70548-8 [DOI] [PubMed] [Google Scholar]
- 3.Wang RY, Lelis A, Mirocha J, et al. Heterozygous Fabry women are not just carriers, but have a significant burden of disease and impaired quality of life. Genet Med 2007;9:34–45. 10.1097/GIM.0b013e31802d8321 [DOI] [PubMed] [Google Scholar]
- 4.Becker J, Rolfs A, Karabul N, et al. D313Y mutation in the differential diagnosis of white matter lesions: Experiences from a multiple sclerosis outpatient clinic. Mult Scler 2016;22:1502–5. 10.1177/1352458516638747 [DOI] [PubMed] [Google Scholar]
- 5.Eng CM, Guffon N, Wilcox WR, et al. Safety and efficacy of recombinant human alpha-galactosidase A replacement therapy in Fabry's disease. N Engl J Med 2001;345:9–16. 10.1056/NEJM200107053450102 [DOI] [PubMed] [Google Scholar]
- 6.Ries M, Kim HJ, Zalewski CK, et al. Neuropathic and cerebrovascular correlates of hearing loss in Fabry disease. Brain 2007;130:143–50. 10.1093/brain/awl310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weisfeld-Adams JD, Katz Sand IB, Honce JM, et al. Differential diagnosis of Mendelian and mitochondrial disorders in patients with suspected multiple sclerosis. Brain 2015;138:517–39. 10.1093/brain/awu397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Böttcher T, Rolfs A, Tanislav C, et al. Fabry disease - underestimated in the differential diagnosis of multiple sclerosis? PLoS One 2013;8:e71894 10.1371/journal.pone.0071894 [DOI] [PMC free article] [PubMed] [Google Scholar]