Early efforts toward human cancer immunotherapy were hindered by the absence of identified antigens to target by vaccination or cell therapy. In the past decade, studies examining CD8+ and CD4+ T cell responses to melanoma and other tumors have uncovered several classes of proteins that give rise to peptides presented by MHC molecules. These include (i) differentiation antigens expressed in the tumor and its normal tissue counterpart e.g., tyrosinase; (ii) cancer-testes antigens expressed in the testes and a variety of malignancies e.g., MAGE-1; and (iii) tumor-specific antigens that arise from mutations in tumor cells e.g., CDK-4 (1–4). The observation that autoimmune depigmentation of the skin (vitiligo) developed in some melanoma patients with tumor regression after IL-2 therapy provided optimism that antigens derived from self-proteins might have utility as targets for immunotherapy (5). Animal model studies demonstrated that T cell immunity could be elicited to tissue-specific self-proteins and promote tumor regression without severe autoimmune injury and provided rationale for clinical vaccine trials targeting self-proteins (6–9). However, with a few exceptions the results of vaccination for human malignancy have been disappointing (10–13). It appears the problem is caused by central and peripheral tolerance mechanisms that limit the repertoire of self-reactive T cells to those of low avidity to prevent autoimmunity, making it difficult to elicit a T cell response sufficient to eradicate tumor (14–16). Recent studies have suggested that the balance between immunity and tolerance is regulated at the immunologic synapse between T cells and specialized bone marrow-derived dendritic cells (DC) that present antigens to T cells (17–19). In a recent issue of PNAS, Fong et al. (20) provide evidence that a key to inducing tumor immunity may lie at this interface. The investigators target carcinoembryonic antigen (CEA), a glycoprotein expressed in normal gastrointestinal (GI) and genitourinary epithelial cells and in most adenocarcinomas of GI origin, 50% of breast cancers, and 70% of non-small-cell lung cancer (21). They demonstrate that vaccination of patients with DC displaying a CEA peptide altered to promote more efficient engagement of T cell receptors elicited high levels of CD8+ cytotoxic T cells (CTL) specific for native CEA. Remarkably, the induction of CTL was associated with tumor regression in some patients with advanced cancer without autoimmunity (20).
The balance between immunity and tolerance is regulated at the immunologic synapse between T cells and specialized bone marrow-derived dendritic cells that present antigens to T cells.
The activation and differentiation of tumor-reactive T cells by vaccination has centered on manipulating two variables, the antigen-presenting cell (APC) and antigen. DC are specialized APCs strategically located in tissues where in their immature form they are proficient at capturing and processing antigen (22). DC maturation is induced by pathogens or inflammatory mediators and is characterized by expression of CCR-7, which promotes trafficking to T cell zones of secondary lymphoid organs, and up-regulation of MHC, costimulatory, and adhesion molecules, which collectively permit the formation of a synapse with naïve T cells expressing a T cell receptor of sufficient avidity (22–24). In animal models, inoculation of DC pulsed with peptides, transfected with RNA- or DNA-encoding tumor antigens, or fused to tumor cells induces tumor-specific immunity (25). Thus, DC have been viewed as the vaccine vehicle of choice for overcoming tolerance to self-antigens.
An obstacle for human studies is obtaining sufficient cells for vaccination because DC comprise <1% of leukocytes in the blood (22). One approach is to administer Flt-3 ligand (Flt3-L), which activates the Flt-3 receptor tyrosine kinase and serves as a growth and differentiation factor for hematopoietic progenitors and expands DC in vivo (26). In normal mice, administration of Flt-3L increased both myeloid and lymphoid DC subsets in blood, lymph nodes, and spleen (27). In mice bearing an immunogenic MCA sarcoma, Flt-3L caused infiltration of DC into the tumor and tumor regression mediated by CD8+ CTL (28). Administration of Flt-3L to normal individuals and patients with cancer also increased DC numbers in the blood and infiltration of DC into tumor metastases but did not cause tumor regression (29, 30). Fong et al. (20) show that administration of Flt-3L can facilitate procurement of DC to permit evaluation of larger cell doses in vaccine studies. The yield of DC obtained by leukapheresis was increased by >60-fold after Flt-3L administration, and with brief in vitro culture the mobilized DC up-regulated expression of CD80, CD83, CD86, MHC, and CCR-7 molecules consistent with acquisition of a mature phenotype (20).
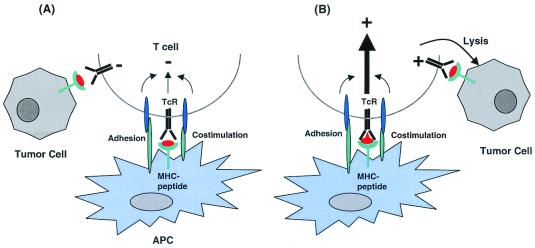
Flt-3L assists in obtaining APCs for vaccination but the formidable task is to display tumor-associated self-antigen in a form that is effective for inducing T cell responses. Insight into how this might be accomplished can be derived from the observation that the quality and duration of T cell receptor signaling at the synapse between DC and T cell influences T cell activation (31). It follows that the ability of a peptide antigen to elicit responses will be related to its affinity for the MHC molecule, determined by the presence of favored amino acids at critical anchor positions involved in MHC binding and by the affinity of the MHC-peptide complex for the T cell receptor. Thus, altered peptide ligands containing amino acid substitutions at residues that anchor the peptide in the MHC binding groove or contact the T cell receptor can inhibit or enhance T cell signaling (Fig. 1) (31–34). The relevance for vaccination was demonstrated in murine tumor models and patients with melanoma in which peptides modified at MHC anchor residues were shown to elicit superior T cell responses to the original unmodified antigen (13, 35). Similarly, vaccination with an altered peptide for a class I-restricted murine tumor antigen that increased the stability of the interaction between MHC-peptide complex and the T cell receptor also enhanced T cell responses and tumor protection when compared with the natural epitope (36).
Figure 1.
Altering the peptide ligand at the immunologic synapse elicits an effector T cell response to a self-antigen. (A) Self-antigens are displayed as peptides bound to MHC on professional APC but because of the low avidity of the T cell repertoire, insufficient signaling is generated to induce an effector T cell response and tumor cells expressing the self-antigen are ignored. (B) Altering amino acid residues of the peptide that protrude out from the MHC molecule and contact the T cell receptor (TcR) improves the affinity of the interaction and promotes signaling and T cell activation. The result is expansion of self-reactive T cells and differentiation to effector cells that have a lower threshold for activation and recognize tumor cells expressing the native self-peptide.
In the study by Fong et al. (20), this principle that peptides modified in T cell receptor contact residues can enhance immunogenicity and break tolerance to a self-protein is extended to a candidate human tumor antigen, CEA. Previous trials using recombinant viruses encoding CEA to vaccinate patients elicited a low frequency of CTL specific for a nonamer CEA peptide (CAP1) presented by HLA A2 (37). Subsequently, DC pulsed with CAP1 was used as a vaccine but failed to induce clinical responses in patients with CEA-positive malignancy (38). CAP1 contains preferred amino acids at anchor positions and binds HLA A2 with high affinity, suggesting alterations to promote MHC binding would not improve immunogenicity. However, an altered CAP1 ligand (CAP1–6D) containing aspartic acid in place of asparagine at position 6, which is predicted by crystallographic data to protrude toward the T cell receptor, was more effective than CAP1 for eliciting tumor-reactive CD8+ CTL in vitro and stimulated greater phosphorylation of the T cell receptor ζ chain and ZAP-70 (39). Fong et al. (20) now show that vaccination of cancer patients with DC pulsed with CAP1–6D and a keyhole limpet hemocyanin antigen to provide T cell help induced CD8+ CTL that lyse CEA-expressing tumor cells. In five patients, the magnitude of CTL responses achieved with vaccination was substantial, exceeding 1% of CD8+ T cells in the blood as measured by staining with a CAP1 or CAP1–6D tetramer. These tetramer-positive cells exhibited a CD45RA+, CD44+, CD27−, and CCR7− phenotype consistent with differentiation to effector T cells.
The striking and hopeful finding in the study by Fong et al. (20) was the regression of metastatic colon cancer in two of the 12 vaccinated patients. One additional patient had a mixed response and two others had stable disease. These clinical responses correlated with increases in tetramer-positive T cells, implicating CD8+ CTL in tumor regression. Colon cancer has not been considered responsive to immunotherapy, and these results are dramatic in view of the advanced stage of the tumors. To build on these results it will be important to discern the contribution to antitumor activity provided by each of the components of the regimen, which included Flt-3L, DC, altered peptide ligand, and the keyhole limpet hemocyanin helper antigen. Obstacles to tumor eradication identified in prior studies may emerge, including outgrowth of antigen loss variants (40), failure of T cells to infiltrate tumor masses (41), anergy or deletion of reactive T cells (42, 43), and autoimmunity if the self-reactive T cell response elicited by vaccination is too vigorous (44, 45). Nevertheless, these provocative findings provide optimism that other interventions at the DC:T cell interface such as augmenting costimulation or reducing inhibitory signals may have utility for human cancer vaccines (46, 47).
Footnotes
See companion article on page 8809 in issue 15 of volume 98.
References
- 1.Boon T, van der Bruggen P. J Exp Med. 1996;183:725–729. doi: 10.1084/jem.183.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg S A. Immunity. 1999;10:281–287. doi: 10.1016/s1074-7613(00)80028-x. [DOI] [PubMed] [Google Scholar]
- 3.Van den Eynde B, Peeters O, De Backer O, Gaugler B, Lucas S, Boon T. J Exp Med. 1995;182:689–698. doi: 10.1084/jem.182.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg S A. Nature (London) 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberg S A, White D E. J Immunother Emphasis Tumor Immunol. 1996;19:81–84. [PubMed] [Google Scholar]
- 6.Morgan D J, Kreuwel H T, Fleck S, Levitsky H I, Pardoll D M, Sherman L A. J Immunol. 1998;160:643–651. [PubMed] [Google Scholar]
- 7.Ohlen C, Kalos M, Hong D J, Shur A C, Greenberg P D. J Immunol. 2001;166:2863–2870. doi: 10.4049/jimmunol.166.4.2863. [DOI] [PubMed] [Google Scholar]
- 8.Overwijk W W, Lee D S, Surman D R, Irvine K R, Touloukian C E, Chan C C, Carroll M W, Moss B, Rosenberg S A, Restifo N P. Proc Natl Acad Sci USA. 1999;96:2982–2987. doi: 10.1073/pnas.96.6.2982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pardoll D M. Proc Natl Acad Sci USA. 1999;96:5340–5342. doi: 10.1073/pnas.96.10.5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu F J, Benike C, Fagnoni F, Liles T M, Czerwinski D, Taidi B, Engleman E G, Levy R. Nat Med. 1996;2:52–58. doi: 10.1038/nm0196-52. [DOI] [PubMed] [Google Scholar]
- 11.Marchand M, Weynants P, Rankin E, Arienti F, Belli F, Parmiani G, Cascinelli N, Bourlond A, Vanwijck R, Humblet Y, et al. Int J Cancer. 1995;63:883–885. doi: 10.1002/ijc.2910630622. [DOI] [PubMed] [Google Scholar]
- 12.Nestle F O, Alijagic S, Gilliet M, Sun Y, Grabbe S, Dummer R, Burg G, Schadendorf D. Nat Med. 1998;4:328–332. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg S A, Yang J C, Schwartzentruber D J, Hwu P, Marincola F M, Topalian S L, Restifo N P, Dudley M E, Schwarz S L, Spiess P J, et al. Nat Med. 1998;4:321–327. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.von Boehmer H, Kisielow P. Science. 1990;248:1369–1373. doi: 10.1126/science.1972594. [DOI] [PubMed] [Google Scholar]
- 15.Rocha B, von Boehmer H. Science. 1991;251:1225–1228. doi: 10.1126/science.1900951. [DOI] [PubMed] [Google Scholar]
- 16.Kamradt T, Mitchison N A. N Engl J Med. 2001;344:655–664. doi: 10.1056/NEJM200103013440907. [DOI] [PubMed] [Google Scholar]
- 17.Lanzavecchia A, Sallusto F. Nat Immunol. 2001;2:487–492. doi: 10.1038/88678. [DOI] [PubMed] [Google Scholar]
- 18.Grakoui A, Bromley S K, Sumen C, Davis M M, Shaw A S, Allen P M, Dustin M L. Science. 1999;285:221–227. [PubMed] [Google Scholar]
- 19.Heath W R, Kurts C, Miller J F, Carbone F R. J Exp Med. 1998;187:1549–1553. doi: 10.1084/jem.187.10.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fong L, Hou Y, Rivas A, Benike C, Yuen A, Fisher G A, Davis M M, Engleman E G. Proc Natl Acad Sci USA. 2001;98:8809–8814. doi: 10.1073/pnas.141226398. . (First Published June 26, 2001; 10.1073/pnas.141226398) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hammarstrom S. Semin Cancer Biol. 1999;9:67–81. doi: 10.1006/scbi.1998.0119. [DOI] [PubMed] [Google Scholar]
- 22.Banchereau J, Steinman R M. Nature (London) 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 23.Cyster J G. J Exp Med. 1999;189:447–450. doi: 10.1084/jem.189.3.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dieu M C, Vanbervliet B, Vicari A, Bridon J M, Oldham E, Ait-Yahia S, Briere F, Zlotnik A, Lebecque S, Caux C. J Exp Med. 1998;188:373–386. doi: 10.1084/jem.188.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu Y J, Pulendran B, Palucka K. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 26.Lyman S D, James L, Vanden Bos T, de Vries P, Brasel K, Gliniak B, Hollingsworth L T, Picha K S, McKenna H J, Splett R R. Cell. 1993;75:1157–1167. doi: 10.1016/0092-8674(93)90325-k. [DOI] [PubMed] [Google Scholar]
- 27.Maraskovsky E, Brasel K, Teepe M, Roux E R, Lyman S D, Shortman K, McKenna H J. J Exp Med. 1996;184:1953–1962. doi: 10.1084/jem.184.5.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lynch D H, Andreasen A, Maraskovsky E, Whitmore J, Miller R E, Schuh J C. Nat Med. 1997;3:625–631. doi: 10.1038/nm0697-625. [DOI] [PubMed] [Google Scholar]
- 29.Maraskovsky E, Daro E, Roux E, Teepe M, Maliszewski C R, Hoek J, Caron D, Lebsack M E, McKenna H J. Blood. 2000;96:878–884. [PubMed] [Google Scholar]
- 30.Morse M A, Nair S, Fernandez-Casal M, Deng Y, St. Peter M, Williams R, Hobeika A, Mosca P, Clay T, Cumming R I, et al. J Clin Oncol. 2000;18:3883–3893. doi: 10.1200/JCO.2000.18.23.3883. [DOI] [PubMed] [Google Scholar]
- 31.Iezzi G, Karjalainen K, Lanzavecchia A. Immunity. 1998;8:89–95. doi: 10.1016/s1074-7613(00)80461-6. [DOI] [PubMed] [Google Scholar]
- 32.Kersh G J, Kersh E N, Fremont D H, Allen P M. Immunity. 1998;9:817–826. doi: 10.1016/s1074-7613(00)80647-0. [DOI] [PubMed] [Google Scholar]
- 33.Kersh G J, Miley M J, Nelson C A, Grakoui A, Horvath S, Donermeyer D L, Kappler J, Allen P M, Fremont D H. J Immunol. 2001;166:3345–3354. doi: 10.4049/jimmunol.166.5.3345. [DOI] [PubMed] [Google Scholar]
- 34.Lucas B, Stefanova I, Yasutomo K, Dautigny N, Germain R N. Immunity. 1999;10:367–376. doi: 10.1016/s1074-7613(00)80036-9. [DOI] [PubMed] [Google Scholar]
- 35.Dyall R, Bowne W B, Weber L W, LeMaoult J, Szabo P, Moroi Y, Piskun G, Lewis J J, Houghton A N, Nikolic-Zugic J. J Exp Med. 1998;188:1553–1561. doi: 10.1084/jem.188.9.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Slansky J E, Rattis F M, Boyd L F, Fahmy T, Jaffee E M, Schneck J P, Margulies D H, Pardoll D M. Immunity. 2000;13:529–538. doi: 10.1016/s1074-7613(00)00052-2. [DOI] [PubMed] [Google Scholar]
- 37.Tsang K Y, Zaremba S, Nieroda C A, Zhu M Z, Hamilton J M, Schlom J. J Natl Cancer Inst. 1995;87:982–990. doi: 10.1093/jnci/87.13.982. [DOI] [PubMed] [Google Scholar]
- 38.Morse M A, Deng Y, Coleman D, Hull S, Kitrell-Fisher E, Nair S, Schlom J, Ryback M E, Lyerly H K. Clin Cancer Res. 1999;5:1331–1338. [PubMed] [Google Scholar]
- 39.Salazar E, Zaremba S, Arlen P M, Tsang K Y, Schlom J. Int J Cancer. 2000;85:829–838. doi: 10.1002/(sici)1097-0215(20000315)85:6<829::aid-ijc16>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 40.Riker A, Cormier J, Panelli M, Kammula U, Wang E, Abati A, Fetsch P, Lee K H, Steinberg S, Rosenberg S, Marincola F. Surgery. 1999;126:112–120. [PubMed] [Google Scholar]
- 41.Hanson H L, Donermeyer D L, Ikeda H, White J M, Shankaran V, Old L J, Shiku H, Schreiber R D, Allen P M. Immunity. 2000;13:265–276. doi: 10.1016/s1074-7613(00)00026-1. [DOI] [PubMed] [Google Scholar]
- 42.Staveley-O'Carroll K, Sotomayor E, Montgomery J, Borrello I, Hwang L, Fein S, Pardoll D, Levitsky H. Proc Natl Acad Sci USA. 1998;95:1178–1183. doi: 10.1073/pnas.95.3.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee P P, Yee C, Savage P A, Fong L, Brockstedt D, Weber J S, Johnson D, Swetter S, Thompson J, Greenberg P D, et al. Nat Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 44.Ludewig B, Ochsenbein A F, Odermatt B, Paulin D, Hengartner H, Zinkernagel R M. J Exp Med. 2000;191:795–804. doi: 10.1084/jem.191.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yee C, Thompson J A, Roche P, Byrd D R, Lee P P, Piepkorn M, Kenyon K, Davis M M, Riddell S R, Greenberg P D. J Exp Med. 2000;192:1637–1644. doi: 10.1084/jem.192.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Elsas A, Hurwitz A A, Allison J P. J Exp Med. 1999;190:355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu M, Terasawa H, Gulley J, Panicali D, Arlen P, Schlom J, Tsang K Y. Cancer Res. 2001;61:3725–3734. [PubMed] [Google Scholar]