Abstract
Genetic and environmental factors contribute to the development of immune-mediated diseases. Although numerous genetic factors contributing to autoimmunity have been identified in recent years, our knowledge on environmental factors contributing to the pathogenesis of autoimmune diseases and the mechanisms involved is still limited. In this context, the diet, microbiome, geographical location, as well as environmental pollutants have been shown to modulate autoimmune disease development. These environmental factors interact with cellular components of the immune system in distinct and defined ways and can influence immune responses at the transcriptional and protein level. Moreover, endogenous metabolites generated from basic cellular processes such as glycolysis and oxidative phosphorylation also contribute to the shaping of the immune response. In this minireview, we highlight recent progress in our understanding of the modulation of the immune response by the aryl hydrocarbon receptor (AhR), a ligand-activated transcription factor whose activity is regulated by small molecules provided by diet, commensal flora, environmental pollutants, and metabolism. We focus on the role of AhR in integrating signals from the diet and the intestinal flora to modulate ongoing inflammation in the central nervous system, and we also discuss the potential therapeutic value of AhR agonists for multiple sclerosis and other autoimmune diseases.
Keywords: aryl hydrocarbon receptor (AhR), astrocyte, immunology, lymphocyte, neuroimmunology
Introduction
Multiple sclerosis (MS) 4 is an autoimmune disease of the central nervous system (CNS). In 85% of the affected individuals, MS initially presents with a relapsing-remitting course characterized by temporally defined relapses, which are followed by a varying degree of remission. Most patients eventually enter the secondary progressive phase of MS, which is characterized by the progressive and irreversible accumulation of neurological deficits (1). Several biological pathways are involved in the regulation of the immune response in MS both in the peripheral and central immune compartment and are thought to play dominant roles in the different stages of the disease. Although the role of several molecules such as cytokines and toll-like receptor agonists in MS pathology has been studied at length, particularly in relation to the relapsing-remitting phase of the disease, only recently has the role of metabolites generated in basic biological processes such as glycolysis and oxidative phosphorylation become appreciated with regard to their relevance for the development, propagation, and resolution of autoimmune inflammation. Prominent examples of endogenous metabolites with central roles in MS pathology include bioactive lipids, reactive oxygen species, and adenosine triphosphate (ATP) (2–13). The effects of these endogenous metabolites are modulated by a multitude of exogenous factors such as pollutants, dietary factors, and products from the commensal flora to trigger transcriptional programs that control the immune response during MS. Ultimately, the understanding of these molecular mechanisms is important for the identification of druggable targets for efficacious and safe therapeutic intervention in MS and other immune-mediated diseases.
Aryl hydrocarbon receptor (AhR) structure and function
AhR is a ligand-dependent transcription factor that can be activated by a broad range of molecules provided by the environment, diet, commensal microbiota, and metabolism (14, 15). Upon ligand binding, AhR translocates to the nucleus and regulates the expression of diverse and ligand-specific target genes involved in detoxification (Cyp1a1 (16)), NF-κB regulation (17), or immune regulation (15, 18–20), among others. In that way, AhR plays an important role in the regulation of autoimmune inflammatory diseases of the gut (e.g. Crohn's disease and ulcerative colitis) (21, 22), connective tissue (rheumatoid arthritis) (23), the skin (psoriasis) (14, 24, 25), and the central nervous system (MS) (2, 6, 7, 9, 10, 26–30). AhR regulates the inflammatory response at multiple levels, acting on immune cells in the periphery (e.g. T-cells, dendritic cells, intraepithelial lymphocytes (IELs)) and locally at the site of ongoing inflammation. Thus, AhR integrates environmental and metabolic signals into systemic and local immune regulation.
AhR is responsive to a variety of ligands that fall into two major classes categorized by chemical structure. The first class is composed of tryptophan derivatives that can be derived from the diet or endogenously produced by the host organism (14, 15, 27, 31, 32). These tryptophan-derived ligands include indoles, 6-formylindolo(3,2-b)carbazole (FICZ), 2-(1H-indol-3-ylcarbonyl)-4-thiazolecarboxylic acid methyl ester, and kynurenine. A second class of AhR ligands contains aromatic hydrocarbons, which are largely derived from environmental toxins (33). Some aromatic hydrocarbon ligands that activate AhR include 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and polychlorinated dibenzofuran (34). Interestingly, in response to these distinct classes of ligands or even within subclasses of each group, AhR exhibits differential cellular responses that may activate distinct downstream pathways, many of which will be discussed in this minireview.
When inactive, AhR is located in the cytosol complexed to the 90-kDa heat-shock protein (HSP90), c-SRC, and AhR-interacting protein (35). Upon binding of an agonist, AhR undergoes conformational changes that lead to its heterodimerization with the AhR nuclear translocator (ARNT) and subsequent translocation to the nucleus. In the nucleus, this AhR-ARNT complex binds to xenobiotic response elements in regulatory regions of responsive genes to induce specific transcriptional programs (5, 14, 29, 30). Through this mechanism, AhR affects the differentiation of several cell types in the immune system relevant for inflammation in the gut and the central nervous system (2, 7, 26, 36). Of note, AhR can also control cellular responses through non-genomic mechanisms that involve the activation of specific kinases in the cytoplasm, also in a ligand-dependent manner (18, 20, 23, 37).
AhR-dependent immune cell regulation
AhR has been shown to affect the transcriptional programs of regulatory- and interleukin-17 (IL-17)-producing T helper cells (Tregs and Th17 cells, respectively) (7, 28, 38), among others. In FoxP3+ Tregs, AhR regulates the transcription and epigenetic status of the Treg master transcription factor FoxP3 (7, 29, 30, 39, 40). In addition, AhR has been shown to participate in the differentiation of FoxP3–IL-10-producing type 1 regulatory T-cells (Tr1 cells) induced by IL-27 (41–43). IL-27 induces the expression of AhR and the transcription factor c-Maf through a STAT3-dependent mechanism (6, 30), suggesting a cross-talk between AhR, IL-27, and c-Maf. Indeed, we detected the presence of AhR-responsive elements in the promoter of Il10 in spatial proximity to c-Maf-binding sites (Maf-responsive elements (MAREs)) (30, 42), and we observed that both AhR and c-Maf are required for the induction of IL-10 expression (8, 44). AhR and c-Maf also cooperatively induce the expression of IL-21, which acts as an autocrine growth factor to stabilize and expand Tr1 cells (30).
AhR has additional roles in the transcriptional control of Tr1 cells, as it mediates the control of their transcriptional program by metabolic and environmental cues. The membrane-bound ectonucleotidase CD39 catalyzes the conversion of extracellular pro-inflammatory adenosine triphosphate (ATP) to adenosine diphosphate (ADP), which is further processed by CD73 to generate the anti-inflammatory metabolite adenosine (45). We found that STAT3–AhR signaling promotes CD39 expression in Tr1 cells (28). Indeed, CD39 boosts Tr1 cell differentiation by depleting extracellular ATP, which promotes AhR degradation and consequently arrests Tr1 cell differentiation, through a mechanism mediated by hypoxia-inducible factor 1-α (HIF1-α). Thus, AhR participates in regulatory feedback loops that promote and stabilize Tr1 cell differentiation (Fig. 1).
Figure 1.
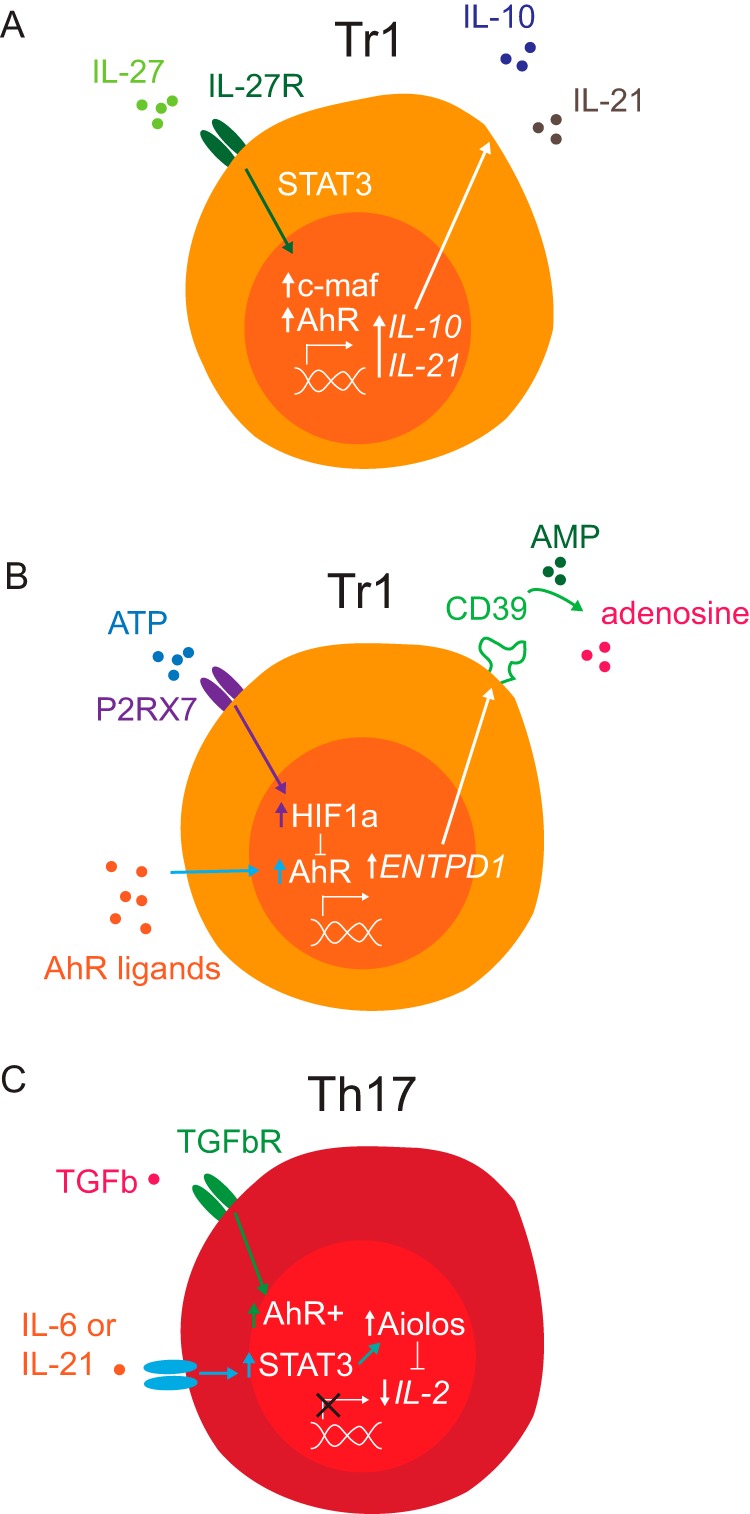
AhR transcriptional mechanisms that regulate the function of T-cell subsets. A, IL-27 induces c-Maf and AhR expression through a STAT3-dependent mechanism in Tr1 cells. AhR and c-Maf cooperate to promote the expression of IL-10 and IL-21. B, metabolic control of Tr1 cell differentiation by ATP and tryptophan metabolites working through HIF1-α and AhR, respectively. AhR promotes CD39 (ENTPD1) expression, which depletes extracellular ATP by catalyzing the conversion of AMP to adenosine. Extracellular ATP suppresses Tr1 cell differentiation by promoting AhR degradation through a mechanism mediated by P2RX7 and HIF1-α. C, AhR and STAT3 cooperate to induce Aiolos expression, which controls the epigenetic status of Il2 and limits its expression during Th17 cell differentiation.
AhR also controls transcriptional programs associated with the epigenetic regulation of cellular responses. Aiolos is a transcription factor of the Ikaros family involved in the development of lymphoid cell lineages (5, 29, 46) through different mechanisms, for example the regulation of the epigenetic status of target genes (47–49). In human Treg cells, for instance, FoxP3 and Aiolos form a heterodimeric complex that binds the Il2 promoter and suppresses IL-2 expression (29). AhR promotes the expression of both FoxP3 and Aiolos (5, 29). For example, AhR activation in a colitis model using the AhR agonist TCDD led to demethylation of CpG islands in the Foxp3 promoter and subsequent methylation of the Il17 promoter in T-cells from the lymph node, increasing the FoxP3+ Treg/Th17 cell balance (40). Moreover, AhR also promotes the expression of the epigenetic modifier Aiolos, which suppresses IL-2 expression in the early stages of Th17 cell differentiation (50, 51), to influence the development of pathogenic and regulatory T-cells in mice and humanized mouse models of inflammatory bowel disease (IBD) (5, 52). Thus, through its effects on the control of epigenetic regulators, AhR plays an important role on the regulation of transcriptional programs that control the immune response.
One study recently demonstrated that AhR can regulate microRNA (miR) signatures, which is an additional mechanism by which AhR can control the epigenetic status of Foxp3 (53). For instance, application of dietary indoles, which are known to activate AhR, induced the differentiation of naïve CD4+ T-cells toward a Treg fate instead of a Th17 fate, but this effect was lost in AhR-deficient mice. Moreover, indole activation of AhR recruited distinct miRs to the promoter of IL17 but prevented miR recruitment to the Foxp3 promoter. However, treatment with FICZ, a known inducer of Th17 cell differentiation through AhR activation (7), caused the silencing of FoxP3 by miRs and the expression of IL17. These interesting results point to yet another mode of AhR-dependent regulation of gene expression, by non-coding miRs, and also highlight the importance of cellular context where ligand-specific effects of AhR signaling regulate critical cellular events. Collectively, these observations suggest that AhR participates in the transcriptional control of T-cell gene expression and cell fate in response to the local milieu, for example within inflamed tissues and exogenous signals, such as pollutants and metabolites.
The gut, for instance, is rich in both AhR ligands and extracellular ATP produced by commensal bacteria (54, 55). AhR activation induces the expression of the receptor tyrosine kinase Kit, which contains AhR-responsive elements in its promoter and plays a central role in innate lymphoid cell (ILC) development (22). Thus, AhR activation by ligands provided by the diet and the commensal flora promotes the development of ILCs that contribute to intestinal homeostasis. Similarly, AhR agonists provided by the diet and the gut flora control the development of IELs that contribute to intestinal homeostasis (21). The specific effects of AhR in each immune cell type, however, are determined by its interaction with additional cell type and tissue-specific transcription factors (e.g. c-Maf, Aiolos, STAT3, and HIF1-α) (5, 6, 30). Considering that deficits in AhR signaling have been linked to experimental and human IBD (56), these findings highlight the important function of AhR as a modulator of the activity of immune cells in response to cues provided by the local microenvironment in health and disease (14, 22, 28). Moreover, they identify AhR as a modulator of commensal/host interactions.
Beyond modulating the function of T-cell subsets in the periphery and lymphocytes in the gut, AhR has been shown to modulate the function of the immune system more generally, for example by modulating the function of B cells, dendritic cells, and monocytes (14, 57–63). One mechanism of increasing interest is the interaction between IL-4 and AhR signaling pathways. For instance, upon engagement of B cell receptors and treatment with IL-4, AhR is up-regulated and promotes efficient cell cycle transitions in proliferative B cells at least in part by regulating cyclin expression (57). Conversely, loss of AhR leads to impaired proliferative capacity in B cells, an important phenotype given the role AhR plays in cancer (58).
In dendritic cells, activation of AhR by endogenous tryptophan-derived agonists led to the suppression of EAE (27) as a result of the induction of a tolerogenic phenotype in dendritic cells that promoted Treg differentiation. In monocytes, however, activation of AhR using the halogenated aromatic hydrocarbon agonist VAF347 caused arrest of monocyte differentiation (64). Together, these results observed in distinct types of immune cells suggest that AhR functions both in a ligand-dependent and a cell type-specific manner to regulate normal and pathogenic cellular events. For summary, see Table 1.
Table 1.
Cell-specific effects of AhR activity
| Astrocytes |
Dendritic cells |
FoxP3+ Tregs |
Tr1 cells |
Th17 cells |
B cells |
Monocytes |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cellular effects | Refs. | Cellular effects | Refs. | Cellular effects | Refs. | Cellular effects | Refs. | Cellular effects | Refs. | Cellular effects | Refs. | Cellular effects | Refs. |
| Anti-inflammatory | 2 | Anti-inflammatory | 27, 28 | Anti-inflammatory | 7 | Promotion of differentiation | 29–30, 43 | Epigenetic regulation | 5, 30 | Regulation of proliferation | 57–58, 62 | Regulation of differentiation | 64 |
| Tolerogenic phenotype | 19, 28 | Epigenetic regulation | 30, 39 | Metabolic remodeling | 6 | Pro-inflammatory | 7, 26 | Epigenetic regulation | 60 | ||||
| Control of differentiation | 61 | Regulation of differentiation | 29 | Induction of IL-10 and IL-22 production | 7, 26, 36, 38 | ||||||||
Astrocytes in MS
Astrocytes are the most abundant cell type in the mammalian brain, outnumbering neurons ∼4:1 (65, 66). In addition to their important roles in neural development, plasticity, metabolism, and neural circuit repair (65, 67–69), astrocytes have been recognized to control inflammatory processes in the central nervous system during MS since the work of Charcot in the 19th century (70, 71), but only recently are the molecular mechanisms being defined (2, 10, 95). Astrocyte activity is regulated by several stimuli relevant for MS (3), including a variety of cytokines and chemokines (65), metabolites such as ATP (72), and apoptotic cell debris (e.g. lipids) (73), among others. Several of these pathways converge on common downstream transcriptional programs that activate pro-inflammatory molecules such as AP-1, NF-κB, and STATs (74). The activation of these pathways contributes to the pathology of MS and other neurological diseases through astrocyte intrinsic and extrinsic mechanisms. For instance, astrocytes interact with inflamed microglia during MS pathogenesis (75) to control their activation status and disease-promoting function (76, 95). Astrocytes also recruit inflammatory monocytes into the CNS through the production of chemokines (77). Astrocytes also interact with neurons (3, 78) through mechanisms that can modulate neuronal death. Indeed, astrocytes secrete neurotoxic molecules such as pro-inflammatory cytokines (e.g. TNF-α), reactive oxygen species, IL-6 and IL-1β, and nitric oxide (3). It was recently reported that microglia induce formation of neurotoxic astrocytes through a combination of IL-1α, TNFα, and complement component 1q, which could be a pathway relevant in MS pathogenesis (97). Astrocytes, however, are also capable of producing a variety of both pro- and anti-inflammatory signals such as CCL2 (77, 79–81), IGF-1 (82), ciliary neurotrophic factor (83), and leukemia inhibitory factor (LIF) (84, 85). These features identify astrocytes as important regulators of inflammation and neurodegeneration.
Role of AhR in astrocyte-mediated inflammatory processes
We recently described a new role for AhR in controlling astrocyte-driven pathology in MS (2). We found that type-I interferons (IFN-Is) induce AhR expression in astrocytes, triggering AhR-dependent anti-inflammatory transcriptional responses. Conditional ablation of AhR in astrocytes led to exacerbated disease and failure to recover during EAE. Mechanistically, AhR deletion as well as lack of AhR-activating ligands resulted in the increased production of pro-inflammatory mediators, including Ccl2, Csf2, and Nos2, reflecting the exacerbated activation of NF-κB. As mentioned previously, AhR activity is regulated by small molecules such as dietary tryptophan generated by commensal bacteria (14, 15). Indeed, we found that AhR agonists provided by the diet and the commensal flora reached the CNS and activated this anti-inflammatory response in astrocytes, limiting CNS inflammation during EAE (Fig. 2).
Figure 2.
Control of astrocyte-driven pathogenesis by AhR signaling. AhR activation by agonists provided by the diet, gut flora, and metabolism limits NF-κB signaling and consequently astrocyte-driven pathogenesis.
In complementary studies using samples taken from MS patients, we detected the up-regulation of AhR in astrocytes, which coincided with activation of the IFN-I pathway in astrocytes (2). Strikingly, we detected decreased AhR activity in MS lesions as compared with controls, reflecting the presence of reduced levels of AhR agonists in serum samples from MS patients. These findings have several implications for our understanding of disease pathogenesis in MS and potentially other inflammatory diseases. First, it identifies AhR activation as a potential therapeutic approach. Indeed, laquinimod, a drug being developed to treat MS (86–88), crosses the blood-brain barrier and ameliorates EAE (and potentially MS) in an AhR-dependent manner (89, 90). In a phase III clinical trial, laquinimod reduced brain atrophy in MS, a process thought to reflect neurodegeneration driven at least partially by astrocytes (91). Second, it suggests that deficits in AhR ligand availability may contribute to MS pathogenesis. The origin of these ligands is unknown. However, it possibly reflects alterations in the uptake and metabolism of physiological AhR agonists in MS. Dietary or probiotic interventions to boost the levels of these AhR agonists may be of therapeutic value for MS patients. Third, these data point to a new gut-brain signaling axis in controlling the development of inflammatory and degenerative pathology in humans. These findings also add to recent advancements made in studies of neuropsychiatric disease where the gut microbiota modulate transcriptional programs that control social behavior (92), modulate synaptic dysfunction (93), and can modulate phenotypes associated with autism (94). It should also be kept in mind that, besides acting as a CNS sensor for immunomodulatory metabolites produced in the gut, based on its effects on gut immunity AhR may also shape the gut flora, impacting the gut-brain axis at multiple levels.
Concluding remarks
AhR integrates environmental and metabolic signals to modulate both peripheral immunity and local CNS inflammation. Thus, investigating the role of AhR signaling in health and disease provides an opportunity to understand the mechanisms by which the environment controls the development of autoimmunity in MS and other diseases. More importantly, AhR offers unique therapeutic opportunities based on the design of AhR targeting synthetic small molecules or the use of dietary or probiotic approaches to modulate the levels of natural AhR ligands.
This work was supported in part by National Institutes of Health Grants NS087867, ES025530, AI126880, and AI093903, National Multiple Sclerosis Society Grants RG4111A1 and JF2161-A-5, American Cancer Society Grant 126566-RSG-14-198-01-LIB), and International Progressive MS Alliance Grant PA-1604-08459 (to F. J.Q.). This is the second article in the Thematic Minireview Series: “Inflammatory transcription confronts homeostatic disruptions.” The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- MS
- multiple sclerosis
- FICZ
- 6-formylindolo(3,2-b)carbazole
- TCDD
- 2,3,7,8-tetrachlorodibenzo-p-dioxin
- AhR
- aryl hydrocarbon receptor
- ARNT
- AhR nuclear translocator
- IEL
- intraepithelial lymphocyte
- IBD
- inflammatory bowel disease
- miR
- microRNA
- ILC
- innate lymphoid cell
- EAE
- experimental autoimmune encephalomyelitis
- IFN-I
- type-I interferon.
References
- 1. Dendrou C. A., Fugger L., and Friese M. A. (2015) Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 15, 545–558 [DOI] [PubMed] [Google Scholar]
- 2. Rothhammer V., Mascanfroni I. D., Bunse L., Takenaka M. C., Kenison J. E., Mayo L., Chao C. C., Patel B., Yan R., Blain M., Alvarez J. I., Kébir H., Anandasabapathy N., Izquierdo G., Jung S., et al. (2016) Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 22, 586–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Glass C. K., Saijo K., Winner B., Marchetto M. C., and Gage F. H. (2010) Mechanisms underlying inflammation in neurodegeneration. Cell 140, 918–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Trapp B. D., and Nave K.-A. (2008) Multiple sclerosis: an immune or neurodegenerative disorder? Annu. Rev. Neurosci. 31, 247–269 [DOI] [PubMed] [Google Scholar]
- 5. Quintana F. J., Jin H., Burns E. J., Nadeau M., Yeste A., Kumar D., Rangachari M., Zhu C., Xiao S., Seavitt J., Georgopoulos K., and Kuchroo V. K. (2012) Aiolos promotes TH17 differentiation by directly silencing Il2 expression. Nat. Immunol. 13, 770–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mascanfroni I. D., Takenaka M. C., Yeste A., Patel B., Wu Y., Kenison J. E., Siddiqui S., Basso A. S., Otterbein L. E., Pardoll D. M., Pan F., Priel A., Clish C. B., Robson S. C., and Quintana F. J. (2015) Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat. Med. 21, 638–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Quintana F. J., Basso A. S., Iglesias A. H., Korn T., Farez M. F., Bettelli E., Caccamo M., Oukka M., and Weiner H. L. (2008) Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453, 65–71 [DOI] [PubMed] [Google Scholar]
- 8. Korn T., Bettelli E., Oukka M., and Kuchroo V. K. (2009) IL-17 and Th17 cells. Annu. Rev. Immunol. 27, 485–517 [DOI] [PubMed] [Google Scholar]
- 9. Farez M. F., Quintana F. J., Gandhi R., Izquierdo G., Lucas M., and Weiner H. L. (2009) Toll-like receptor 2 and poly(ADP-ribose) polymerase 1 promote central nervous system neuroinflammation in progressive EAE. Nat. Immunol. 10, 958–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mayo L., Trauger S. A., Blain M., Nadeau M., Patel B., Alvarez J. I., Mascanfroni I. D., Yeste A., Kivisäkk P., Kallas K., Ellezam B., Bakshi R., Prat A., Antel J. P., Weiner H. L., et al. (2014) Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat. Med. 20, 1147–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Quintana F. J., Farez M. F., Viglietta V., Iglesias A. H., Merbl Y., Izquierdo G., Lucas M., Basso A. S., Khoury S. J., Lucchinetti C. F., Cohen I. R., and Weiner H. L. (2008) Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc. Natl. Acad. Sci. U.S.A. 105, 18889–18894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bakshi R., Yeste A., Patel B., Tauhid S., Tummala S., Rahbari R., Chu R., Regev K., Kivisäkk P., Weiner H. L., and Quintana F. J. (2016) Serum lipid antibodies are associated with cerebral tissue damage in multiple sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 3, e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Quintana F. J., Yeste A., Weiner H. L., and Covacu R. (2012) Lipids and lipid-reactive antibodies as biomarkers for multiple sclerosis. J. Neuroimmunol. 248, 53–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stockinger B., Di Meglio P., Gialitakis M., and Duarte J. H. (2014) The aryl hydrocarbon receptor: multitasking in the immune system. Annu. Rev. Immunol. 32, 403–432 [DOI] [PubMed] [Google Scholar]
- 15. Quintana F. J., and Sherr D. H. (2013) Aryl hydrocarbon receptor control of adaptive immunity. Pharmacol. Rev. 65, 1148–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chang C. Y., and Puga A. (1998) Constitutive activation of the aromatic hydrocarbon receptor. Mol. Cell. Biol. 18, 525–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Patel R. D., Murray I. A., Flaveny C. A., Kusnadi A., and Perdew G. H. (2009) Ah receptor represses acute-phase response gene expression without binding to its cognate response element. Lab. Invest. 89, 695–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Quintana F. J. (2013) Regulation of central nervous system autoimmunity by the aryl hydrocarbon receptor. Semin. Immunopathol. 35, 627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takenaka M. C., Robson S., and Quintana F. J. (2016) Regulation of the T cell response by CD39. Trends Immunol. 37, 427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quintana F. J. (2013) The aryl hydrocarbon receptor: a molecular pathway for the environmental control of the immune response. Immunology 138, 183–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Y., Innocentin S., Withers D. R., Roberts N. A., Gallagher A. R., Grigorieva E. F., Wilhelm C., and Veldhoen M. (2011) Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 147, 629–640 [DOI] [PubMed] [Google Scholar]
- 22. Kiss E. A., Vonarbourg C., Kopfmann S., Hobeika E., Finke D., Esser C., and Diefenbach A. (2011) Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 334, 1561–1565 [DOI] [PubMed] [Google Scholar]
- 23. Nguyen N. T., Nakahama T., and Kishimoto T. (2013) Aryl hydrocarbon receptor and experimental autoimmune arthritis. Semin. Immunopathol. 35, 637–644 [DOI] [PubMed] [Google Scholar]
- 24. Colonna M. (2014) AHR: Making the keratinocytes thick skinned. Immunity 40, 863–864 [DOI] [PubMed] [Google Scholar]
- 25. Cibrian D., Saiz M. L., de la Fuente H., Sánchez-Díaz R., Moreno-Gonzalo O., Jorge I., Ferrarini A., Vázquez J., Punzón C., Fresno M., Vicente-Manzanares M., Daudén E., Fernández-Salguero P. M., Martín P., and Sánchez-Madrid F. (2016) CD69 controls the uptake of l-tryptophan through LAT1-CD98 and AhR-dependent secretion of IL-22 in psoriasis. Nat. Immunol. 17, 985–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Veldhoen M., Hirota K., Westendorf A. M., Buer J., Dumoutier L., Renauld J. C., and Stockinger B. (2008) The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453, 106–109 [DOI] [PubMed] [Google Scholar]
- 27. Quintana F. J., Murugaiyan G., Farez M. F., Mitsdoerffer M., Tukpah A. M., Burns E. J., and Weiner H. L. (2010) An endogenous aryl hydrocarbon receptor ligand acts on dendritic cells and T cells to suppress experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. U.S.A. 107, 20768–20773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mascanfroni I. D., Yeste A., Vieira S. M., Burns E. J., Patel B., Sloma I., Wu Y., Mayo L., Ben-Hamo R., Efroni S., Kuchroo V. K., Robson S. C., and Quintana F. J. (2013) IL-27 acts on DCs to suppress the T cell response and autoimmunity by inducing expression of the immunoregulatory molecule CD39. Nat. Immunol. 14, 1054–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gandhi R., Kumar D., Burns E. J., Nadeau M., Dake B., Laroni A., Kozoriz D., Weiner H. L., and Quintana F. J. (2010) Activation of the aryl hydrocarbon receptor induces human type 1 regulatory T cell-like and Foxp3+ regulatory T cells. Nat. Immunol. 11, 846–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Apetoh L., Quintana F. J., Pot C., Joller N., Xiao S., Kumar D., Burns E. J., Sherr D. H., Weiner H. L., and Kuchroo V. K. (2010) The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol. 11, 854–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Song J., Clagett-Dame M., Peterson R. E., Hahn M. E., Westler W. M., Sicinski R. R., and DeLuca H. F. (2002) A ligand for the aryl hydrocarbon receptor isolated from lung. Proc. Natl. Acad. Sci. U.S.A. 99, 14694–14699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Busbee P. B., Rouse M., Nagarkatti M., and Nagarkatti P. S. (2013) Use of natural AhR ligands as potential therapeutic modalities against inflammatory disorders. Nutr. Rev. 71, 353–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mandal P. K. (2005) Dioxin: a review of its environmental effects and its aryl hydrocarbon receptor biology. J. Comp. Physiol. B 175, 221–230 [DOI] [PubMed] [Google Scholar]
- 34. Denison M. S., and Nagy S. R. (2003) Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu. Rev. Pharmacol. Toxicol. 43, 309–334 [DOI] [PubMed] [Google Scholar]
- 35. Tian J., Feng Y., Fu H., Xie H. Q., Jiang J. X., and Zhao B. (2015) The aryl hydrocarbon receptor: a key bridging molecule of external and internal chemical signals. Environ. Sci. Technol. 49, 9518–9531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Veldhoen M., Hirota K., Christensen J., O'Garra A., and Stockinger B. (2009) Natural agonists for aryl hydrocarbon receptor in culture medium are essential for optimal differentiation of Th17 T cells. J. Exp. Med. 206, 43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bessede A., Gargaro M., Pallotta M. T., Matino D., Servillo G., Brunacci C., Bicciato S., Mazza E. M., Macchiarulo A., Vacca C., Iannitti R., Tissi L., Volpi C., Belladonna M. L., Orabona C., et al. (2014) Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 511, 184–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gagliani N., Amezcua Vesely M. C., Iseppon A., Brockmann L., Xu H., Palm N. W., de Zoete M. R., Licona-Limón P., Paiva R. S., Ching T., Weaver C., Zi X., Pan X., Fan R., Garmire L. X., et al. (2015) Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature 523, 221–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mezrich J. D., Fechner J. H., Zhang X., Johnson B. P., Burlingham W. J., and Bradfield C. A. (2010) An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 185, 3190–3198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Singh N. P., Singh U. P., Singh B., Price R. L., Nagarkatti M., Nagarkatti P. S. (2011) Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PLoS ONE 6, e23522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roncarolo M. G., Gregori S., Battaglia M., Bacchetta R., Fleischhauer K., and Levings M. K. (2006) Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 212, 28–50 [DOI] [PubMed] [Google Scholar]
- 42. Pot C., Jin H., Awasthi A., Liu S. M., Lai C. Y., Madan R., Sharpe A. H., Karp C. L., Miaw S. C., Ho I. C., and Kuchroo V. K. (2009) Cutting edge: IL-27 induces the transcription factor c-Maf cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J. Immunol. 183, 797–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wu H. Y., Quintana F. J., da Cunha A. P., Dake B. T., Koeglsperger T., Starossom S. C., and Weiner H. L. (2011) In vivo induction of Tr1 cells via mucosal dendritic cells and AHR signaling. PLoS ONE 6, e23618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Torchinsky M. B., and Blander J. M. (2010) T helper 17 cells: discovery, function, and physiological trigger. Cell. Mol. Life Sci. 67, 1407–1421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Antonioli L., Blandizzi C., Pacher P., and Haskó G. (2013) Immunity, inflammation and cancer: a leading role for adenosine. Nat. Rev. Cancer 13, 842–857 [DOI] [PubMed] [Google Scholar]
- 46. Rebollo A., and Schmitt C. (2003) Ikaros, Aiolos and Helios: transcription regulators and lymphoid malignancies. Immunol. Cell Biol. 81, 171–175 [DOI] [PubMed] [Google Scholar]
- 47. Bandyopadhyay S., Duré M., Paroder M., Soto-Nieves N., Puga I., and Macián F. (2007) Interleukin 2 gene transcription is regulated by Ikaros-induced changes in histone acetylation in anergic T cells. Blood 109, 2878–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pan F., Yu H., Dang E. V., Barbi J., Pan X., Grosso J. F., Jinasena D., Sharma S. M., McCadden E. M., Getnet D., Drake C. G., Liu J. O., Ostrowski M. C., and Pardoll D. M. (2009) Eos mediates Foxp3-dependent gene. Science 325, 1142–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Koipally J., Renold A., Kim J., and Georgopoulos K. (1999) Repression by Ikaros and Aiolos is mediated through histone deacetylase complexes. EMBO J. 18, 3090–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Korn T., Bettelli E., Gao W., Awasthi A., Jäger A., Strom T. B., Oukka M., and Kuchroo V. K. (2007) I. L. -21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature 448, 484–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bettelli E., Carrier Y., Gao W., Korn T., Strom T. B., Oukka M., Weiner H. L., and Kuchroo V. K. (2006) Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 [DOI] [PubMed] [Google Scholar]
- 52. Goettel J. A., Gandhi R., Kenison J. E., Yeste A., Murugaiyan G., Sambanthamoorthy S., Griffith A. E., Patel B., Shouval D. S., Weiner H. L., Snapper S. B., and Quintana F. J. (2016) AHR activation is protective against colitis driven by T cells in humanized mice. Cell Rep. 17, 1318–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Singh N. P., Singh U. P., Rouse M., Zhang J., Chatterjee S., Nagarkatti P. S., and Nagarkatti M. (2016) Dietary indoles suppress delayed-type hypersensitivity by inducing a switch from proinflammatory Th17 cells to anti-inflammatory regulatory T cells through regulation of MicroRNA. J. Immunol. 196, 1108–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Atarashi K., Nishimura J., Shima T., Umesaki Y., Yamamoto M., Onoue M., Yagita H., Ishii N., Evans R., Honda K., and Takeda K. (2008) ATP drives lamina propria T(H)17 cell differentiation. Nature 455, 808–812 [DOI] [PubMed] [Google Scholar]
- 55. Zelante T., Iannitti R. G., Cunha C., De Luca A., Giovannini G., Pieraccini G., Zecchi R., D'Angelo C., Massi-Benedetti C., Fallarino F., Carvalho A., Puccetti P., and Romani L. (2013) Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39, 372–385 [DOI] [PubMed] [Google Scholar]
- 56. Richard M. L., Leducq V., Pham H. P., Michel M. L., Da Costa G., Bridonneau C., Jegou S., Hoffmann T. W., Natividad J. M., Brot L., Taleb S., Couturier-Maillard A., Nion-Larmurier I., Merabtene F., et al. (2016) CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 22, 598–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Villa M., Gialitakis M., Tolaini M., Ahlfors H., Henderson C. J., Wolf C. R., Brink R., and Stockinger B. (2017) Aryl hydrocarbon receptor is required for optimal B-cell proliferation. EMBO J. 36, 116–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sherr D. H., and Monti S. (2013) The role of the aryl hydrocarbon receptor in normal and malignant B cell development. Semin. Immunopathol. 35, 705–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Vogel C. F., Wu D., Goth S. R., Baek J., Lollies A., Domhardt R., Grindel A., and Pessah I. N. (2013) Aryl hydrocarbon receptor signaling regulates NF-κB RelB activation during dendritic-cell differentiation. Immunol. Cell Biol. 91, 568–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liao W.-T., Lu J. H., Wang W. T,. Hung C. H., Sheu C. C., and Huang S. K. (2017) Epigenetic synergism between interleukin-4 and aryl-hydrocarbon receptor in human macrophages. J. Mol. Med. 95, 395–404 [DOI] [PubMed] [Google Scholar]
- 61. Aguilera-Montilla N., Chamorro S., Nieto C., Sánchez-Cabo F., Dopazo A., Fernández-Salguero P. M., Rodríguez-Fernández J. L., Pello O. M., Andrés V., Cuenda A., Alonso B., Domínguez-Soto A., Sánchez-Ramón S., and Corbí A. L. (2013) Aryl hydrocarbon receptor contributes to the MEK/ERK-dependent maintenance of the immature state of human dendritic cells. Blood 121, e108–e117 [DOI] [PubMed] [Google Scholar]
- 62. Tanaka G., Kanaji S., Hirano A., Arima K., Shinagawa A., Goda C., Yasunaga S., Ikizawa K., Yanagihara Y., Kubo M., Kuriyama-Fujii Y., Sugita Y., Inokuchi A., and Izuhara K. (2005) Induction and activation of the aryl hydrocarbon receptor by IL-4 in B cells. Int. Immunol. 17, 797–805 [DOI] [PubMed] [Google Scholar]
- 63. Marcus R. S., Holsapple M. P., and Kaminski N. E. (1998) Lipopolysaccharide activation of murine splenocytes and splenic B cells increased the expression of aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator. J. Pharmacol. Exp. Ther. 287, 1113–1118 [PubMed] [Google Scholar]
- 64. Platzer B., Richter S., Kneidinger D., Waltenberger D., Woisetschläger M., and Strobl H. (2009) Aryl hydrocarbon receptor activation inhibits in vitro differentiation of human monocytes and Langerhans dendritic cells. J. Immunol. 183, 66–74 [DOI] [PubMed] [Google Scholar]
- 65. Khakh B. S., and Sofroniew M. V. (2015) Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 18, 942–952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ludwin S. K., Rao V. Ts., Moore C. S., and Antel J. P. (2016) Astrocytes in multiple sclerosis. Mult. Scler. 22, 1114–1124 [DOI] [PubMed] [Google Scholar]
- 67. Clarke L. E., and Barres B. A. (2013) Emerging roles of astrocytes in neural circuit development. Nat. Rev. Neurosci. 14, 311–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Eroglu C., and Barres B. (2010) Regulation of synaptic connectivity by glia. Nature 468, 223–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sofroniew M. V. (2015) Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 16, 249–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Charcot J.-M. (1868) Histologie de la sclérose en plaque. Gazette des hopitaux (Paris) 41, 554–555 [Google Scholar]
- 71. Charcot J.-M. (1868) Comptes rendus des séances et mémoires lus à la société de Biologie, Vol. 33, Société de biologie, Paris [Google Scholar]
- 72. Di Virgilio F., Ceruti S., Bramanti P., and Abbracchio M. P. (2009) Purinergic signalling in inflammation of the central nervous system. Trends Neurosci. 32, 79–87 [DOI] [PubMed] [Google Scholar]
- 73. Husemann J., Loike J. D., Anankov R., Febbraio M., and Silverstein S. C. (2002) Scavenger receptors in neurobiology and neuropathology: their role on microglia and other cells of the nervous system. Glia 40, 195–205 [DOI] [PubMed] [Google Scholar]
- 74. De Keyser J., Laureys G., Demol F., Wilczak N., Mostert J., and Clinckers R. (2010) Astrocytes as potential targets to suppress inflammatory demyelinating lesions in multiple sclerosis. Neurochem. Int. 57, 446–450 [DOI] [PubMed] [Google Scholar]
- 75. Calabrese M., Magliozzi R., Ciccarelli O., Geurts J. J., Reynolds R., and Martin R. (2015) Exploring the origins of grey matter damage in multiple sclerosis. Nat. Rev. Neurosci. 16, 147–158 [DOI] [PubMed] [Google Scholar]
- 76. Erny D., Hrabě de Angelis A. L., Jaitin D.,, Wieghofer P., Staszewski O., David E., Keren-Shaul H., Mahlakoiv T., Jakobshagen K., Buch T., Schwierzeck V., Utermöhlen O., Chun E., Garrett W. S., McCoy K. D., et al. (2015) Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rothhammer V., and Quintana F. J. (2016) Environmental control of autoimmune inflammation in the central nervous system. Curr. Opin. Immunol. 43, 46–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nair A., Frederick T. J., and Miller S. D. (2008) Astrocytes in multiple sclerosis: a product of their environment. Cell. Mol. Life Sci. 65, 2702–2720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Brambilla R., Dvoriantchikova G., Barakat D., Ivanov D., Bethea J. R., and Shestopalov V. I. (2012) Transgenic inhibition of astroglial NF-κB protects from optic nerve damage and retinal ganglion cell loss in experimental optic neuritis. J. Neuroinflammation 9, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Moreno M., Bannerman P., Ma J., Guo F., Miers L., Soulika A. M., and Pleasure D. (2014) Conditional ablation of astroglial CCL2 suppresses CNS accumulation of M1 macrophages and preserves axons in mice with MOG peptide EAE. J. Neurosci. 34, 8175–8185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Paul D., Ge S., Lemire Y., Jellison E. R., Serwanski D. R., Ruddle N. H., and Pachter J. S. (2014) Cell-selective knockout and 3D confocal image analysis reveals separate roles for astrocyte-and endothelial-derived CCL2 in neuroinflammation. J. Neuroinflammation 11, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Clarner T., Parabucki A., Beyer C., and Kipp M. (2011) Corticosteroids impair remyelination in the corpus callosum of cuprizone-treated mice. J. Neuroendocrinol. 23, 601–611 [DOI] [PubMed] [Google Scholar]
- 83. Hesp Z. C., Goldstein E. Z., Goldstein E. A., Miranda C. J., Kaspar B. K., Kaspar B. K., and McTigue D. M. (2015) Chronic oligodendrogenesis and remyelination after spinal cord injury in mice and rats. J. Neurosci. 35, 1274–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Ishibashi T., Dakin K. A., Stevens B., Lee P. R., Kozlov S. V., Stewart C. L., and Fields R. D. (2006) Astrocytes promote myelination in response to electrical impulses. Neuron 49, 823–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fischer R., Wajant H., Kontermann R., Pfizenmaier K., and Maier O. (2014) Astrocyte-specific activation of TNFR2 promotes oligodendrocyte maturation by secretion of leukemia inhibitory factor. Glia 62, 272–283 [DOI] [PubMed] [Google Scholar]
- 86. Brück W., and Vollmer T. (2013) Multiple sclerosis: oral laquinimod for MS–bringing the brain into focus. Nat. Rev. Neurol. 9, 664–665 [DOI] [PubMed] [Google Scholar]
- 87. Palma J.-A., and Pagola I. (2012) Oral laquinimod for multiple sclerosis. N. Engl. J. Med. 366, 2527. [DOI] [PubMed] [Google Scholar]
- 88. Varrin-Doyer M., Zamvil S. S., and Schulze-Topphoff U. (2014) Laquinimod, an up-and-coming immunomodulatory agent for treatment of multiple sclerosis. Exp. Neurol. 262, 66–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Berg J., Mahmoudjanlou Y., Duscha A., Massa M. G., Thöne J., Esser C., Gold R., and Haghikia A. (2016) The immunomodulatory effect of laquinimod in CNS autoimmunity is mediated by the aryl hydrocarbon receptor. J. Neuroimmunol. 298, 9–15 [DOI] [PubMed] [Google Scholar]
- 90. Kaye J., Piryatinsky V., Birnberg T., Hingaly T., Raymond E., Kashi R., Amit-Romach E., Caballero I. S., Towfic F., Ator M. A., Rubinstein E., Laifenfeld D., Orbach A., Shinar D., Marantz Y., et al. (2016) Laquinimod arrests experimental autoimmune encephalomyelitis by activating the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. U.S.A. 113, E6145–E6152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Vollmer T. L., Sorensen P. S., Selmaj K., Zipp F., Havrdova E., Cohen J. A., Sasson N., Gilgun-Sherki Y., Arnold D. L., and BRAVO Study Group. (2014) A randomized placebo-controlled phase III trial of oral laquinimod for multiple sclerosis. J. Neurol. 261, 773–783 [DOI] [PubMed] [Google Scholar]
- 92. Gacias M., Gaspari S., Santos P. M., Tamburini S., Andrade M., Zhang F., Shen N., Tolstikov V., Kiebish M. A., Dupree J. L., Zachariou V., Clemente J. C., and Casaccia P. (2016) Microbiota-driven transcriptional changes in prefrontal cortex override genetic differences in social behavior. Elife 5, e13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Buffington S. A., Di Prisco G. V., Auchtung T. A., Ajami N. J., Petrosino J. F., and Costa-Mattioli M. (2016) Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cell 165, 1762–1775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hsiao E. Y., McBride S. W., Hsien S., Sharon G., Hyde E. R., McCue T., Codelli J. A., Chow J., Reisman S. E., Petrosino J. F., Patterson P. H., and Mazmanian S. K. (2013) Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 155, 1451–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Liddelow S. A., Guttenplan K. A., Clarke L. E., Bennett F. C., Bohlen C. J., Schirmer L., Bennett M. L., Münch A. E., Chung W. S., Peterson T. C., Wilton D. K., Frouin A., Napier B. A., Panicker N., Kumar M., et al. (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]



