Abstract
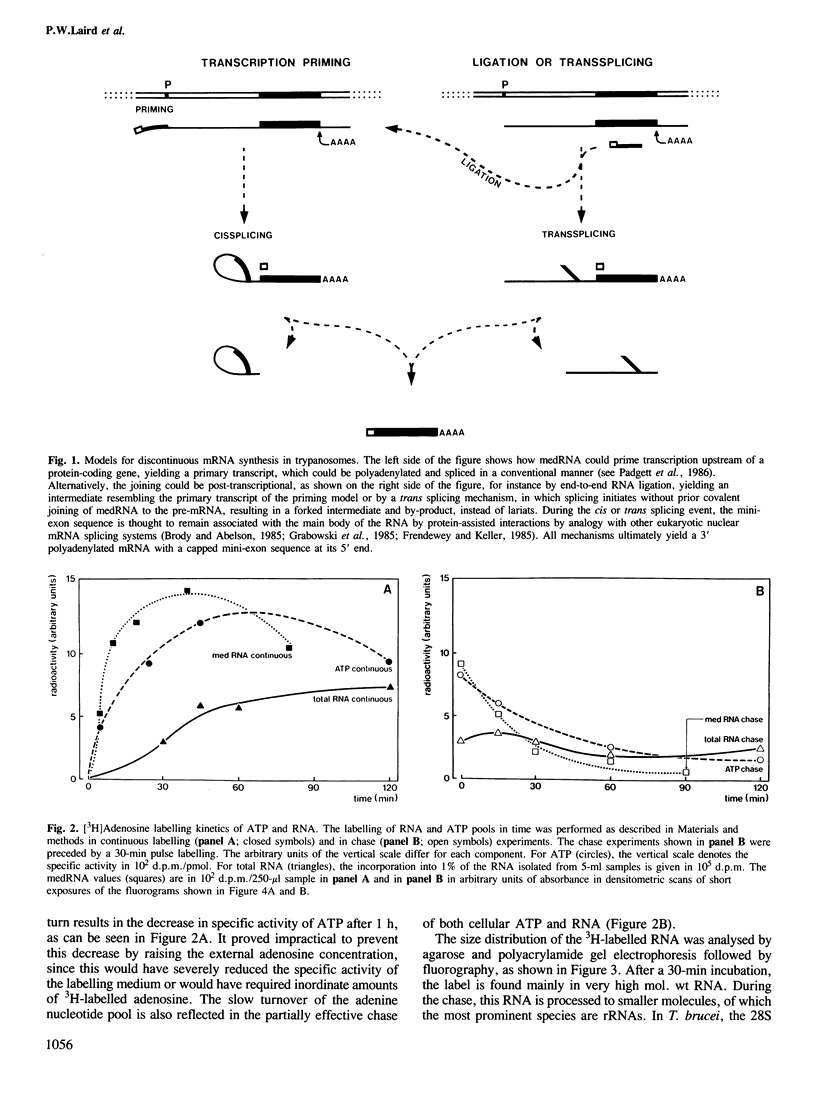
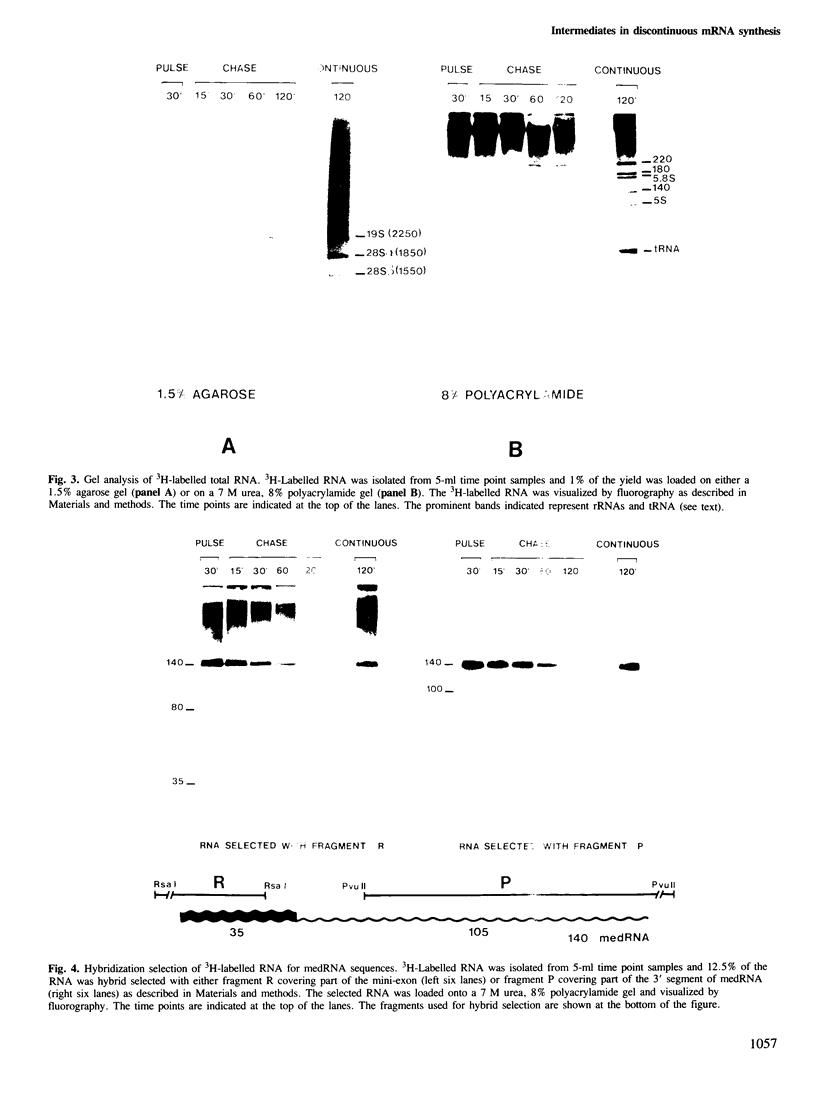
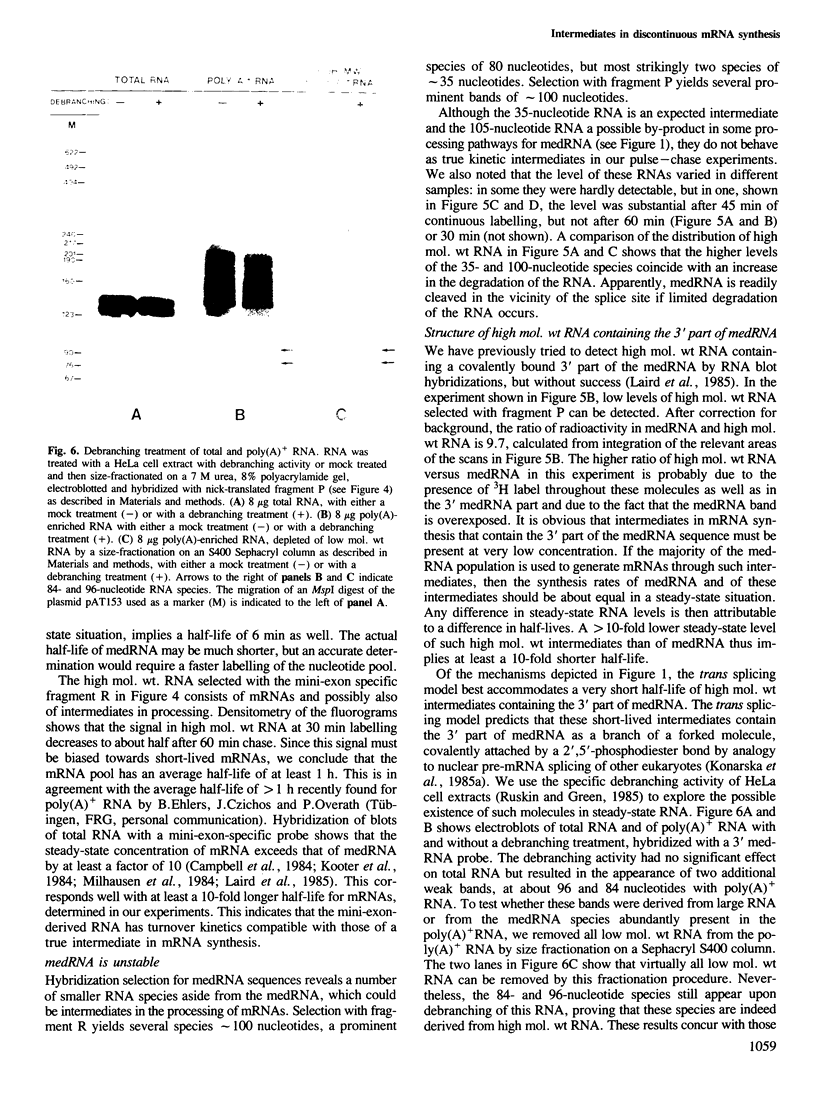
Discontinuous mRNA synthesis in trypanosomes is thought to involve a 140-nucleotide precursor, called the mini-exon-derived RNA or medRNA, which contributes its 5' 35 nucleotides to the 5' end of nascent mRNAs. We used in vivo labelling of RNA to show that medRNA has a half-life of less than 6 min, whereas putative high mol. wt intermediates containing the 3' part of the medRNA have an average half-life of less than 1 min. This eliminates priming of pre-mRNA synthesis by intact medRNA as the main mode of discontinuous mRNA synthesis. Potential intermediates of 35 and 105 nucleotides were labelled in parallel with medRNA, but their significance could not be assessed in RNA preparations containing medRNA, as they are also produced by artefactual cleavage of medRNA. We show, however, that high mol. wt RNA, free of medRNA, can release medRNA segments upon a debranching treatment. These results are consistent with a trans splicing mechanism involving short-lived forked intermediates, analogous to lariats in cis splicing systems.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothroyd J. C. Antigenic variation in African trypanosomes. Annu Rev Microbiol. 1985;39:475–502. doi: 10.1146/annurev.mi.39.100185.002355. [DOI] [PubMed] [Google Scholar]
- Boothroyd J. C., Cross G. A. Transcripts coding for variant surface glycoproteins of Trypanosoma brucei have a short, identical exon at their 5' end. Gene. 1982 Dec;20(2):281–289. doi: 10.1016/0378-1119(82)90046-4. [DOI] [PubMed] [Google Scholar]
- Borst P. Discontinuous transcription and antigenic variation in trypanosomes. Annu Rev Biochem. 1986;55:701–732. doi: 10.1146/annurev.bi.55.070186.003413. [DOI] [PubMed] [Google Scholar]
- Brody E., Abelson J. The "spliceosome": yeast pre-messenger RNA associates with a 40S complex in a splicing-dependent reaction. Science. 1985 May 24;228(4702):963–967. doi: 10.1126/science.3890181. [DOI] [PubMed] [Google Scholar]
- Brun R., Schönenberger Cultivation and in vitro cloning or procyclic culture forms of Trypanosoma brucei in a semi-defined medium. Short communication. Acta Trop. 1979 Sep;36(3):289–292. [PubMed] [Google Scholar]
- Campbell D. A., Thornton D. A., Boothroyd J. C. Apparent discontinuous transcription of Trypanosoma brucei variant surface antigen genes. 1984 Sep 27-Oct 3Nature. 311(5984):350–355. doi: 10.1038/311350a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordingley J. S. Nucleotide sequence of the 5S ribosomal RNA gene repeat of Trypanosoma brucei. Mol Biochem Parasitol. 1985 Dec;17(3):321–330. doi: 10.1016/0166-6851(85)90006-4. [DOI] [PubMed] [Google Scholar]
- Cordingley J. S., Turner M. J. 6.5 S RNA; preliminary characterisation of unusual small RNAs in Trypanosoma brucei. Mol Biochem Parasitol. 1980 Apr;1(2):91–96. doi: 10.1016/0166-6851(80)90003-1. [DOI] [PubMed] [Google Scholar]
- Cornelissen A. W., Verspieren M. P., Toulmé J. J., Swinkels B. W., Borst P. The common 5' terminal sequence on trypanosome mRNAs: a target for anti-messenger oligodeoxynucleotides. Nucleic Acids Res. 1986 Jul 25;14(14):5605–5614. doi: 10.1093/nar/14.14.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross G. A. Identification, purification and properties of clone-specific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitology. 1975 Dec;71(3):393–417. doi: 10.1017/s003118200004717x. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- De Lange T., Berkvens T. M., Veerman H. J., Frasch A. C., Barry J. D., Borst P. Comparison of the genes coding for the common 5' terminal sequence of messenger RNAs in three trypanosome species. Nucleic Acids Res. 1984 Jun 11;12(11):4431–4443. doi: 10.1093/nar/12.11.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lange T., Liu A. Y., Van der Ploeg L. H., Borst P., Tromp M. C., Van Boom J. H. Tandem repetition of the 5' mini-exon of variant surface glycoprotein genes: a multiple promoter for VSG gene transcription? Cell. 1983 Oct;34(3):891–900. doi: 10.1016/0092-8674(83)90546-9. [DOI] [PubMed] [Google Scholar]
- De Lange T., Michels P. A., Veerman H. J., Cornelissen A. W., Borst P. Many trypanosome messenger RNAs share a common 5' terminal sequence. Nucleic Acids Res. 1984 May 11;12(9):3777–3790. doi: 10.1093/nar/12.9.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman D. M., Donelson J. E. Characterization of the 1.35 kilobase DNA repeat unit containing the conserved 35 nucleotides at the 5'-termini of variable surface glycoprotein mRNAs in Trypanosoma brucei. Nucleic Acids Res. 1984 Jun 25;12(12):4907–4920. doi: 10.1093/nar/12.12.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish W. R., Marr J. J., Berens R. L. Purine metabolism in Trypanosoma brucei gambiense. Biochim Biophys Acta. 1982 Feb 25;714(3):422–428. doi: 10.1016/0304-4165(82)90149-0. [DOI] [PubMed] [Google Scholar]
- Frendewey D., Keller W. Stepwise assembly of a pre-mRNA splicing complex requires U-snRNPs and specific intron sequences. Cell. 1985 Aug;42(1):355–367. doi: 10.1016/s0092-8674(85)80131-8. [DOI] [PubMed] [Google Scholar]
- Grabowski P. J., Seiler S. R., Sharp P. A. A multicomponent complex is involved in the splicing of messenger RNA precursors. Cell. 1985 Aug;42(1):345–353. doi: 10.1016/s0092-8674(85)80130-6. [DOI] [PubMed] [Google Scholar]
- Guyaux M., Cornelissen A. W., Pays E., Steinert M., Borst P. Trypanosoma brucei: a surface antigen mRNA is discontinuously transcribed from two distinct chromosomes. EMBO J. 1985 Apr;4(4):995–998. doi: 10.1002/j.1460-2075.1985.tb03729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond D. J., Gutteridge W. E. Purine and pyrimidine metabolism in the Trypanosomatidae. Mol Biochem Parasitol. 1984 Nov;13(3):243–261. doi: 10.1016/0166-6851(84)90117-8. [DOI] [PubMed] [Google Scholar]
- Hasan G., Turner M. J., Cordingley J. S. Ribosomal RNA genes of Trypanosoma brucei: mapping the regions specifying the six small ribosomal RNAs. Gene. 1984 Jan;27(1):75–86. doi: 10.1016/0378-1119(84)90240-3. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Borst P., van den Burg J., Weissmann C., Cross G. A. The isolation of plasmids containing DNA complementary to messenger RNA for variant surface glycoproteins of Trypanosoma brucei. Gene. 1980 Mar;8(4):391–417. doi: 10.1016/0378-1119(80)90043-8. [DOI] [PubMed] [Google Scholar]
- James D. M., Born G. V. Uptake of purine bases and nucleosides in African trypanosomes. Parasitology. 1980 Oct;81(2):383–393. doi: 10.1017/s0031182000056110. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C., Jones C. W., Efstratiadis A. Determination of nucleic acid sequence homologies and relative concentrations by a dot hybridization procedure. Nucleic Acids Res. 1979 Nov 24;7(6):1541–1552. doi: 10.1093/nar/7.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarska M. M., Grabowski P. J., Padgett R. A., Sharp P. A. Characterization of the branch site in lariat RNAs produced by splicing of mRNA precursors. Nature. 1985 Feb 14;313(6003):552–557. doi: 10.1038/313552a0. [DOI] [PubMed] [Google Scholar]
- Konarska M. M., Padgett R. A., Sharp P. A. Trans splicing of mRNA precursors in vitro. Cell. 1985 Aug;42(1):165–171. doi: 10.1016/s0092-8674(85)80112-4. [DOI] [PubMed] [Google Scholar]
- Kooter J. M., Borst P. Alpha-amanitin-insensitive transcription of variant surface glycoprotein genes provides further evidence for discontinuous transcription in trypanosomes. Nucleic Acids Res. 1984 Dec 21;12(24):9457–9472. doi: 10.1093/nar/12.24.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooter J. M., De Lange T., Borst P. Discontinuous synthesis of mRNA in trypanosomes. EMBO J. 1984 Oct;3(10):2387–2392. doi: 10.1002/j.1460-2075.1984.tb02144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird P. W., Kooter J. M., Loosbroek N., Borst P. Mature mRNAs of Trypanosoma brucei possess a 5' cap acquired by discontinuous RNA synthesis. Nucleic Acids Res. 1985 Jun 25;13(12):4253–4266. doi: 10.1093/nar/13.12.4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M. J., Dorfman D. M., Donelson J. E. The spliced leader sequence of Trypanosoma brucei has a potential role as a cap donor structure. Mol Cell Biol. 1985 Sep;5(9):2487–2490. doi: 10.1128/mcb.5.9.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M. J., Dorfman D. M., Reddy L. V., Donelson J. E. Characterization of the Trypanosoma brucei 5S ribosomal RNA gene and transcript: the 5S rRNA is a spliced-leader-independent species. Gene. 1985;35(1-2):131–141. doi: 10.1016/0378-1119(85)90165-9. [DOI] [PubMed] [Google Scholar]
- Milhausen M., Nelson R. G., Sather S., Selkirk M., Agabian N. Identification of a small RNA containing the trypanosome spliced leader: a donor of shared 5' sequences of trypanosomatid mRNAs? Cell. 1984 Oct;38(3):721–729. doi: 10.1016/0092-8674(84)90267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy W. J., Watkins K. P., Agabian N. Identification of a novel Y branch structure as an intermediate in trypanosome mRNA processing: evidence for trans splicing. Cell. 1986 Nov 21;47(4):517–525. doi: 10.1016/0092-8674(86)90616-1. [DOI] [PubMed] [Google Scholar]
- Nelson R. G., Parsons M., Barr P. J., Stuart K., Selkirk M., Agabian N. Sequences homologous to the variant antigen mRNA spliced leader are located in tandem repeats and variable orphons in trypanosoma brucei. Cell. 1983 Oct;34(3):901–909. doi: 10.1016/0092-8674(83)90547-0. [DOI] [PubMed] [Google Scholar]
- Nelson R. G., Parsons M., Selkirk M., Newport G., Barr P. J., Agabian N. Sequences homologous to variant antigen mRNA spliced leader in Trypanosomatidae which do not undergo antigenic variation. Nature. 1984 Apr 12;308(5960):665–667. doi: 10.1038/308665a0. [DOI] [PubMed] [Google Scholar]
- Opperdoes F. R. Biochemical peculiarities of trypanosomes, African and South American. Br Med Bull. 1985 Apr;41(2):130–136. doi: 10.1093/oxfordjournals.bmb.a072039. [DOI] [PubMed] [Google Scholar]
- Opperdoes F. R., Borst P. Localization of nine glycolytic enzymes in a microbody-like organelle in Trypanosoma brucei: the glycosome. FEBS Lett. 1977 Aug 15;80(2):360–364. doi: 10.1016/0014-5793(77)80476-6. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Parsons M., Nelson R. G., Watkins K. P., Agabian N. Trypanosome mRNAs share a common 5' spliced leader sequence. Cell. 1984 Aug;38(1):309–316. doi: 10.1016/0092-8674(84)90552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruskin B., Green M. R. An RNA processing activity that debranches RNA lariats. Science. 1985 Jul 12;229(4709):135–140. doi: 10.1126/science.2990042. [DOI] [PubMed] [Google Scholar]
- Sogin M. L., Elwood H. J., Gunderson J. H. Evolutionary diversity of eukaryotic small-subunit rRNA genes. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1383–1387. doi: 10.1073/pnas.83.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solnick D. Trans splicing of mRNA precursors. Cell. 1985 Aug;42(1):157–164. doi: 10.1016/s0092-8674(85)80111-2. [DOI] [PubMed] [Google Scholar]
- Sutton R. E., Boothroyd J. C. Evidence for trans splicing in trypanosomes. Cell. 1986 Nov 21;47(4):527–535. doi: 10.1016/0092-8674(86)90617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullis R. H., Rubin H. Calcium protects DNase I from proteinase K: a new method for the removal of contaminating RNase from DNase I. Anal Biochem. 1980 Sep 1;107(1):260–264. doi: 10.1016/0003-2697(80)90519-9. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Liu A. Y., Michels P. A., De Lange T., Borst P., Majumder H. K., Weber H., Veeneman G. H., Van Boom J. RNA splicing is required to make the messenger RNA for a variant surface antigen in trypanosomes. Nucleic Acids Res. 1982 Jun 25;10(12):3591–3604. doi: 10.1093/nar/10.12.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder J. A., Eder P. S., Engman D. M., Brentano S. T., Walder R. Y., Knutzon D. S., Dorfman D. M., Donelson J. E. The 35-nucleotide spliced leader sequence is common to all trypanosome messenger RNA's. Science. 1986 Aug 1;233(4763):569–571. doi: 10.1126/science.3523758. [DOI] [PubMed] [Google Scholar]
- White T. C., Rudenko G., Borst P. Three small RNAs within the 10 kb trypanosome rRNA transcription unit are analogous to domain VII of other eukaryotic 28S rRNAs. Nucleic Acids Res. 1986 Dec 9;14(23):9471–9489. doi: 10.1093/nar/14.23.9471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Korte D., Haverkort W. A., Roos D., van Gennip A. H. Anion-exchange high performance liquid chromatography method for the quantitation of nucleotides in human blood cells. Clin Chim Acta. 1985 Jun 14;148(3):185–196. doi: 10.1016/0009-8981(85)90145-7. [DOI] [PubMed] [Google Scholar]